Abstract
Aims
The experiments explored for atrial arrhythmogenesis and its possible physiological background in recently developed hetero-(RyR2+/S) and homozygotic (RyR2S/S) RyR2-P2328S murine models for catecholaminergic polymorphic ventricular tachycardia (VT) for the first time. They complement previous clinical and experimental reports describing increased ventricular arrhythmic tendencies associated with physical activity, stress, or catecholamine infusion, potentially leading to VT and ventricular fibrillation.
Methods and results
Atrial arrhythmogenic properties were compared at the whole animal, Langendorff-perfused heart, and single, isolated atrial myocyte levels using electrophysiological and confocal fluorescence microscopy methods. This demonstrated that: (i) electrocardiographic parameters in intact anaesthetized wild-type (WT), RyR2+/S and RyR2S/S mice were statistically indistinguishable both before and after addition of isoproterenol apart from increases in heart rates. (ii) Bipolar electrogram and monophasic action potential recordings showed significantly higher incidences of arrhythmogenesis in isolated perfused RyR2S/S, but not RyR2+/S, relative to WT hearts during either regular pacing or programmed electrical stimulation. The addition of isoproterenol increased such incidences in all three groups. (iii) However, there were no accompanying differences in cardiac anatomy or action potential durations at 90% repolarization and refractory periods. (iv) In contrast, episodes of diastolic Ca2+ release were observed under confocal microscopy in isolated fluo-3-loaded RyR2S/S, but not RyR2+/S or WT, atrial myocytes. The introduction of isoproterenol resulted in significant diastolic Ca2+ release in all three groups.
Conclusions
These findings establish acute atrial arrhythmogenic properties in RyR2-P2328S hearts and correlate these with altered Ca2+ homeostasis in an absence of repolarization abnormalities for the first time.
Keywords: RyR2, Atrial arrhythmia, Calcium, Electrocardiography, Monophasic action potentials
1. Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an arrhythmogenic cardiac disorder associated with a characteristic PVT induced by physical activity, stress, or catecholamine infusion, which can deteriorate into ventricular fibrillation (VF). Patients typically present with recurrent syncope, seizures, or sudden death after physical activity or emotional stress.1
Two major genes have been implicated in CPVT. One is transmitted as an autosomal dominant trait caused by mutations in the RyR2 gene.2 More than 70 distinct RyR2 mutations have been reported.2–5 The other is a recessive form caused by mutations in the cardiac-specific isoform of CASQ2.6,7 Mutant RyR22 and CASQ26 may influence myocyte Ca2+ homeostasis resulting in inappropriate ‘leakiness’ of RyR2-Ca2+ release channels, causing increases in their basal activity, alterations in their phosphorylation status or defective interactions of RyR2 with other molecules or ions, including FKBP12.68, CASQ2, or Mg2+, or its abnormal activation by extra- or intraluminal Ca2+.9 Such changes recently have been investigated using murine CPVT models containing either R4497C10 or P2328S modifications in the RyR2 gene.11
There are also reports associating clinical atrial as opposed to ventricular arrhythmias with RyR2 mutations. A large in-frame deletion in the N-terminal region of RyR2 has recently been correlated with progressive atrial ventricular block, sino-atrial node dysfunction, atrial fibrillation (AF), and atrial standstill in addition to the typical CPVT.12 Supraventricular arrhythmias including isolated atrial ectopic beats, non-sustained supraventricular tachycardia, and short runs of AF have been reported during exercise with an onset pattern similar to that of the ventricular arrhythmias.1,13,14 So have episodes of sinus bradycardia.1,14,15
However, apart from a recent, important report on the RyR2-R176Q heterozygotic system, there have been few studies of atrial arrhythmogenesis in genetically modified RyR2 mouse models.16 In addition, all these studies were confined to heterozygotic, omitting homozygotic variants resulting from the genetic modifications. The present study accordingly explores for such arrhythmogenic properties and their possible physiological background in a recently developed RyR2-P2328S murine model for CPVT11 for the first time, studying both heterozygotic and homozygotic systems. They thus extend previous findings on ventricular arrhythmogenic properties in the P2328S system. We evaluated the presence or absence of anatomical abnormalities, spontaneous and atrial arrhythmogenicity, and alterations in action potential recovery in intact anaesthetized animals, isolated perfused hearts, and altered Ca2+ homeostasis in atrial myocytes.
2. Methods
2.1. Animals
Heterozygote RyR2+/S, homozygote RyR2S/S, and wild-type (WT) mice (aged 3–6 months) with an inbred 129/Sv genetic background (Harlan, UK) were generated as described on a previous occasion.11 They were kept in plastic cages at room temperature in an animal facility, given free access to sterile rodent chow and water and subjected to 12h light/dark cycles. All procedures were performed in institutional premises approved under the UK Animals (Scientific Procedures) Act (1986), under UK Home Office project licence no. PPL No. 80/1974, approved by a university ethics review board, accordingly also in conformity with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. In vivo surface electrocardiographic recordings
Mice were anaesthetized using avertin (2,2,2-tribromo-ethanol: Sigma, Poole, Dorset, UK) in a 24 mg/mL solution at 0.10 mL/(10 g body weight) dosage injected into the left peritoneal cavity 5 min before electrical recording. They were placed on a heating pad with body temperature monitoring for three-lead electrocardiographic (ECG) measurements using subcutaneous needle electrodes employing a Powerlab 26T system (AD Instruments, Hastings, UK). The digital recordings (16 bit, 2 kHz/channel) were analysed using the Chart v6.0 program (AD Instruments, Oxfordshire, UK) giving signal-averaged ECGs and ECG parameters in which corrected, QT was given as QTc = QT/(RR/100)1/2.17 This choice of injected rather than inhalational anaesthesia permitted experiments using oesophageal-stimulating electrodes. Preliminary experiments on WT animals also explored ketamine–xylazine anaesthetic (1.8 mL ketamine hydrochloride at 100 mg/mL, 0.35 mL xylazine hydrochloride at 23.32 mg/mL, and 2.85 mL of sterile phosphate base solution; administration at 0.10 mL/(10 g body weight). However, this gave greater bradycardic effects (heart rates 260.6 ± 18.6, n = 12 vs. 410.4 ± 14.5 b.p.m., n = 19; P < 0.01) in agreement with previous reports.18 An isoproterenol stress protocol involved recording the baseline ECG in lead II for 10 min, followed by intraperitoneal injection of 2.0 mg/kg isoproterenol.
2.3. In vivo stimulation studies
A further series of in vivo experiments then applied programmed stimulation procedures both before and after the application of isoproterenol during the ECG recordings in the anaesthetized mice. This used an ultraminiature octapolar 1.1F electrophysiological oesophageal catheter that permitted connections to stimulating leads (EPR-800, Millar Instrument, Inc., Houston, TX, USA). The stimulation protocols were performed after 5 min of stable simultaneous recordings of the baseline surface ECG. The stimulation protocols first applied oesophageal pacing at a 100 ms cycle length (CL) for 30 s. Secondly, a burst pacing procedure involved pacing hearts at a 40 ms CL of 40 ms in bursts of 2 s duration. This CL was then decreased in successive 2 s bursts in 2ms decrements following sets of three cycles until a CL of 20 ms was reached.
2.4. Electrophysiological studies in Langendorff-perfused hearts
Langendorff-perfused hearts showing clear-cut 1:1 atrioventricular conduction during intrinsic activity following cannulation were studied as described previously19,20 using Krebs–Henseleit (K–H) solution (in mM: NaCl 119, NaHCO3 25, KCl 4.0, KH2PO4 1.2, MgCl2 1.0, CaCl2 1.8, glucose 10, and sodium pyruvate 2.0) maintained at pH 7.4 by bubbling with 95% O2–5% CO2 (British Oxygen, Manchester, UK) using the following recording methods: (i) bipolar electrogram (BEG) electrodes with 1mm interpole spacings were placed on the left atrium and left ventricle. Hearts were initially paced for >5 min at 10 Hz to permit them to regain a steady state. The resulting electrogram signals were amplified for band-pass filtering between 30 Hz and 1 kHz. (ii) Left atrial monophasic action potentials (MAPs) were recorded with a microMAP electrode (Linton Instruments, Harvard Apparatus, UK) for band-pass filtering between 0.5 Hz and 1 kHz. All signals were recorded using an NL100AK head stage and NL104 amplifier unit, then band-pass filtered (Neurolog NL 125/6 Filter, Neurolog, Hertfordshire, UK), then digitized at 5 kHz by a micro 1401plus MKII laboratory interface (Cambridge Electronic Design, Cambridge, UK) using Spike2 software (Cambridge Electronic Design), and action potential durations corresponding to the time at 90% recovery of the MAPs to baseline (APD90) were determined.
Three types of pacing protocols were used. (i) Hearts were studied at their intrinsic rates in the absence of stimulation. (ii) Regular pacing at 10 Hz used 2ms square-wave stimuli set at twice the excitation threshold (Grass S48 stimulator; Grass-Telefactor, Slough, UK). (iii) Programmed electrical stimulation (PES) began using standard baseline pacing stimuli at frequencies of 10 Hz for 20 s. Drive trains of eight paced beats (S1) were each followed by an extra-stimulus (S2) every ninth beat, initially at an S1S2 interval equal to the pacing interval. Each subsequent cycle reduced the S1S2 interval by 1 ms until atrial refractoriness was reached.
2.5. Atrial myocyte isolation
Atrial myocyte isolation used methods described previously.20 Briefly, hearts were rapidly excised and cannulated in ice-cold K–H solution before Langendorff perfusion with a succession of buffers (in mmol/L): NaCl 125; KCl 4.75; MgSO4 1.2; KH2PO4 1.2; HEPES 30; glucose 10; taurine 50, titrated to pH 7.4 with NaOH to which was added 750 μM Ca2+, 5 mM nitrilotriacetic acid, 1 mg/ml collagenase type II (Worthington, NJ, USA), and 1 mg/mL hyaluronidase, respectively, at a stable temperature of 37 °C. The heart was then removed and the atrial appendages excised and chopped into pieces in buffer with reduced enzyme. These were incubated for 5–10 min with gentle manual agitation. Cells were then separated from the enzymatic solution by centrifuging at 243g for 3 min. The isolated cells were washed using BSA-containing wash buffer, followed after 5 min by centrifugation at 30g for 2 min. The cells were resuspended in normal K–H solution and, after 5 min, centrifuged again at 30g for 2 min. The cells were maintained at room temperature for the experiments that followed and used within 4–6h.
2.6. Measurements of Ca2+ transients
Cells placed on a Grade 1 circular laminin-coated cover slip (Menzel, Glasbearbeitungswerk, Germany) forming the floor of a 1.5 mL perfusion chamber were incubated with 5 µM Fluo-3 AM (Molecular Probes, Leiden, The Netherlands) in K–H buffer (1.2 mM CaCl2) for 10–20 min in the dark before washout. Cells were scanned using a Leica TCS SP5 confocal scanning laser microscopy system incorporating a Leica 6000 CS inverted microscope with a ×63 water-immersion objective lens (numerical aperture 1.2; confocal aperture 600 μm; slice thickness 20.5 μm). The Fluo-3 was excited using a 488 nm argon laser, and emission collected between 505 and 550 nm. Series of 250 frames (128 × 64 pixels/frame) were collected at 25 ms/frame. Images were analysed using custom-made software. Fluorescence, corrected for background signal in regions outside the cells, was measured within defined regions of interest (ROIs; F) and normalized to resting (F0) values. Peak F/F0 values were calculated through each time series for each myocyte and a mean peak F/F0 was calculated for that series. Where indicated, cells were paced at 1 Hz (5 V above threshold of 30–60 V for 2 ms) with two field electrodes. Ca2+ transients were measured both from ROIs covering entire cells, as well as localized ROIs.
Myocytes loaded with Fluo-3 AM were selected for the study of spontaneous Ca2+ release events (sparks) on the basis of their clear striation pattern, contraction on electrical field stimulation by 5–15% of their resting length, and an absence of spontaneous contractions during a preliminary 1 min observation period. Sparks were recorded in the line scan imaging mode before and after addition of isoproterenol at resting condition along the long axis of the myocyte. Each scan consisted of 512 pixels (=40 μm) scanned at intervals of 1 ms over 5 s. Regions were selected for such imaging with the aid of series of preliminary frame scans obtained at intervals of 25 ms over 5 s from which a presence of spontaneous Ca2+ events could be reconstructed. The frequencies (in s−1) of Ca2+ sparks were analysed using an in-house custom-made software program. All fluorescence studies were performed at room temperature.
2.7. Statistical analysis
Data are expressed as means ± SEM. The numbers, n, denote either numbers of whole hearts, the number of cells, or peak F/F0 values. Different experimental genotype groups were compared using one-way ANOVA for correlated samples analysis of variance (SPSS software) and Student's unpaired t-test as appropriate, with P < 0.05 considered statistically significant. Categorical variables were compared using χ2 and Fisher's exact tests, with P < 0.05 considered significant. All chemical agents were purchased from Sigma-Aldrich (Poole, UK) except where otherwise indicated.
3. Results
3.1. ECG characteristics of anaesthetized WT, RyR2+/S, and RyR2S/S mice
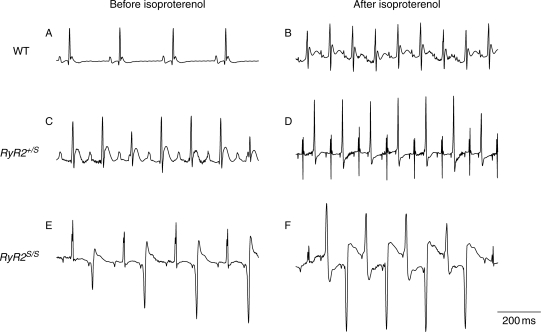
Gross morphological and light microscopic (haematoxylin and eosin: Supplementary material online, Figure S1A, C, and E; Masson trichrome: Supplementary material online, Figure S1B, D, and F) examination showed no obvious structural differences and indistinguishable heart to body weight ratios between WT (Supplementary material online, Figure S1A and B), RyR2+/S (Supplementary material online, Figure S1C and D), and RyR2S/S (Supplementary material online, Figure S1E and F) groups. Figure 1 shows typical ECG records from steadily beating anaesthetized WT (Figure 1A and B), RyR2+/S (Figure 1C and D), and RyR2S/S hearts (Figure 1E and F) both before (Figure 1A, C, and E) and after addition of isoproterenol (2.0 mg/kg; Figure 1B, D, and F). Table 1 summarizes ECG parameters when hearts were in normal sinus rhythm. These were statistically indistinguishable between all three groups, under both conditions, apart from increases in heart rates (P < 0.01, 0.01, and 0.05 in WT, RyR2+/S, and RyR2S/S, respectively; n = 8 mice in each group) produced by isoproterenol. However, there were contrasting atrial and ventricular arrhythmogenic features. None of the WT studied (n = 8) showed arrhythmias whether before or after addition of isoproterenol (Figure 1A and B). In contrast, VT and VF did occur in the RyR2+/S and RyR2S/S confirming earlier results.11 Furthermore, of the RyR2+/S (n = 8), one heart showed spontaneous bigeminy lasting for 13.2 s before and three showed ventricular arrhythmias after addition of isoproterenol. All three of the latter showed episodes of bigeminy lasting 0.4–5.8 s (Figure 1D), two showed multiple episodes of bidirectional VT (BVT) lasting from 0.5 to 2.5 s. Finally, of the eight RyR2S/S mice, one showed episodes of spontaneous bigeminy (Figure 1E) and BVT both before and after addition of isoproterenol (Figure 1F). In addition, after addition of isoproterenol, all the RyR2S/S mice showed multiple ventricular ectopic beats. Four showed episodes of bigeminy lasting from 0.5 to 7.4 s, five showed BVT lasting for 0.5–59.4 s, two showed monomorphic VT lasting from 0.8 to 23.0 s, and one showed multiple PVT episodes lasting from 0.5 to 28.7 s. In contrast, none of the WT, RyR2+/S, or RyR2S/S showed spontaneous atrial tachycardia (AT) and AF whether in the presence or absence of isoproterenol.
Figure 1.
In vivo ECG records from anaesthetized WT, RyR2+/S, and RyR2S/S, before and after addition of isoproterenol. (A and B) WT hearts in normal sinus rhythm under both conditions. (C) Normal sinus rhythm before and (D) episodes of bigeminy following addition of isoproterenol in RyR2+/S. Episodes of (E) bigeminy before and (F) BVT after adding isoproterenol in RyR2S/S.
Table 1.
Comparison of in vivo ECG parameters in anaesthetized WT, RyR2+/S, and RyR2S/S, before and after addition of isoproterenol
| Conditions | Genotype | n | Heart rate (b.p.m.) | PR interval (ms) | P-wave duration (ms) | QRS duration (ms) | QT interval (ms) | QTc interval (ms) |
|---|---|---|---|---|---|---|---|---|
| Control | WT | 8 | 422.4 ± 17.1 | 45.2 ± 3.6 | 9.2 ± 0.8 | 12.6 ± 0.7 | 38.9 ± 4.5 | 32.3 ± 3.6 |
| RyR2+/S | 8 | 391.4 ± 14.8 | 49.6 ± 3.0 | 10.3 ± 1.2 | 12.3 ± 0.9 | 38.0 ± 2.7 | 30.6 ± 2.4 | |
| RyR2S/S | 8 | 422.2 ± 28.5 | 43.7 ± 2.2 | 9.4 ± 0.8 | 11.1 ± 0.8 | 42.8 ± 7.1 | 34.5 ± 4.7 | |
| Isoproterenol | WT | 8 | 503.1 ± 26.5** | 53.4 ± 6.4 | 10.0 ± 1.0 | 14.2 ± 1.0 | 47.5 ± 4.3 | 43.3 ± 3.9 |
| RyR2+/S | 8 | 464.1 ± 17** | 57.4 ± 6.3 | 9.8 ± 1.0 | 13.4 ± 1.0 | 40.6 ± 1.8 | 35.6 ± 1.5 | |
| RyR2S/S | 8 | 513.0 ± 26.1* | 49.8 ± 5.7 | 11.6 ± 2.2 | 11.3 ± 1.0 | 43.2 ± 5.1 | 39.1 ± 3.7 |
Control vs. isoproterenol addition *P < 0.05, **P < 0.01.
3.2. In vivo stimulation studies
The subsequent experiments then investigated for the presence or absence of arrhythmic properties during programmed stimulation of the in vivo preparation. First, oesophageal pacing was applied at a 100 ms CL for 30 s. Five animals were studied in each group. None of the WT or heterozygotes showed atrial arrhythmia whether before and after addition of isoproterenol. One RyR2+/S mouse showed bigeminy both before and after addition of isoproterenol. Of the RyR2S/S, one animal showed ventricular arrhythmias before addition of isoproterenol and two showed ventricular arrhythmias after addition of isoproterenol, one of which was a BVT and the other was bigeminy. However, none of these showed atrial arrhythmias.
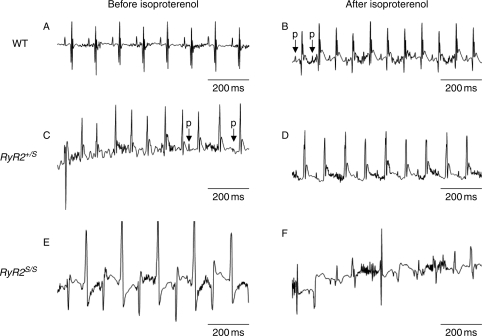
Secondly, burst pacing was applied to three mice from each group. Hearts initially showing intrinsic activity were paced at a CL of 40 ms in bursts of 2 s duration. This CL was then decreased in successive 2 s bursts in 2ms decrements following sets of three cycles until a CL of 20 ms was reached. Cardiac electrical activity was then measured immediately following such stimulation when hearts were then permitted to resume intrinsic activity for 5 min. Results were compared in hearts both before and after the addition of isoproterenol. None of the three WT mice studied showed either AT and AF whether or not isoproterenol was present (Figure 2A and B). However, two mice showed isolated ectopic ventricular beats after burst pacing after addition of isoproterenol. In contrast, of the three RyR2+/S mice studied, one showed atrioventricular block after burst pacing both before and after addition of isoproterenol. One showed an AT episode following burst pacing lasting 1.78 s (Figure 2C) and one animal showed spontaneous ventricular bigeminy before addition of isoproterenol. However, there were no atrial arrhythmic episodes after addition of isoproterenol (Figure 2D). Of the three RyR2S/S mice studied, two showed spontaneous bigeminy and sustained BVT (Figure 2E) before addition of isoproterenol. All three RyR2S/S mice showed BVT after burst pacing both before and after addition of isoproterenol (Figure 2F); this made it difficult to clarify the atrial phenotype.
Figure 2.
ECG records obtained during oesophageal stimulation. Typical results from (A and B) WT hearts, (C and D) RyR2+/S, and (E and F) RyR2S/S hearts before (A, C, and E), and following (B, D, and F) addition of isoproterenol. The letter ‘p’ indicates P-wave.
3.3. Comparison of atrial arrhythmogenicity in isolated WT, RyR2+/S, and RyR2S/S hearts
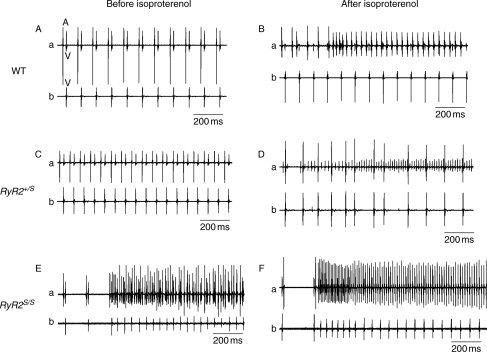
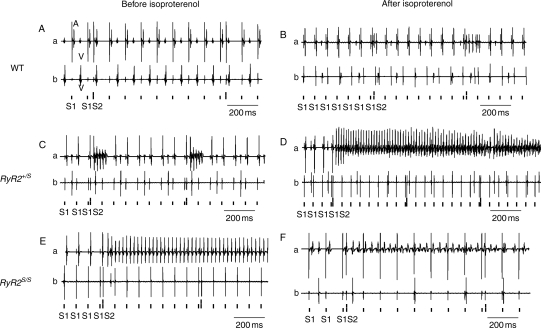
Experiments in isolated Langendorff-perfused hearts then explored for spontaneous and provoked atrial arrhythmias. A comparison of simultaneously recorded bipolar atrial and ventricular traces made it possible to attribute observed arrhythmias to either atrial or ventricular activity. Of intrinsically beating WT (Figure 3A and B), RyR2+/S (Figure 3C and D), and RyR2S/S (Figure 3E and F), before (Figure 3A, C, and E) and after introduction of isoproterenol (100 nM; Figure 3B, D, and F), atrial arrhythmias occurred in isoproterenol-treated WT and RyR2+/S (Figure 3A–D) and in RyR2S/S both before and after isoproterenol administration (Figure 3E and F). Similar recordings were made during PES in WT (Figure 4A and B), RyR2+/S (Figure 4C and D) and RyR2S/S (Figure 4E and F) before (Figure 4A, C, and E) and after (Figure 4B, D, and F) isoproterenol treatment. Figure 4 exemplifies non-sustained (Figure 4C) and sustained (Figure 4D) atrial arrhythmias from RyR2+/S and sustained arrhythmias in RyR2S/S (Figure 4E and F) hearts. MAP recordings differentiated these phenomena into AT, as exemplified in an RyR2+/S heart (Figure 5A and B) or AF, as exemplified in an RyR2S/S heart (Figure 5C and D) both before (Figure 5A and C) and after (Figure 5B and D) addition of isoproterenol.
Figure 3.
BEG records from both the atria and ventricles of WT, RyR2+/S, and RyR2S/S hearts before and after introduction of isoproterenol. Atrial (a) and ventricular (b) BEG records showing normal sinus rhythm before (A and C), but episodes of AT after addition of isoproterenol (B and D) in intrinsically beating WT (A and B) and RyR2+/S (C and D). Episodes of AT in intrinsically beating RyR2S/S (E and F) both before (E) and following addition of isoproterenol (F). In panel (A), contributions from atrial activation are labelled ‘A’ and those from ventricular activation are labelled ‘V’.
Figure 4.
BEG records obtained from atria and ventricles during programmed electrical stimulation in WT, RyR2+/S and RyR2S/S before and after introduction of isoproterenol. Atrial (a) and ventricular (b) BEG records showing normal (A) non-sustained (B and C) and sustained (D) AT before (A and C) and after (B and D) introduction of isoproterenol in WT and RyR2+/S. Episodes of sustained AT occurred in both conditions in RyR2S/S (E and F). The short vertical bars below the traces give the timings of the S1, and the long bars the S2 stimuli in the PES sequences.
Figure 5.
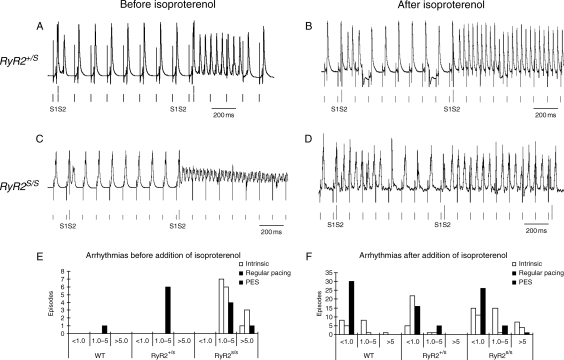
MAP recordings from RyR2+/S and RyR2S/S during PES before and after introduction of isoproterenol. (A) Non-sustained and (B) sustained tachycardia observed before (A) and after (B) introduction of isoproterenol in RyR2+/S. Episodes of sustained AT were observed both before (C) and after (D) introduction of isoproterenol arrhythmias in RyR2S/S following PES. (E and F) Numbers of episodes sorted by durations of <1.0, 1.0–5.0, and >5.0 s before (E) and following (F) addition of isoproterenol. There were larger numbers of arrhythmic episodes of longer duration in the RyR2S/S before addition of isoproterenol (E) than the remaining groups. An increased incidence particularly of brief arrhythmic episodes (<1.0 s) occurred in all experimental groups (F) following addition of isoproterenol.
Table 2 summarizes the incidence of hearts showing atrial arrhythmogenesis (Columns 3 and 7) for which different experimental groups were tested against each other for significance. The remaining columns provide details concerning the number of episodes observed as well as their durations. This demonstrates that under conditions of intrinsic pacing, there were no significant differences in the incidence of atrial arrhythmic episodes between groups. However, during both regular pacing and PES, the RyR2S/S, but not the RyR2+/S, was more arrhythmogenic than WT in the absence of isoproterenol. In contrast, both the RyR2+/S and the RyR2S/S were more arrhythmogenic than untreated WT in the presence of isoproterenol.
Table 2.
Summary of atrial arrhythmogenic properties in WT, RyR2+/S, and RyR2S/S before and after addition of isoproterenol
| Pacing protocol | Genotype | Incidence of hearts showing AT and AF before addition of isoproterenol |
Incidence of hearts showing AT and AF after addition of isoproterenol |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Arrhythmic incidence in n hearts | No. of episodes | Duration of episodes (range) (s) | Duration of episodes (mean ± SEM) | Arrhythmic incidence in n hearts | No. of episodes | Duration of episodes (range) (s) | Duration of episodes (mean ± SEM) | ||
| Intrinsic | WT | 0/14 | 0 | 0 | 0 | 1/14 | 10 | 0.15–8.24 | 1.20 ± 2.51 |
| RyR2+/S | 0/16 | 0 | 0 | 0 | 2/15 | 6 | 0.13–5.04 | 1.28 ± 1.87 | |
| RyR2S/S | 3/18 | 28 | 0.17–5.99 | 0.89 ± 1.23 | 3/17 | 36 | 0.28–8.01 | 2.77 ± 2.29 | |
| Regular | WT | 0/14 | 0 | 0 | 0 | 2/14 | 17 | 0.26–26.53 | 2.51 ± 6.22 |
| RyR2+/S | 1/16 | 1 | 0.21 | 0.21 ± 0.00 | 5/15 | 21 | 0.14–3.33 | 0.31 ± 0.69 | |
| RyR2S/S | 5/18* | 30 | 0.22–8.54 | 2.3 ± 3.15 | 4/17 | 10 | 0.1–7.38 | 2.84 ± 3.57 | |
| PES | WT | 3/14 | 27 | 0.09–2.72 | 0.31 ± 0.05 | 6/12 | 29 | 0.1–0.72 | 0.28 ± 0.18 |
| RyR2+/S | 6/14 | 20 | 0.15–3.73 | 0.99 ± 1.03 | 5/14¶ | 10 | 0.1–4.86 | 1.68 ± 1.89 | |
| RyR2S/S | 12/16** | 36 | 0.1–5.59 | 0.59 ± 1.25 | 9/12¶ | 29 | 0.13–6.03 | 0.83 ± 1.27 | |
Note: vs. WT *P < 0.05, **P < 0.01; vs. WT before addition of isoproterenol ¶P < 0.05.
Figure 5E and F classifies the numbers of such episodes into durations of <1.0, 1.0–5.0, and >5.0 s. Results compared before (Figure 5E) and after (Figure 5F) addition of isoproterenol demonstrate larger numbers of arrhythmic episodes of greater duration in the RyR2S/S than the remaining groups. Isoproterenol increased the incidence of arrhythmic episodes, particularly of brief episodes (<1.0 s) in all the experimental groups.
3.4. Atrial arrhythmogenesis in isolated RyR2+/S and RyR2S/S hearts takes place in the absence of alterations in action potential waveforms and refractory periods
Previous studies have implicated shortenings of the APD90, and of the atrial effective refractory periods (AERPs) in re-entrant atrial arrhythmogenicity in murine hearts.21 The AERP in particular appeared important as the determinant of the time period in which a given myocardial region is capable of re-excitation in response to a stimulus, whether appropriate and originating from the sinus node, or abnormal ectopic or re-entrant activity.22 In contrast, prolongations in these parameters conferred protection from such arrhythmic effects in studies of murine hearts modelling genetic modifications in the Na+ channel.23 In contrast, the atrial arrhythmogenicity associated with RyR2 modification observed here were not accompanied by alterations in either of these parameters, and therefore appear to reflect a contrasting mechanism. Table 3 (Columns 2 and 3) thus demonstrates statistically indistinguishable APD90 values obtained from hearts in each of the three groups during regular pacing; Table 3 (Columns 4 and 5) demonstrates statistically identical AERPs, given by the shortest S1S2 interval that failed to elicit an action potential. None of these parameters were significantly altered by the introduction of isoproterenol.
Table 3.
Action potential parameters obtained in WT, RyR2+/S and RyR2S/S before and after introduction of isoproterenol
| Genotype | APD90 (ms) |
AERP (ms) |
||
|---|---|---|---|---|
| Control (mean ± SEM) (n) | Isoproterenol (mean ± SEM) (n) | Control (mean ± SEM) (n) | Isoproterenol (mean ± SEM) (n) | |
| WT | 20.26 ± 1.57 (6) | 17.14 ± 0.84 (5) | 24.62 ± 1.30 (13) | 23.31 ± 1.48 (13) |
| RyR2+/S | 18.61 ± 1.33 (9) | 19.86 ± 0.54 (5) | 23.64 ± 1.64 (14) | 25.18 ± 2.71 (17) |
| RyR2S/S | 19.02 ± 1.06 (7) | 19.87 ± 1.12 (6) | 26.05 ± 1.95 (20) | 25.95 ± 0.95 (19) |
3.5. Atrial arrhythmogenesis in isolated RyR2+/S and RyR2S/S hearts is associated with abnormal Ca2+ homeostasis
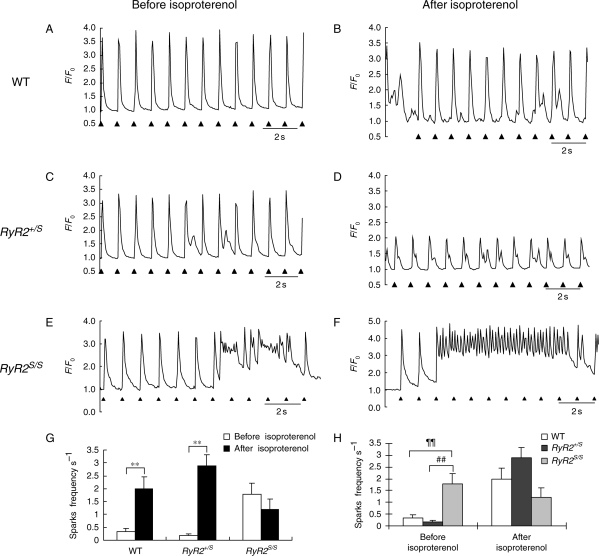
In contrast, confocal fluorescence microscope studies in fluo-3-loaded atrial myocytes demonstrated contrasting patterns in Ca2+ homeostasis between the three groups. Figure 6 shows typical fluorescence traces obtained from WT (Figure 6A and B), RyR2+/S (Figure 6C and D), and RyR2S/S (Figure 6E and F) myocytes regularly field-stimulated at 1.0 Hz before (Figure 6A, C, and E) and after introduction of isoproterenol (100 nM; Figure 6B, D, and F). In contrast to WT and RyR2+/S, RyR2S/S myocytes showed a significantly greater incidence of evoked Ca2+ responses that showed episodes of diastolic Ca2+ release in the absence of isoproterenol. The introduction of isoproterenol resulted in an increase in such incidences in all the groups, despite an absence of significant difference in peak F/F0 values evoked by the regular stimulation (Table 4). All these findings are in exact parallel with the incidence of atrial arrhythmia displayed in Table 2. Such diastolic phenomena would be expected to result in a formation of delayed after-depolarization events resulting from increased electrogenic Na+–Ca2+ exchanger following the consequent elevations in cytoplasmic Ca2+. These could potentially alter membrane voltage to threshold to give rise to extrasystolic triggered activity.
Figure 6.
Ca2+ transients from regularly stimulated fluo-3-loaded WT, RyR2+/S, and RyR2S/S atrial myocytes studied using confocal microscopy before and after addition of isoproterenol. Left (A, C, and E) and right (B, D, and F) columns illustrate WT (A, B), RyR2+/S (C and D), and RyR2S/S (E and F) Ca2+ transients before (A, C, and E) and following addition of isoproterenol (B, D, and F). Regular Ca2+ transients without (A) and showing diastolic Ca2+ release (B) before (A) and following (B) addition of isoproterenol in WT (A and B). Diastolic Ca2+ release (C) whose incidence was increased by isoproterenol (D) in RyR2+/S. Multiple episodes of diastolic Ca2+ release (E) accentuated by addition of isoproterenol (F) in RyR2S/S. Markers below the traces indicating timing of the regular stimulation. (G and H) Frequencies of Ca2+ sparks among the three genotypes; **denote differences to a significance level of P < 0.01 (G), and before and after addition of isoproterenol; ¶¶ and ## both denote differences to a significance level of P < 0.01 (H).
Table 4.
Results of confocal fluorescence microscopy of fluo-3-loaded atrial myocytes during regular stimulation
| Genotype | Control |
Isoproterenol |
||||||
|---|---|---|---|---|---|---|---|---|
| Number of cells (n) | Number of peaks | Peak fluorescence expressed as F/F0 | Number of episodes of diastolic release | Number of cells (n) | Number of peaks | Peak fluorescence expressed as F/F0 | Number of episodes of diastolic release | |
| WT | 9 | 141 | 3.06 ± 0.17 | 11 | 7 | 84 | 3.18 ± 0.42 | 13*** |
| RyR2+/S | 15 | 179 | 3.64 ± 0.25 | 12 | 11 | 132 | 2.91 ± 0.27 | 82###,**** |
| RyR2S/S | 10 | 115 | 2.81 ± 0.28 | 39###,¶¶¶ | 14 | 168 | 2.94 ± 0.23 | 224###,¶¶¶,*** |
Note: vs. control ***P < 0.001; vs. WT ###P < 0.001; vs. RyR2+/S ¶¶¶P < 0.001.
The above findings correlated with our estimates of spontaneous Ca2+ release events obtained in quiescent atrial myocytes. The presence of these was clearly demonstrable in the preliminary frame scan images obtained from cells from all three experimental groups both before and after addition of isoproterenol. Furthermore, analysis of their frequencies correlated closely with the corresponding arrhythmic tendencies that were observed at the level of whole hearts. Thus, Figure 5G and H, Supplementary material online, Figure S2, and Table 5 demonstrate significantly higher event frequencies in RyR2S/S (P < 0.01) but not RyR2+/S compared with WT and in RyR2S/S compared with RyR2+/S in the absence of isoproterenol. These differences disappeared following addition of isoproterenol, an effect attributable to its action in significantly (P < 0.01) increasing event frequency in WT and RyR2+/S but not RyR2S/S.
Table 5.
Frequencies of spontaneous Ca2+ release events in WT, RyR2+/S, and RyR2S/S atrial myocytes before and after addition of isoproterenol
| WT (n) | RyR2+/S (n) | RyR2S/S (n) | |
|---|---|---|---|
| Before isoproterenol | 0.35 ± 0.12 (12) | 0.17 ± 0.07 (9) | 1.78 ± 0.44¶¶,## (10) |
| After isoproterenol | 2.00 ± 0.47 (14)** | 2.89 ± 0.43** (11) | 1.20 ± 0.40 (10) |
**P < 0.01 when compared with the corresponding findings obtained before addition of isoproterenol.
¶¶P < 0.01 when compared with findings obtained from WT before addition of isoproterenol.
##P < 0.01 when compared with findings obtained from RyR2+/S before addition of isoproterenol.
4. Discussion
The present experiments explored for and characterized acute atrial arrhythmogenic properties in recently developed hetero-(RyR2+/S) and homozygotic (RyR2S/S) RyR2-P2328S murine models for CPVT, and investigated their physiological background. The RyR2-P2328S mutation was first described in a Finnish family with CPVT.3,24 However, clinical reports also associate occasional acute atrial arrhythmias in patients carrying RyR2 mutations.12,25 Thus, in reports including a family with RyR2-P2328S, although all carriers exhibited polymorphic VT and/or VF, three patients also showed non-sustained AT.24 The present experiments demonstrated acute arrhythmogenic properties in structurally normal atria of RyR2-P2328S hearts. They correlated these with altered Ca2+ homeostasis in an absence of abnormalities in action potential repolarization for the first time.
First, in intact anaesthetized animals, mutations in RyR2-P2328S did not alter baseline ECG parameters during normal sinus rhythm. ECG parameters in WT, RyR2+/S, and RyR2S/S hearts were statistically indistinguishable. None of three groups showed significant differences in RR, P-wave, QRS complex, and QTc intervals whether before and after addition of isoproterenol apart from increased heart rates. These findings paralleled previous clinical reports of the family of RyR2-P2328S mutation carriers24 and other clinical examples.15 The latter thus also showed normal RR intervals, atrioventricular conduction, QRS complex morphology, ST-segment, and T-wave pattern, although QTc intervals of affected individuals were slightly longer than those of controls.24 The present findings in RyR2-P2328S hearts also agreed with baseline ECG parameters in the alternative RyR2-R4496C and RyR2-R176Q mouse models.10,26
RyR2+/S and RyR2S/S but not WT hearts showed episodic spontaneous ventricular arrhythmogenesis in both the absence and the presence of isoproterenol. Such ventricular arrhythmias often took the form of BVTs in common with clinical findings, which also describe exercise-induced supraventricular arrhythmias that precede, but which are less common than ventricular tachyarrhythmias in CPVT,1,27,28 making it less likely they are their direct cause. The present results similarly showed no incidents of spontaneous supraventricular arrhythmogenesis. The observed disruptions with the mutant RyR2 described below therefore likely reflect acute atrial arrhythmogenesis rather than representing chronic arrhythmogenic changes following electrical remodeling.29
Secondly, isolated RyR2S/S hearts showed atrial arrhythmogenicity in the absence of alterations in action potential waveforms and AERPs. Thus, BEG and MAP recordings in isolated perfused preparations demonstrated significantly higher arrhythmogenic incidences in RyR2S/S, but not RyR2+/S, relative to WT hearts during both regular pacing and PES, even before addition of isoproterenol. The addition of isoproterenol increased such incidences in all three groups. These findings were in contrast to the ECG findings in the intact mice. There are thus detailed differences between the murine and the previously described clinical findings, with the murine homozygote, but not heterozygote, showing an atrial arrhythmic phenotype, but then, in the absence of catecholaminergic challenge.
Thirdly, APD90 and AERPs were indistinguishable between the three groups. This contrasts with predictions of a re-entrant arrhythmogenic substrate resulting from abnormalities in action potential recovery as has been associated with gain-of-function KCNQ1 mutations. The latter involves the Kv7.1 α-subunit responsible for the slow delayed rectifier K+. It results in a shortened APD90 and AERP.30
Finally, studies in regularly stimulated isolated fluo-3-loaded RyR2S/S, but not RyR2+/S or WT, atrial myocytes contrastingly showed episodes of diastolic Ca2+ release under confocal microscopy. RyR2 mutation thus alters atrial Ca2+ homeostasis. The introduction of isoproterenol then resulted in significant diastolic Ca2+ release in all three groups. These findings directly parallel the corresponding observations of the presence or absence of increased atrial arrhythmogenesis in the isolated perfused hearts described above. These findings thus implicate alterations in Ca2+ homeostasis in the observed atrial arrhythmic properties. This could then take place through ectopic firing that might contribute to triggering of arrhythmia.31 These findings additionally directly parallel our earlier reports which correlated acute atrial arrhythmogenesis in intact WT hearts with effects of altered Ca2+ homeostasis in atrial myocytes following pharmacological manipulation.20
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Medical Research Council, Wellcome Trust, British Heart Foundation, the Biotechnology and Biological Sciences Research Council, and Natural Science Foundation of China (30371571, 30830051, and 30672209). Funding to pay the open access publication charge was provided by The Wellcome Trust.
Supplementary Material
Acknowledgements
Paul Frost and Alan Catell provided skilled assistance.
Conflict of interest: none declared.
References
- 1.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 3.Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- 4.Lehnart SE, Ackerman MJ, Benson DW, Jr, Brugada R, Clancy CE, Donahue JK, et al. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation. 2007;116:2325–2345. doi: 10.1161/CIRCULATIONAHA.107.711689. doi:10.1161/CIRCULATIONAHA.107.711689. [DOI] [PubMed] [Google Scholar]
- 5.Kauferstein S, Kiehne N, Neumann T, Pitschner HF, Bratzke H. Cardiac gene defects can cause sudden cardiac death in young people. Dtsch Arztebl Int. 2009;106:41–47. doi: 10.3238/arztebl.2009.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. doi:10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. doi:10.1161/01.RES.0000038886.18992.6B. [DOI] [PubMed] [Google Scholar]
- 8.Lehnart SE, Wehrens XH, Marks AR. Calstabin deficiency, ryanodine receptors, and sudden cardiac death. Biochem Biophys Res Commun. 2004;322:1267–1279. doi: 10.1016/j.bbrc.2004.08.032. doi:10.1016/j.bbrc.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 9.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. doi:10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 10.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. doi:10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 11.Goddard CA, Ghais NS, Zhang Y, Williams AJ, Colledge WH, Grace AA, et al. Physiological consequences of the P2328S mutation in the ryanodine receptor (RyR2) gene in genetically modified murine hearts. Acta Physiol (Oxf) 2008;194:123–140. doi: 10.1111/j.1748-1716.2008.01865.x. doi:10.1111/j.1748-1716.2008.01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders M, et al. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007;116:1569–1576. doi: 10.1161/CIRCULATIONAHA.107.711606. doi:10.1161/CIRCULATIONAHA.107.711606. [DOI] [PubMed] [Google Scholar]
- 13.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. doi:10.1161/01.CIR.0000020013.73106.D8. [DOI] [PubMed] [Google Scholar]
- 14.Sumitomo N, Harada K, Nagashima M, Yasuda T, Nakamura Y, Aragaki Y, et al. Catecholaminergic polymorphic ventricular tachycardia: electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart. 2003;89:66–70. doi: 10.1136/heart.89.1.66. doi:10.1136/heart.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann G, et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42:863–870. doi: 10.1136/jmg.2004.028993. doi:10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274:H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 18.Hart CY, Burnett JC, Jr, Redfield MM. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am J Physiol Heart Circ Physiol. 2001;281:H1938–H1945. doi: 10.1152/ajpheart.2001.281.5.H1938. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Fraser JA, Schwiening C, Zhang Y, Killeen MJ, Grace AA, et al. Acute atrial arrhythmogenesis in murine hearts following enhanced extracellular Ca2+ entry depends on intracellular Ca2+ stores. Acta Physiol (Oxf) 2010;198:143–158. doi: 10.1111/j.1748-1716.2009.02055.x. doi:10.1111/j.1748-1716.2009.02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Schwiening C, Killeen MJ, Ma A, Lei M, Grace AA, et al. Pharmacological changes in cellular Ca2+ homeostasis parallel initiation of atrial arrhythmogenesis in murine Langendorff-perfused hearts. Clin Exp Pharmacol Physiol. 2009;36:969–980. doi: 10.1111/j.1440-1681.2009.05170.x. doi:10.1111/j.1440-1681.2009.05170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nattel S, Shiroshita-Takeshita A, Brundel BJ, Rivard L. Mechanisms of atrial fibrillation: lessons from animal models. Prog Cardiovasc Dis. 2005;48:9–28. doi: 10.1016/j.pcad.2005.06.002. doi:10.1016/j.pcad.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Dobrev D. Atrial Ca2+ signaling in atrial fibrillation as an antiarrhythmic drug target. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:195–206. doi: 10.1007/s00210-009-0457-1. [DOI] [PubMed] [Google Scholar]
- 23.Dautova Y, Zhang Y, Sabir I, Grace AA, Huang CL. Atrial arrhythmogenesis in wild-type and Scn5a + /delta murine hearts modelling LQT3 syndrome. Pflugers Arch. 2009;458:443–457. doi: 10.1007/s00424-008-0633-z. doi:10.1007/s00424-008-0633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swan H, Piippo K, Viitasalo M, Heikkila P, Paavonen T, Kainulainen K, et al. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol. 1999;34:2035–2042. doi: 10.1016/s0735-1097(99)00461-1. doi:10.1016/S0735-1097(99)00461-1. [DOI] [PubMed] [Google Scholar]
- 25.Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita M, et al. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J. 2007;71:1606–1609. doi: 10.1253/circj.71.1606. doi:10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 26.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. doi:10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 27.Fisher JD, Krikler D, Hallidie-Smith KA. Familial polymorphic ventricular arrhythmias: a quarter century of successful medical treatment based on serial exercise-pharmacologic testing. J Am Coll Cardiol. 1999;34:2015–2022. doi: 10.1016/s0735-1097(99)00438-6. doi:10.1016/S0735-1097(99)00438-6. [DOI] [PubMed] [Google Scholar]
- 28.Liu N, Ruan Y, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. Prog Cardiovasc Dis. 2008;51:23–30. doi: 10.1016/j.pcad.2007.10.005. doi:10.1016/j.pcad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Nattel S, Shiroshita-Takeshita A, Cardin S, Pelletier P. Mechanisms of atrial remodeling and clinical relevance. Curr Opin Cardiol. 2005;20:21–25. [PubMed] [Google Scholar]
- 30.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. doi:10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 31.Dobrev D, Nattel S. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J Cardiovasc Pharmacol. 2008;52:293–299. doi: 10.1097/FJC.0b013e318171924d. doi:10.1097/FJC.0b013e318171924d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.