Abstract
Cardiac fibroblasts account for about 75% of all cardiac cells, but because of their small size contribute only ∼10–15% of total cardiac cell volume. They play a crucial role in cardiac pathophysiology. For a long time, it has been recognized that fibroblasts and related cell types are the principal sources of extracellular matrix (ECM) proteins, which organize cardiac cellular architecture. In disease states, fibroblast production of increased quantities of ECM proteins leads to tissue fibrosis, which can impair both mechanical and electrical function of the heart, contributing to heart failure and arrhythmogenesis. Atrial fibrosis is known to play a particularly important role in atrial fibrillation (AF). This review article focuses on recent advances in understanding the molecular electrophysiology of cardiac fibroblasts. Cardiac fibroblasts express a variety of ion channels, in particular voltage-gated K+ channels and non-selective cation channels of the transient receptor potential (TRP) family. Both K+ and TRP channels are important determinants of fibroblast function, with TRP channels acting as Ca2+-entry pathways that stimulate fibroblast differentiation into secretory myofibroblast phenotypes producing ECM proteins. Fibroblasts can couple to cardiomyocytes and substantially affect their cellular electrical properties, including conduction, resting potential, repolarization, and excitability. Co-cultured preparations of cardiomyocytes and fibroblasts generate arrhythmias by a variety of mechanisms, including spontaneous impulse formation and rotor-driven reentry. In addition, the excess ECM proteins produced by fibroblasts can interrupt cardiomyocyte-bundle continuity, leading to local conduction disturbances and reentrant arrhythmias. A better understanding of the electrical properties of fibroblasts should lead to an improved comprehension of AF pathophysiology and a variety of novel targets for antiarrhythmic intervention.
Keywords: Atrial fibrillation, Fibroblasts, Fibrosis, Remodelling, Extracellular matrix
1. Introduction
Cardiac fibrosis is a pathological response that causes abnormalities in cardiac conduction and mechanical function, thereby contributing to the pathophysiology of a variety of cardiac conditions, including hypertrophy, failure, and arrhythmias.1 Atrial fibrillation (AF) is the most common sustained clinical arrhythmia and is a major cause of population morbidity and mortality.2 Recent studies have demonstrated that structural remodelling, involving prominent fibrotic changes, is a fundamental determinant of the perpetuation of AF, and contributes synergistically with electrical remodelling to the AF substrate.3,4 Atrial fibrosis is a hallmark feature of arrhythmogenic structural remodelling in clinical AF.5 Increased amounts of fibrous tissue occur not only in AF patients with identifiable cardiac diseases,6,7 but also in those with lone AF.6,8 There is a positive correlation between the amount of fibrosis and the persistence of AF,7 suggesting that AF may itself cause structural remodelling that in turn promotes AF. Evidence supporting this idea comes from animal studies showing that even when the ventricular rate is well-controlled, rapid atrial activation causes atrial fibrosis,9 and from work indicating that rapidly activated atrial-derived cardiomyocytes secrete substances that enhance collagen synthesis by atrial fibroblasts.10
Fibrosis represents excessive deposition of extracellular matrix (ECM) proteins synthesized by fibroblast-related cells, particularly myofibroblasts, upon stimulation.11 Fibroblasts are the most abundant cell type by number in the myocardium, about 75% of all cells.12 Because of their small size, they comprise 5–10% of the total mammalian heart volume,13 but up to 75% of sinoatrial node (SAN) volume.14 In contrast to cardiomyocytes,15 cardiac fibroblasts are electrically non-excitable,16 and were initially thought to serve a predominantly structural role.17,18 Over the past 10 years, however, a growing body of evidence suggests that cardiac fibroblasts importantly modify cardiac electrical function.
Myofibroblasts are rarely found in normal cardiac tissue.19 In response to pathological stimuli, such as myocardial injury, oxidative stress, mechanical stretch, enhanced autocrine–paracrine mediator production, and inflammatory stimuli, fibroblasts proliferate, migrate, and undergo phenotypic changes involving differentiation into myofibroblasts,20,21 which are the principal ECM-secretory cell type. Myofibroblasts may also be derived from cardiac endothelial cells22 and from circulating precursors.23 By producing growth factors, cytokines, chemokines, ECM proteins, and proteases,24,25 myofibroblasts play a pivotal role in the fibrotic process. Cytokines and other growth factors produced by myofibroblasts can further stimulate fibroblasts, perpetuating a fibrogenic cascade. Fibroblasts produce two types of fibrosis, reactive and reparative.4,26 Reparative fibrosis fills in tissue and maintains structural integrity after cardiomyocyte death, whereas reactive fibrosis is a response to excessive myocardial loads or inflammation and causes ECM expansion between muscle bundles4 (Figure 1). The goal of this paper is to review recent developments in understanding the pathophysiology of cardiac fibroblasts and their role in AF development and perpetuation, with a particular focus on the properties of fibroblasts relevant to cardiac and more specifically atrial electrical function.
Figure 1.
Schematic diagram of cardiac fibrogenesis cascade and AF. In response to a variety of stimuli, cardiac fibroblasts proliferate, differentiate, synthesize extracellular matrix (ECM) proteins, and produce cytokines that in turn stimulate fibroblasts, thereby providing positive feedback and perpetuating the fibrogenesis cascade. ECM accumulation causes fibrosis that favours the occurrence and maintenance of AF. There are two types of fibrosis, reactive and reparative, which can be caused by many common stimuli, with cardiomyocyte death required for reparative fibrosis. TRPM7-mediated Ca2+ signals are critical for fibroblast proliferation, differentiation, and ECM production in fibroblasts from AF patients.67 Fibroblast Ca2+ signalling may be an effective target for the prevention of fibrogenesis.
2. Differences between atrial and ventricular fibroblasts
A number of observations suggest that the atria are more susceptible to fibrosis than the ventricles.27–31 While various mechanisms, including differential stretch and mechanical-loading properties, may contribute to the atrial fibrotic susceptibility, there is evidence that there are fundamental differences between atrial and ventricular fibroblasts.32 In culture, atrial fibroblasts show faster increases in cell-surface area typical of myofibroblast differentiation and a distinct morphology at confluence.32 Atrial fibroblasts also show proliferative responses that are greater than those of ventricular fibroblasts upon exposure to a range of growth stimuli, including foetal–bovine serum, platelet-derived growth factor (PDGF), basic fibroblast growth factor, angiotensin II (AngII), endothelin-1, and transforming growth factor (TGF)-β1.32 Atrial fibroblasts express PDGF and PDGF-receptor mRNA more strongly than ventricular fibroblasts, congestive heart failure (CHF) differentially enhances atrial vs. ventricular PDGF and PDGF-receptor gene expression, and PDGF-receptor inhibition suppresses atrial-specific responses, pointing to a role for PDGF in atrial-selective fibrosis.32
3. Electrophysiological properties of cardiac fibroblasts
The resting membrane potential (RMP) of cardiac fibroblasts is much less negative than that of cardiomyocytes. RMP in fibroblasts can be measured by whole-cell patch clamp (under current clamp mode), with limitations of altering cell contents by dialysis and the need for junction potential compensation, or by classical fine-tipped microelectrodes, which is technically challenging and can cause mechanical cell damage. Values between −31 and −16 mV have been recorded in situ with standard sharp microelectrodes in atrial fibroblasts,33,34 vs. −70 to −80 mV for atrial cardiomyocytes. Patch clamp recordings in isolated rat atrial fibroblasts indicate a RMP of about −37 mV.35 The fibroblast RMP becomes hyperpolarized during atrial relaxation and depolarized during atrial contraction,35 presumably due to activation and inactivation of a mechanosensitive non-selective cation channel.35
Table 1 provides a summary of the information presently available about cardiac fibroblast ionic-current properties. Recent studies have demonstrated the presence of several K+ current types in rat ventricular fibroblasts,36 with the RMP controlled by an inward-rectifying K+ current, likely encoded by Kir 2.1.36 A Ca2+-activated large-conductance K+ channel (BKCa) recorded in human cardiac fibroblasts seems to contribute to myocyte–fibroblast coupling as predicted by modelling.37 Several depolarization-elicited K+ currents have been recorded in rat ventricular fibroblasts, including transient outward K+ current (Ito),38 and two types of kinetically distinct delayed-rectifier K+ currents.38,39 Immunoblot analysis reveals the presence of Kv1.2, Kv1.4, Kv1.5, and Kv2.1 α-subunits, but not Kv4.2 or Kv1.6 α-subunits, in neonatal fibroblasts;38 whereas RT–PCR results indicate that Kv1.6 may constitute the molecular basis of delayed-rectifier outward current (IK) in adult rat fibroblasts.39 Interestingly, Li et al.40 recently reported that two types of voltage-gated Na currents (tetrodotoxin, TTX, sensitive and resistant: INa.TTX and INa.TTXR), IK, Ito, Ca-activated K+ current (BKCa), inward-rectifier (Kir-type) current, and swelling-induced Cl− current are all present in commercially available cultured human ventricular fibroblasts (ScienCell). The presence of a voltage-gated proton (H+)-permeable current has also been demonstrated in human atrial fibroblasts.41 It will be important to determine whether and how the various ion channels in fibroblasts contribute to basic fibroblast function, as well as to fibroblast–cardiomyocyte interactions.
Table 1.
Currents recorded in cardiac fibroblasts
| Na+ currents | Voltage-gated K+ currents | Inward rectifier | Ca2+-activated K+ currents | CL− currents | H+ currents | |
|---|---|---|---|---|---|---|
| Human cardiac fibroblasts | INa.TTX, INa.TTXR40 | IKDR, Ito40 | Kir40 | BKCa37,40 | ICl-swell40 | Voltage-gated H+ current41 |
| Neonatal rat cardiac fibroblasts | ND | Ito,38,105 IKf, and IKS38 | ND | ND | ND | |
| Adult rat cardiac fibroblasts | ND | IK36,39 | Kir36 | IKCa106 | ND | ND |
ND, not determined.
4. Ca2+ signalling and fibroblast function
Ca2+ signals are essential for diverse cellular functions including differentiation, gene expression, cell proliferation, growth, and death.42,43 Several lines of evidence suggest that Ca2+ entry is essential for fibroblast function. Chelating external Ca2+ by EGTA prevents substance P-induced proliferation of cultured rat cardiac fibroblasts.18,44 Eliminating external Ca2+ to prevent Ca2+ influx attenuates H2O2-induced IL-6 mRNA expression.45 In mechanical stimulation or stretch-induced membrane potential-change studies, both Ca2+ entry and Ca2+ release are important mediators.46,47 A stretch-induced fibroblast current can be blocked by gadolinium (Gd3+),35 a non-selective cation channel blocker. Recently, it has been shown that intracellular Ca2+ changes contribute to AngII-induced proliferation of cardiac fibroblasts.48 In an in vivo study, mibefradil, a mixed L/T-type Ca2+-channel blocker, reduced collagen production and fibroblast differentiation in rats receiving AngII or aldosterone.49 These studies suggest that Ca2+ entry through Ca2+-permeable ion channels is important for fibroblast responsiveness and fibrosis generation.
Fibroblasts appear to lack voltage-gated Ca2+ channels.15 Transient receptor potential (TRP) channels are responsible for Ca2+ entry in various cell types.50–52 TRP channels are not voltage gated but are activated by a variety of stimuli including membrane-receptor stimulation, oxidative stress, mechanical stretch, cell-metabolite accumulation, and thermal or sensory stimuli.50–52 These unique properties suggest that TRP channels are potential candidates for mediators of Ca2+ signalling in cardiac fibroblasts.
5. TRP channels and Ca2+ signalling in cardiac fibroblasts
5.1. Overview
The 28 mammalian TRP channel genes fall into six sequence-homology subfamilies.50–52 The TRPC (canonical) subfamily contains seven members (TRPC1–7). The TRPM (melastatin) subfamily includes eight channel types (TRPM1–8), the TRPV (vanilloid) subfamily has six members (TRPV1–6), and the TRPA (ankyrin) subfamily has only one member, TRPA1. The TRPP (polycystin) and TRPML (mucolipin) families, each with three members, are likely intracellular ion channels.53
TRP channels consist of putative six transmembrane polypeptide subunits assembling as homo- or hetero-tetramers to form cation-permeable pores. TRP channels have few charged amino acids in the S4 domain, making them only weakly voltage responsive.50,51 Some TRP channels are constitutively open, others open upon Gq-linked receptor activation.50–52 All functionally characterized TRP channels are permeable to Ca2+ except TRPM4 and TRPM5. TRPM6 and TRPM7 are also permeable to Mg2+.
5.2. TRP channels in the heart
TRP channels are highly expressed in heart.54 Aortic banding upregulates TRPC155 and TRPC3 in rats.56 TRPC6 is essential for cardiac hypertrophy.57 Cardiac overexpression of dominant-negative TRPC3 attenuates hypertrophic responses to neuroendocrine agonists or pressure overload.58 Both TRPC3 and TRPC6 play a role in AngII-induced NFAT nuclear translocation,59 a crucial step in cardiac hypertrophy.60 TRPC4 and TRPC5 are upregulated in mouse cardiomyocytes with down-regulation of SERCA2a.61 TRPC1 knockout protects against haemodynamic stress-induced hypertrophy.62 TRPC5 and TRPC6 are up-regulated in patients with severe heart failure.56,57
Many TRP channels have been detected in specific cardiac tissues and cells (Table 2).63 TRPC1, 2, 3, 4, 6, and 7 transcripts are present in SAN tissue, and TRPC1, 2, 4, and 6 proteins are immunodetectable in SAN cardiomyocytes.64 TRPC1–3 and TRPC5–7 mRNA were detected in rat cardiac fibroblasts by RT–PCR, and the presence of TRPM7-like65 and TRPV4 current has been demonstrated by whole-cell current recording. TRPC6 opening and TRPC6-mediated Ca2+ entry are enhanced by activation of Gα12/13.66 TRPC1, TRPC4, TRPC6, TRPV2, TRPV4, TRPM4, and TRPM7 mRNA are expressed in human atrial fibroblasts.67 TRPM7 current was evident at whole-cell and single-channel levels, whereas TRPC6-, TRPV2-, and TRPV4-like currents were not elicited.67
Table 2.
Expression of TRP channels in cardiac fibroblasts and myocytes
| TRPC1 | TRPC2 | TRPC3 | TRPC4 | TRPC5 | TRPC6 | TRPV2 | TRPV4 | TRPV6 | TRPM3 | TRPM4 | TRPM6 | TRPM7 | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human atrial fibroblasts | PCR | PCR | PCR | PCR, WB, WC, SC | 67 | |||||||||
| Mouse ventricle fibroblasts | PCR | PCR | PCR | PCR | PCR | PCR | PCR | PCR | PCR | PCR | PCR | PCR, WB, WC, SC | 67 | |
| Rat ventricle fibroblasts | PCR | PCR | PCR | PCR | PCR, WC107 | PCR | PCR, WC65 | 68,65,107 | ||||||
| Human cardiomyocytes | PCR, WB | PCR | PCR, SC | 57,108,109 | ||||||||||
| Mouse cardiomyocytes | PCR, WB | PCR | PCR, WB | PCR, WB, WC57 | WB | PCR | PCR, WB, SC110 | PCR | 57,110 | |||||
| Rat cardiomyocytes | PCR, WB | PCR, WB | PCR | PCR, WB | PCR, WB | PCR | PCR, SC111 | 55,56,59,111,112 |
PCR, RT–PCR or qPCR; WB, western blot; WC, whole-cell current recording; SC, single-channel current recording.
TRPC, canonical or classical TRP channel subfamily.
TRPV, vanilloid-related TRP channel subfamily.
TRPM, melastatin-related TRP channel subfamily.
A non-selective cation current (NSCC) was elicited by CNP (C-type natriuretic peptide) and cANP in freshly isolated rat ventricular fibroblasts.68 NSCC can also be activated by OAG (1-oleoyl-2-acety-sn-glycerol), suggesting that TRPC3, TRPC6, or TRPC3/TRPC6 heteromers may underlie NSCC in rat ventricular fibroblasts.68 Similar NSCC current was not obtained in human atrial fibroblasts.67
5.3. TRPM7 and atrial fibrillation
TRPM7, a Ca2+-permeable channel/kinase,50 plays a vital role in embryonic development69 and anoxic cell death.70 TRPM7 is constitutively active under physiological conditions and conducts small inward current.50 Extracellular acidosis potentiates TRPM7 inward currents.71,72 TRPM7 is up-regulated about three- to five-fold in AF.67 TRPM7 knockdown suppresses endogenous TRPM7 currents and Ca2+ influx in atrial fibroblasts and inhibits TGF-β1-induced fibroblast proliferation, differentiation, and collagen production.67 Thus, TRPM7-mediated Ca2+ signals likely play a pivotal role in fibroblast differentiation and fibrosis in human AF. TRPM7 or other TRP channels may thus be effective targets for atrial fibrosis prevention and AF prophylaxis (Figure 1).
6. How do fibroblasts affect cardiac electrical function?
Cardiac fibrosis plays a central role in the pathophysiology of AF.4 Myofibroblast content increases are clearly involved in the fibrotic process. CHF enhances myofibroblast content in the atria much more than in the ventricles, a phenomenon clearly related to the atrial selectivity of fibrotic processes and their role in AF.32 The precise mechanisms by which fibrosis promotes AF are a subject of active interest, and particular attention has been paid to the involvement of atrial fibroblasts in atrial electrical dysfunction and arrhythmogenesis.
6.1. Cardiomyocyte–fibroblast electrical interactions and arrhythmogenesis
Fibroblast coupling to cardiomyocytes can produce a variety of changes in cardiomyocyte action potential (AP) properties,15,73–77 including changes in pace-making function.15,78 Fibroblasts are capable of coupling electrically to cardiomyocytes.79 The complement of fibroblast ion channels produces a moderately polarized (negative intracellular) potential (about −30 mV), without active depolarization properties mediated by voltage-gated Na+ or Ca2+ channels. Fibroblasts modulate cardiomyocyte electrical activity by depolarizing or hyperpolarizing cardiomyocytes depending on the relative values of cardiomyocyte vs. fibroblast transmembrane potential. Lacking active phase 0 depolarization, fibroblast cell membranes possess a capacitance in parallel to their resistance, making them leaky capacitors. They also display a variety of ion channels that show voltage- and time-dependent conductance.73 When cardiomyocytes are depolarized to voltages positive to about −30 mV (the normal fibroblast resting potential), the gap-junctional flow of positive ions towards the more negatively charged fibroblast produces repolarizing cardiomyocyte current flow that can mimic transient outward current.76 Conversely, when the cardiomyocyte transmembrane potential is negative to −30 mV, positively charged gap-junctional current flow from fibroblasts will depolarize cardiomyocytes. As a result, cardiomyocyte–fibroblast coupling depolarizes cardiomyocyte RMP, slows conduction by drawing off excitatory current flow during phase 0, and can increase or decrease AP duration (APD) depending on fibroblast resting potential and cardiomyocyte–fibroblast coupling properties.76,77
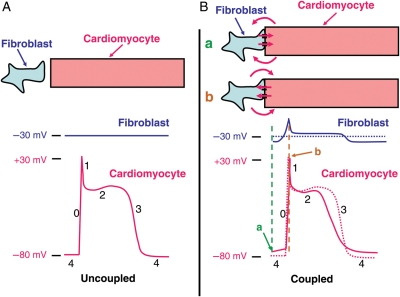
Figure 2 schematically illustrates the effects of coupled fibroblasts on cardiomyocyte electrical activity. Figure 2A shows recordings of fibroblast and cardiomyocyte transmembrane potential as they would look if the cell types were not electrically coupled, with a typical cardiomyocyte AP and the fibroblast at a constant transmembrane potential determined by its intrinsic ionic permeabilities. Figure 2B shows the effects of coupling the cell types. Whenever the cardiomyocyte membrane potential is different from −30 mV, current flows through gap junctions between the cells (as well as in the extracellular fluid around them, as indicated in the figure), depolarizing cardiomyocytes and hyperpolarizing fibroblasts when the cardiomyocyte potential is negative to −30 mV, and conversely hyperpolarizing cardiomyocytes and depolarizing fibroblasts when the cardiomyocyte potential is positive to −30 mV. Prior to activation, during phase 4 when the cardiomyocyte is normally at rest (Figure 2Ba), current flow from fibroblasts depolarizes the cardiomyocyte cell membrane. Theoretically, this could initiate spontaneous phase 0 depolarization and automaticity if the cardiomyocyte reaches threshold potential. Phase 0 depolarization in cardiomyocytes is slowed because the phase 4 depolarization resulting from coupling to fibroblasts inactivates cardiomyocyte Na+ channels. In addition, when the cardiomyocyte potential is positive to −30 mV, cardiomyocytes experience an outward (hyperpolarizing) current flow to fibroblasts which oppose the effect of inward Na+ current (Figure 2Bb). Throughout phases 1 and 2, this outward cardiomyocyte current flow continues and accelerates repolarization. Phase 3 is initially accelerated, but when the cardiomyocyte returns to −30 mV the direction of current flow is reversed and the cardiomyocyte is now depolarized by the attached fibroblast, slowing repolarization. Current flow across the fibroblast cell membrane is inversely parallel to that in the cardiomyocyte, producing changes in fibroblast transmembrane potential that are qualitatively similar to, but smaller than, those in the cardiomyocyte. Net effects on cardiomyocyte APD will depend on whether initial repolarization accelerating effects (positive to the fibroblast intrinsic potential) predominate, shortening APD, or whether terminal repolarization delaying effects (negative to fibroblast intrinsic potential) predominate, increasing APD. Conduction will be slowed, because of the reduced phase 0 slope. In pathological conditions, intervening fibroblasts can actually couple otherwise-uncoupled cardiomyocytes, producing extremely slow conduction that can greatly facilitate the occurrence of reentry.80
Figure 2.
A schematic illustration of the effects of fibroblast coupling to cardiomyocytes on cardiomyocyte action potentials (APs). (A) Intracellular electrical recordings from uncoupled fibroblasts (blue) and cardiomyocytes (red). (B) Changes resulting from coupling between fibroblasts and cardiomyocytes. Transmembrane recordings from uncoupled cells are reproduced in dotted lines; solid lines show recordings resulting from the cell-to-cell current flow that occurs with cell coupling. The dashed horizontal black line in the lower panel indicates the normal fibroblast resting potential (−30 mV). Directions of (positive) current flow between cardiomyocytes and fibroblasts through gap junctions are shown with red arrows, at cardiomyocyte maximum diastolic potential (a) and peak overshoot (b).
The effects of fibroblasts on cardiomyocyte electrical activity will clearly depend on a variety of factors. Based on the cellular actions shown in Figure 2, one would expect fibroblasts to be able to induce both abnormal spontaneous impulse formation (abnormally enhanced automaticity) and reentry in cardiac tissues. Abnormal automatic activity is induced by depolarization of cardiomyocytes towards their threshold potential, as well as by the induction of voltage- and time-dependent inward Na+ and/or Ca2+ currents at depolarized potentials. Reentry results from conduction slowing and APD abbreviation in cardiomyocytes. Both abnormal automaticity and reentry have been shown to occur in co-cultures of cardiomyocytes and fibroblasts.81,82 Based on modelling predictions, factors that determine the ability of fibroblasts to modulate cardiomyocyte electrical activity include the number of resident fibroblasts in any given cardiac region and the effectiveness of their coupling to cardiomyocytes.73–77 Fibroblasts are small cells. Although they are numerous in the heart, their much smaller size means that many fibroblasts need to be coupled to cardiomyocytes to affect their electrical activity—significant effects are observed with ∼10–30 fibroblasts per cardiomyocyte in computer modelling.15,75,83 This result needs to be considered cautiously, because size estimates may be inaccurate if, as some believe, cell isolation damages fibroblasts so that they are isolated as nucleated fragments rather than intact cells. The in vivo contexts in which fibroblast coupling significantly modulates cardiomyocyte electrical activity remain unknown, although the large numbers of fibroblasts in the SA node suggest that they may be particularly important in regulating SA node pacemaker activity.14,79 Similarly, the degree, types, and effectiveness of cardiomyocyte–fibroblast coupling in various pathological contexts is uncertain, although it is known that fibroblasts expressing connexins 40 and 43 appear in large numbers in evolving myocardial infarctions.84 There is a paucity of data about cardiomyocyte–fibroblast gap-junction coupling from in situ preparations, whereas in vitro data indicate extensive formation of Cx43- and Cx45-based inter-myofibroblast and myofibroblast–cardiomyocyte coupling.80 A mathematical model incorporating fibroblast–cardiomyocyte interactions accounted well for the effects of tissue fibrosis on electrical propagation during AF in sheep with CHF induced by chronic rapid ventricular pacing.85 An interesting recent finding has been that cardiac fibroblasts can produce paracrine factors that affect cardiac electrical activity.86 Neonatal-rat cardiomyocyte monolayers exposed for 24 h to conditioned media from cardiac fibroblasts showed significant conduction-slowing, APD prolongation and reduced follow frequency. It is therefore possible that cardiomyocyte electrical activity is affected by diffusible factors from adjacent fibroblasts in fibrotic tissues. Overall, while it is clear from studies in model systems that fibroblast–cardiomyocyte interactions can produce AF-promoting electrophysiological changes, the extent to which they contribute to clinical arrhythmias like AF is at present still an unresolved issue of great interest.
6.2. ‘Barrier' function of fibrotic tissue and altered cardiac electrical continuity
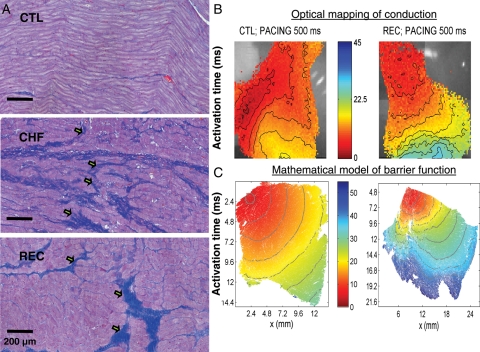
Another way in which fibroblasts can promote arrhythmogenesis is by producing large quantities of ECM proteins, particularly collagen, that alter cardiomyocyte architecture and disturb electrical continuity. Tissue fibrosis in AF develops in parallel with local conduction disturbances and increases in AF persistence, during both the development and resolution of experimental CHF, in contrast to a variety of other CHF-related changes.87,88 ECM alterations in fibrotic tissue could lead to conduction abnormalities and AF promotion in a variety of ways. Loss of side-to-side cardiomyocyte connections due to insulating collagen within cardiomyocyte bundles has been suggested to produce zigzag conduction patterns and promote atrial microreentry with ageing.89 The potential effects of fibrosis on longitudinal conduction, the dominant conduction form in highly anisotropic atrial tissue,90,91 are illustrated in Figure 3. The normal architecture of cardiomyocyte bundles is shown schematically in Figure 3A. Figure 3B illustrates the effects of reactive fibrosis, which increases the collagen content in the interstitial spaces between fibres.4 Fibrosis occurs around cardiomyocyte bundles. Longitudinal conduction is uninterrupted, and could even be accelerated due to better insulation of cable-like bundles. Figure 3C illustrates the effect of reparative fibrosis, in which dead cardiomyocytes are replaced by fibrous tissue,4 which then physically separates cardiomyocytes in the longitudinal direction and interferes with longitudinal conduction. Experimental observations in a canine CHF model are shown in Figure 4A, and indicate that both during CHF and following full recovery from CHF fibrotic tissue interrupts cardiomyocyte-bundle continuity in the longitudinal direction.88 Optical mapping shows conduction abnormalities in atrial tissues of dogs recovered from CHF (Figure 4B). A mathematical model assuming that fibrous tissue impairs atrial conduction by physically interrupting cardiomyocyte muscle–bundle connections fully accounts for CHF-related conduction abnormalities (Figure 4C).88 Furthermore, AF promotion tracked both conduction abnormalities and fibrosis in this CHF model, arguing for an important role of fibrotic conduction barriers in AF maintenance.88 These results support the notion that fibrosis impairs conduction by acting as a barrier to continuous longitudinal conduction in cardiomyocyte bundles. It has been suggested that the maintenance of transmural conduction in chronically infarcted regions of rabbit hearts may be due to cardiomyocyte–fibroblast coupling in scarred myocardium.92An interesting recent tissue-engineering study showed that inserting a layer of fibroblast-derived NIH3T3 cells between two sheets of neonatal rat cardiomyocytes restores electrical conduction between the cardiomyocyte sheets, suggesting the time-dependent development of electrical coupling across the fibroblast sheet.93
Figure 3.
A schematic illustration of the expected effects of tissue fibrosis on conduction in bundles of cardiomyocyte (red cylinders). (A) Normal tissue. (B) Fibrosis occurring parallel to cardiomyocyte-bundle sheaths (as would occur in reactive fibrosis). Longitudinal cell-to-cell connections are intact and longitudinal conduction is unaffected. (C) Fibrosis parallel to and across cardiomyocyte bundles, as would occur in reparative fibrosis. End-to-end connections are prevented by the physical separation resulting from replacement of dead cardiomyocytes by fibroblasts and fibrotic extracellular matrix proteins, producing local impairments in longitudinal conduction.
Figure 4.
(A) Longitudinal sections of Masson trichrome-stained atrial tissue from a control dog (top), a dog in which 2-week tachypacing at 240b.p.m. led to CHF (middle) and a dog tachypaced into CHF and then allowed to recover for 4 weeks without tachypacing (REC), which permitted full haemodynamic recovery (bottom). Collagen is stained blue, cardiomyocytes are pink. Arrows point to regions in which transverse fibrosis interrupts cardiomyocytes in their longitudinal direction. (B) Optical maps of electrical conduction in right atrial tissue preparations from a control (left) and a REC dog (right). Tissues were paced at 2 Hz at the upper left corner. The activation colour scale is in ms. (C) Results of mathematical modelling of conduction in simulated tissues with the same fibrosis distribution as the preparations shown immediately above, assuming that fibrosis acts as a non-conductive barrier. The mathematical model accounted well for the electrical propagation changes observed experimentally. Reproduced from the reference88 with permission of the American Heart Association.
7. Therapeutic implications
Given the important role of atrial fibrosis in AF pathophysiology, therapeutic approaches that target fibroblast function are potentially of interest. Angiotensin converting-enzyme inhibitors, which suppress renin–angiotensin–aldosterone system (RAAS) activation, were the first agents shown experimentally to suppress atrial fibrosis, prevent atrial conduction abnormalities, and attenuate associated AF promotion.94 Subsequent experimental evidence indicated similar effects from angiotensin type-1 receptor antagonists95 and aldosterone inhibitors.96 Statins suppress atrial fibroblast proliferation and suppress atrial fibrosis/AF in experimental CHF97 and omega-3 fatty acids have similar antifibrotic/AF-suppressing actions,98 possibly via anti-inflammatory/antioxidant actions. Pirfenidone, an antifibrotic drug that appears to act by interfering with TGF-β signalling, also suppresses fibrosis, conduction abnormalities, and AF promotion resulting from CHF.99 Heat-shock protein inducers like geranylgeranylacetone also attenuate atrial remodelling and CHF-related fibrosis.100,101 Clinical data are still insufficient to draw definite conclusions, but support the concept that RAAS inhibition can prevent AF, at least in some patient populations.102,103
The electrical properties of fibroblasts also open up exciting and novel therapeutic possibilities. If Ca2+ entry through TRP channels plays a central role in fibroblast activation, as suggested by experimental data,67,104 then TRP channel blockers have the potential to be useful AF-preventing drugs. The prevention of fibroblast–cardiomyocyte interactions, by targeting fibroblast ion channels or fibroblast–cardiomyocyte coupling, offers additional novel and exciting possibilities in AF therapy. For example, if fibroblast coupling to cardiomyocytes causes atrial ectopic activity or reentry that underlies AF occurrence, specifically targeting cardiomyocyte–fibroblast coupling mechanisms could suppress AF. There may be ways of stopping fibroblast-induced abnormal automaticity or reentry by developing drugs that selectively inhibit or activate fibroblast ion channels. We are only beginning to understand the detailed function and pathophysiology of atrial fibroblasts and how they contribute to AF development, but the new insights and therapeutic possibilities that they present are exciting.
8. Conclusions
Fibroblasts are quantitatively and qualitatively a very important cell type in the heart. Extensive evidence suggests that tissue fibrosis, involving proliferation of fibroblasts and increased fibroblast-derived ECM protein expression, plays an important role in AF. Rapidly accumulating information suggests that the electrical properties of cardiac fibroblasts are important in governing both their ECM protein-producing properties and arrhythmogenic events related to cardiomyocyte–fibroblast interaction. Work in this exciting field promises to lead to new mechanistic insights and therapeutic options for AF.
Funding
This work was supported by NIH grant HL078960 (L.Y.), a bio-medical grant from the Department of Public Health of Connecticut (L.Y.), the European-North American AF-research network (ENAFRA) award from Fondation Leducq (S.N.), Canadian Institutes of Health Research grants (MGP6957 and MOP44365), the MITCS Network, and the Quebec Heart and Stroke Foundation (S.N.).
Acknowledgments
The authors thank France Theriault for invaluable secretarial help.
Conflict of interest: none declared.
References
- 1.Weber K. Cardiac interstitium. In: Poole-Wilson P, Colucci W, Massie B, Chatterjee K, Coats A, editors. Heart Failure. New York, NY: Churchill Livingstone; 1997. pp. p13–31. [Google Scholar]
- 2.Benjamin EJ, Chen P-S, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: Report from a National Heart, Lung, and Blood Institute Workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. doi:10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. doi:10.1016/S0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 4.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. doi:10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 5.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. doi:10.1016/S0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 6.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. doi:10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109:363–368. doi: 10.1161/01.CIR.0000109495.02213.52. doi:10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 8.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 9.Avitall B, Bi J, Mykytsey A, Chicos A. Atrial and ventricular fibrosis induced by atrial fibrillation: evidence to support early rhythm control. Heart Rhythm. 2008;5:839–845. doi: 10.1016/j.hrthm.2008.02.042. doi:10.1016/j.hrthm.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Burstein B, Qi XY, Yeh YH, Calderone A, Nattel S. Atrial cardiomyocyte tachycardia alters cardiac fibroblast function: a novel consideration in atrial remodeling. Cardiovasc Res. 2007;76:442–452. doi: 10.1016/j.cardiores.2007.07.013. doi:10.1016/j.cardiores.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. doi:10.1161/01.RES.0000046452.67724.B8. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. doi:10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 13.Shiraishi I, Takamatsu T, Minamikawa T, Fujita S. 3-D observation of actin filaments during cardiac myofibrinogenesis in chick embryo using a confocal laser scanning microscope. Anat Embryol (Berl) 1992;185:401–408. doi: 10.1007/BF00188551. [DOI] [PubMed] [Google Scholar]
- 14.Shiraishi I, Takamatsu T, Minamikawa T, Onouchi Z, Fujita S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation. 1992;85:2176–2184. doi: 10.1161/01.cir.85.6.2176. [DOI] [PubMed] [Google Scholar]
- 15.Kohl P, Noble D. Mechanosensitive connective tissue: potential influence on heart rhythm. Cardiovasc Res. 1996;32:62–68. [PubMed] [Google Scholar]
- 16.Carver W, Nagpal ML, Nachtigal M, Borg TK, Terracio L. Collagen expression in mechanically stimulated cardiac fibroblasts. Circ Res. 1991;69:116–122. doi: 10.1161/01.res.69.1.116. [DOI] [PubMed] [Google Scholar]
- 17.Weber KT, Sun Y, Katwa LC, Cleutjens JP. Connective tissue: a metabolic entity? J Mol Cell Cardiol. 1995;27:107–120. doi: 10.1016/s0022-2828(08)80011-9. doi:10.1016/S0022-2828(08)80011-9. [DOI] [PubMed] [Google Scholar]
- 18.Kumaran C, Shivakumar K. Calcium- and superoxide anion-mediated mitogenic action of substance P on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2002;282:H1855–H1862. doi: 10.1152/ajpheart.00747.2001. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. doi:10.1016/S0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 20.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 21.Weber KT, Sun Y, Tyagi SC, Cleutjens JP. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:279–292. doi: 10.1006/jmcc.1994.1036. doi:10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- 22.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. doi:10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 23.van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214:377–386. doi: 10.1002/path.2281. doi:10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 24.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 25.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. doi:10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 26.Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. Patterns of myocardial fibrosis. J Mol Cell Cardiol. 1989;21(Suppl. 5):121–131. doi: 10.1016/0022-2828(89)90778-5. doi:10.1016/0022-2828(89)90778-5. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 28.Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63:236–244. doi: 10.1016/j.cardiores.2004.03.026. doi:10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Jr., Kasi VS, et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 31.Verheule S, Sato T, Everett T, Engle SK, Otten D, Rubart-von der Lohe M, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. doi:10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. doi:10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 33.Kamkin A, Kiseleva I, Wagner KD, Pylaev A, Leiterer KP, Theres H, et al. A possible role for atrial fibroblasts in postinfarction bradycardia. Am J Physiol Heart Circ Physiol. 2002;282:H842–H849. doi: 10.1152/ajpheart.00240.2001. [DOI] [PubMed] [Google Scholar]
- 34.Kamkin A, Kiseleva I, Wagner KD, Lammerich A, Bohm J, Persson PB, et al. Mechanically induced potentials in fibroblasts from human right atrium. Exp Physiol. 1999;84:347–356. doi:10.1017/S0958067099017947. [PubMed] [Google Scholar]
- 35.Kamkin A, Kiseleva I, Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc Res. 2003;57:793–803. doi: 10.1016/s0008-6363(02)00775-7. doi:10.1016/S0008-6363(02)00775-7. [DOI] [PubMed] [Google Scholar]
- 36.Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, et al. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H2931–H2939. doi: 10.1152/ajpheart.01220.2004. doi:10.1152/ajpheart.01220.2004. [DOI] [PubMed] [Google Scholar]
- 37.Wang YJ, Sung RJ, Lin MW, Wu SN. Contribution of BK(Ca)-channel activity in human cardiac fibroblasts to electrical coupling of cardiomyocytes-fibroblasts. J Membr Biol. 2006;213:175–185. doi: 10.1007/s00232-007-0027-8. doi:10.1007/s00232-007-0027-8. [DOI] [PubMed] [Google Scholar]
- 38.Walsh KB, Zhang J. Neonatal rat cardiac fibroblasts express three types of voltage-gated K+ channels: regulation of a transient outward current by protein kinase C. Am J Physiol Heart Circ Physiol. 2008;294:H1010–H1017. doi: 10.1152/ajpheart.01195.2007. doi:10.1152/ajpheart.01195.2007. [DOI] [PubMed] [Google Scholar]
- 39.Shibukawa Y, Chilton EL, Maccannell KA, Clark RB, Giles WR. K+ currents activated by depolarization in cardiac fibroblasts. Biophys J. 2005;88:3924–3935. doi: 10.1529/biophysj.104.054429. doi:10.1529/biophysj.104.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li GR, Sun HY, Chen JB, Zhou Y, Tse HF, Lau CP. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS ONE. 2009;4:e7307. doi: 10.1371/journal.pone.0007307. doi:10.1371/journal.pone.0007307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Chemaly A, Guinamard R, Demion M, Fares N, Jebara V, Faivre JF, et al. A voltage-activated proton current in human cardiac fibroblasts. Biochem Biophys Res Commun. 2006;340:512–516. doi: 10.1016/j.bbrc.2005.12.038. doi:10.1016/j.bbrc.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 42.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. doi:10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 43.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. doi:10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shivakumar K, Kumaran C. L-type calcium channel blockers and EGTA enhance superoxide production in cardiac fibroblasts. J Mol Cell Cardiol. 2001;33:373–377. doi: 10.1006/jmcc.2000.1309. doi:10.1006/jmcc.2000.1309. [DOI] [PubMed] [Google Scholar]
- 45.Colston JT, Chandrasekar B, Freeman GL. A novel peroxide-induced calcium transient regulates interleukin-6 expression in cardiac-derived fibroblasts. J Biol Chem. 2002;277:23477–23483. doi: 10.1074/jbc.M108676200. doi:10.1074/jbc.M108676200. [DOI] [PubMed] [Google Scholar]
- 46.Kiseleva I, Kamkin A, Kohl P, Lab MJ. Calcium and mechanically induced potentials in fibroblasts of rat atrium. Cardiovasc Res. 1996;32:98–111. [PubMed] [Google Scholar]
- 47.Kiseleva I, Kamkin A, Pylaev A, Kondratjev D, Leiterer KP, Theres H, et al. Electrophysiological properties of mechanosensitive atrial fibroblasts from chronic infarcted rat heart. J Mol Cell Cardiol. 1998;30:1083–1093. doi: 10.1006/jmcc.1998.0673. doi:10.1006/jmcc.1998.0673. [DOI] [PubMed] [Google Scholar]
- 48.Olson ER, Shamhart PE, Naugle JE, Meszaros JG. Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase C{delta} and intracellular calcium in adult rat cardiac fibroblasts. Hypertension. 2008;51:704–711. doi: 10.1161/HYPERTENSIONAHA.107.098459. doi:10.1161/HYPERTENSIONAHA.107.098459. [DOI] [PubMed] [Google Scholar]
- 49.Ramires FJ, Sun Y, Weber KT. Myocardial fibrosis associated with aldosterone or angiotensin II administration: attenuation by calcium channel blockade. J Mol Cell Cardiol. 1998;30:475–483. doi: 10.1006/jmcc.1997.0612. doi:10.1006/jmcc.1997.0612. [DOI] [PubMed] [Google Scholar]
- 50.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. doi:10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 51.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:1–24. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 52.Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J Neurochem. 2010;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. doi:10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dietrich A, Kalwa H, Fuchs B, Grimminger F, Weissmann N, Gudermann T. In vivo TRPC functions in the cardiopulmonary vasculature. Cell Calcium. 2007;42:233–244. doi: 10.1016/j.ceca.2007.02.009. doi:10.1016/j.ceca.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, et al. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. doi:10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 56.Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. doi:10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 57.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. doi:10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci. 2010;107:7000–7005. doi: 10.1073/pnas.1001825107. doi:10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. doi:10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. doi:10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 61.Seth M, Sumbilla C, Mullen SP, Lewis D, Klein MG, Hussain A, et al. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signaling mechanism in cardiac myocytes. Proc Natl Acad Sci USA. 2004;101:16683–16688. doi: 10.1073/pnas.0407537101. doi:10.1073/pnas.0407537101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seth M, Zhang Z-S, Mao L, Graham V, Burch J, Stiber J, et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. doi:10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther. 2008;118:337–351. doi: 10.1016/j.pharmthera.2008.03.008. doi:10.1016/j.pharmthera.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Ju Y-K, Chu Y, Chaulet H, Lai D, Gervasio OL, Graham RM, et al. Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circ Res. 2007;100:1605–1614. doi: 10.1161/CIRCRESAHA.107.152181. doi:10.1161/CIRCRESAHA.107.152181. [DOI] [PubMed] [Google Scholar]
- 65.Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 66.Nishida M, Onohara N, Sato Y, Suda R, Ogushi M, Tanabe S, et al. Galpha12/13-mediated up–regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem. 2007;282:23117–23128. doi: 10.1074/jbc.M611780200. doi:10.1074/jbc.M611780200. [DOI] [PubMed] [Google Scholar]
- 67.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, et al. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. doi:10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rose RA, Hatano N, Ohya S, Imaizumi Y, Giles WR. C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling. J Physiol. 2007;580:255–274. doi: 10.1113/jphysiol.2006.120832. doi:10.1113/jphysiol.2006.120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. doi:10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. doi:10.1016/S0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 71.Jiang J, Li M, Yue L. Potentiation of TRPM7 Inward Currents by Protons. J Gen Physiol. 2005;126:137–150. doi: 10.1085/jgp.200409185. doi:10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li M, Du J, Jiang J, Ratzan W, Su L-T, Runnels LW, et al. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007;282:25817–25830. doi: 10.1074/jbc.M608972200. doi:10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J. 2007;92:4121–4132. doi: 10.1529/biophysj.106.101410. doi:10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: insights from a microstructure model. Am J Physiol Heart Circ Physiol. 2008;294:H2040–H2052. doi: 10.1152/ajpheart.01298.2007. doi:10.1152/ajpheart.01298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sachse FB, Moreno AP, Seemann G, Abildskov JA. A model of electrical conduction in cardiac tissue including fibroblasts. Ann Biomed Eng. 2009;37:874–889. doi: 10.1007/s10439-009-9667-4. doi:10.1007/s10439-009-9667-4. [DOI] [PubMed] [Google Scholar]
- 76.Xie Y, Garfinkel A, Weiss JN, Qu Z. Cardiac alternans induced by fibroblast-myocyte coupling: mechanistic insights from computational models. Am J Physiol Heart Circ Physiol. 2009;297:H775–H784. doi: 10.1152/ajpheart.00341.2009. doi:10.1152/ajpheart.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. Electrotonic coupling between human atrial myocytes and fibroblasts alters myocyte excitability and repolarization. Biophys J. 2009;97:2179–2190. doi: 10.1016/j.bpj.2009.07.054. doi:10.1016/j.bpj.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fahrenbach JP, Mejia-Alvarez R, Banach K. The relevance of non-excitable cells for cardiac pacemaker function. J Physiol. 2007;585:565–578. doi: 10.1113/jphysiol.2007.144121. doi:10.1113/jphysiol.2007.144121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. doi:10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 80.Rohr S. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm. 2009;6:848–856. doi: 10.1016/j.hrthm.2009.02.038. doi:10.1016/j.hrthm.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 81.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 82.Zlochiver S, Munoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys J. 2008;95:4469–4480. doi: 10.1529/biophysj.108.136473. doi:10.1529/biophysj.108.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacquemet V, Henriquez CS. Modelling cardiac fibroblasts: interactions with myocytes and their impact on impulse propagation. Europace. 2007;9(Suppl. 6):vi29–vi37. doi: 10.1093/europace/eum207. doi:10.1093/europace/eum207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res. 2004;62:415–425. doi: 10.1016/j.cardiores.2004.01.027. doi:10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. doi:10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 86.Pedrotty DM, Klinger RY, Kirkton RD, Bursac N. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc Res. 2009;83:688–697. doi: 10.1093/cvr/cvp164. doi:10.1093/cvr/cvp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, et al. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–418. doi: 10.1161/01.CIR.0000109501.47603.0C. doi:10.1161/01.CIR.0000109501.47603.0C. [DOI] [PubMed] [Google Scholar]
- 88.Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh YH, et al. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res. 2009;105:1213–1222. doi: 10.1161/CIRCRESAHA.108.183400. doi:10.1161/CIRCRESAHA.108.183400. [DOI] [PubMed] [Google Scholar]
- 89.Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res. 1986;58:356–371. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- 90.Zhao J, Trew ML, Legrice IJ, Smaill BH, Pullan AJ. A tissue-specific model of reentry in the right atrial appendage. J Cardiovasc Electrophysiol. 2009;20:675–684. doi: 10.1111/j.1540-8167.2008.01420.x. doi:10.1111/j.1540-8167.2008.01420.x. [DOI] [PubMed] [Google Scholar]
- 91.Xie Y, Garfinkel A, Camelliti P, Kohl P, Weiss JN, Qu Z. Effects of fibroblast-myocyte coupling on cardiac conduction and vulnerability to reentry: a computational study. Heart Rhythm. 2009;6:1641–1649. doi: 10.1016/j.hrthm.2009.08.003. doi:10.1016/j.hrthm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker NL, Burton FL, Kettlewell S, Smith GL, Cobbe SM. Mapping of epicardial activation in a rabbit model of chronic myocardial infarction. J Cardiovasc Electrophysiol. 2007;18:862–868. doi: 10.1111/j.1540-8167.2007.00858.x. doi:10.1111/j.1540-8167.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 93.Haraguchi Y, Shimizu T, Yamato M, Okano T. Electrical interaction between cardiomyocyte sheets separated by non-cardiomyocyte sheets in heterogeneous tissues. J Tissue Eng Regen Med. 2010;4:291–299. doi: 10.1002/term.241. doi:10.1002/term.241. [DOI] [PubMed] [Google Scholar]
- 94.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–2614. doi: 10.1161/hc4601.099402. doi:10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 95.Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003;41:2197–2204. doi: 10.1016/s0735-1097(03)00464-9. doi:10.1016/S0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- 96.Milliez P, Deangelis N, Rucker-Martin C, Leenhardt A, Vicaut E, Robidel E, et al. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J. 2005;26:2193–2199. doi: 10.1093/eurheartj/ehi478. doi:10.1093/eurheartj/ehi478. [DOI] [PubMed] [Google Scholar]
- 97.Shiroshita-Takeshita A, Brundel BJ, Burstein B, Leung TK, Mitamura H, Ogawa S, et al. Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res. 2007;74:75–84. doi: 10.1016/j.cardiores.2007.01.002. doi:10.1016/j.cardiores.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Sakabe M, Shiroshita-Takeshita A, Maguy A, Dumesnil C, Nigam A, Leung TK, et al. Omega-3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation. 2007;116:2101–2109. doi: 10.1161/CIRCULATIONAHA.107.704759. doi:10.1161/CIRCULATIONAHA.107.704759. [DOI] [PubMed] [Google Scholar]
- 99.Lee KW, Everett T, Rahmutula D, Guerra JM, Wilson E, Ding C, et al. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114:1703–1712. doi: 10.1161/CIRCULATIONAHA.106.624320. doi:10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brundel BJJM, Ke L, Dijkhuis A-J, Qi XY, Shiroshita-Takeshita A, Nattel S, et al. Heat shock proteins as molecular targets for intervention in atrial fibrillation. Cardiovasc Res. 2008;78:422–428. doi: 10.1093/cvr/cvn060. doi:10.1093/cvr/cvn060. [DOI] [PubMed] [Google Scholar]
- 101.Shiroshita-Takeshita A, Sakabe M, Maguy A, Cardin S, Brundel BJJM, Nattel S. Heat shock protein induction attenuates atrial remodeling and atrial fibrillation promotion in dogs with ventricular tachycardiomyopathy. Circulation. 2007;116(Suppl. II) II-251 (abstract) [Google Scholar]
- 102.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–2307. doi: 10.1016/j.jacc.2010.01.043. doi:10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 103.Gao X, Peng L, Adhikari CM, Lin J, Zuo Z. Spironolactone reduced arrhythmia and maintained magnesium homeostasis in patients with congestive heart failure. J Card Fail. 2007;13:170–177. doi: 10.1016/j.cardfail.2006.11.015. doi:10.1016/j.cardfail.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 104.Harada M, Maguy A, Ordog B, Dawson K, Murohara T, Kamiya K, et al. Role of TRP channels in cardiac fibroblast proliferation- New target for AF preventation? Heart Rhythm. 2010;7:S52. (abstract) doi:10.1016/j.hrthm.2009.09.023. [Google Scholar]
- 105.Rook MB, van Ginneken AC, de Jonge B, el Aoumari A, Gros D, Jongsma HJ. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol Cell Physiol. 1992;263:C959–C977. doi: 10.1152/ajpcell.1992.263.5.C959. [DOI] [PubMed] [Google Scholar]
- 106.Saito T, Fujiwara Y, Fujiwara R, Hasegawa H, Kibira S, Miura H, et al. Role of augmented expression of intermediate-conductance Ca2+-activated K+ channels in postischaemic heart. Clin Exp Pharmacol Physiol. 2002;29:324–329. doi: 10.1046/j.1440-1681.2002.03652.x. doi:10.1046/j.1440-1681.2002.03652.x. [DOI] [PubMed] [Google Scholar]
- 107.Hatano N, Itoh Y, Muraki K. Cardiac fibroblasts have functional TRPV4 activated by 4alpha-phorbol 12,13-didecanoate. Life Sci. 2009;85:808–814. doi: 10.1016/j.lfs.2009.10.013. doi:10.1016/j.lfs.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 108.Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, et al. Functional characterization of a Ca(2+)-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol. 2004;558:75–83. doi: 10.1113/jphysiol.2004.063974. doi:10.1113/jphysiol.2004.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, et al. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest. 2009;119:2737–2744. doi: 10.1172/JCI38292. doi:10.1172/JCI38292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Demion M, Bois P, Launay P, Guinamard R. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res. 2007;73:531–538. doi: 10.1016/j.cardiores.2006.11.023. doi:10.1016/j.cardiores.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 111.Guinamard R, Demion M, Magaud C, Potreau D, Bois P. Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension. 2006;48:587–594. doi: 10.1161/01.HYP.0000237864.65019.a5. doi:10.1161/01.HYP.0000237864.65019.a5. [DOI] [PubMed] [Google Scholar]
- 112.Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. doi:10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]