Abstract
Corticogeniculate neurones make more synapses in the lateral geniculate nucleus (LGN) than retinal ganglion cells, yet we know relatively little about the functions of corticogeniculate feedback for visual processing. In primates, feedforward projections from the retina to the LGN and from the LGN to primary visual cortex are organized into anatomically and physiologically distinct parallel pathways. Recent work demonstrates a close relationship between these parallel streams of feedforward projections and the corticogeniculate feedback pathway. Here, we review the evidence for stream-specific feedback in the primate and consider the implications of parallel streams of feedback for vision.
Farran Briggs received her BA in Biology at Darmouth College and her PhD in Biology at the University of California, San Diego working with Dr Edward Callaway. She is currently a postdoctoral fellow with Dr Usrey. W. Martin Usrey is Professor of Neurobiology, Physiology & Behavior and Neurology at the University of California, Davis. He received his PhD from Duke University working with Dr David Fitzpatrick and performed his postdoctoral training with Dr R. Clay Reid at the Rockefeller University and Harvard Medical School. His current research programme is focused on understanding the functional interactions between feedforward and feedback pathways for vision.
Introduction
A dense network of feedforward and feedback projections interconnects neurones in the lateral geniculate nucleus (LGN) of the thalamus and primary visual cortex (V1). In the feedforward pathway, LGN neurones receive visual signals from the retina and relay these signals to V1. In the feedback pathway, corticogeniculate neurones provide synaptic input to the LGN as well to the overlying cortical layers targeted by LGN projections. As a consequence of this organization, corticogeniculate neurones are in a strategic position to influence the transmission and processing of visual information en route from retina to cortex. While corticogeniculate feedback is ubiquitous across mammals, recent work in the primate is providing new insight into the possible functions of this pathway for vision. In the sections below, we review the anatomical organization and physiological properties of corticogeniculate neurones in the primate followed by a discussion of their potential contributions to visual processing. Where appropriate, results will also be presented from studies examining other animal models.
Parallel processing streams from retina to cortex
There is a striking relationship between the organization of corticogeniculate neurones and their feedback projections and the feedforward parallel processing streams. Parallel processing streams are robust in the primate visual system and are particularly evident in the LGN where three classes of neurones – the magnocellular, parvocellular and koniocellular neurones – are segregated into distinct layers (Fig. 1). These three classes of LGN neurones receive input from separate classes of retinal ganglion cells, give rise to axons that terminate in different cortical laminae, and display distinct visual physiology (reviewed in Schiller & Logothetis, 1990; Shapley, 1992; Merigan & Maunsell, 1993; Casagrande & Kaas, 1994; Casagrande, 1994; Hendry & Calkins, 1998; Hendry & Reid, 2000; Rathbun & Usrey, 2008; Briggs & Usrey, 2009a; Nassi & Callaway, 2009).
Figure 1. Laminar organization of the LGN in five different primates: galago, squirrel monkey, macaque monkey, chimpanzee, and human.
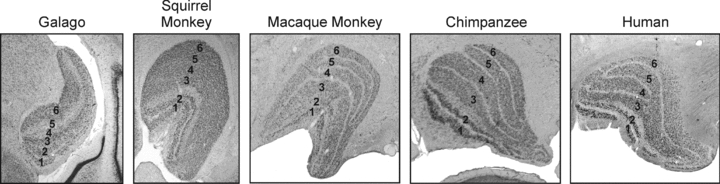
In each, neurones in the magnocellular, parvocellular and koniocellular streams occupy distinct laminae. In the galago, magnocellular neurones occupy layers 1 and 2, parvocellular neurones occupy layers 3 and 6, and koniocellular neurones occupy layers 4 and 5 and the intercalated zones. In the squirrel monkey, macaque monkey, chimpanzee and human, magnocellular neurones occupy layers 1 and 2, parvocellular neurones occupy layers 3, 4, 5 and 6, and koniocellular neurones occupy the intercalated layers below and between each of the magnocellular and parvocellular layers. Although the squirrel monkey lacks clear intercalated zones between the parvocellular layers, koniocellular neurones have been reported between the layers.
The physiology of magnocellular and parvocellular LGN neurones has been studied extensively (partial list: Schiller & Malpeli, 1978; Kaplan & Shapley, 1982, 1986; Derrington & Lennie, 1984; Norton et al. 1988; Benardete et al. 1992; Reid & Shapley, 1992; O’Keefe et al. 1998; Maunsell et al. 1999; Solomon et al. 1999; Usrey & Reid, 2000; Levitt et al. 2001; Movshon et al. 2005; Alitto & Usrey, 2008; Alitto et al. 2010). Compared to parvocellular neurones, magnocellular neurones respond better to low contrast stimuli, are more sensitive to stimuli modulated at high temporal frequencies (but see Spear et al. 1994; Hawken et al. 1996), display greater extraclassical surround suppression, and respond with a shorter latency following stimulus presentation. In addition, magnocellular neurones lack colour selectivity, while most parvocellular neurones in old world monkeys have long- (L) and medium- (M) wavelength opponent receptive fields. Less is known about the physiology of koniocellular neurones; however, existing evidence indicates that many are selectively modulated by short- (S) wavelength inputs and have visual responses (e.g. contrast gain, temporal-frequency tuning) that are generally intermediate to those of magnocellular and parvocellular neurones (Hendry & Reid, 2000; White et al. 2001; Chatterjee & Callaway, 2003; Tailby et al. 2008; Roy et al. 2009).
The three major classes of LGN neurones provide stream-specific input to V1 with magnocellular axons targeting layer 4Cα, parvocellular axons targeting layer 4Cβ, and koniocellular axons targeting the cytochrome-oxidase rich blobs, layer 1 and, in a subset of species including the macaque monkey, layer 4A (Fig. 2). In addition to providing input to layers 4Cα and 4Cβ, magnocellular and parvocellular LGN axons also provide input to layer 6 (described below). As a consequence of these projection patterns, corticogeniculate neurones in the primate have the opportunity to receive direct geniculate input onto both their basal dendrites in layer 6 as well as their apical dendrites in the overlying cortical layers. Recent results from the cat, however, indicate that the majority of synapses from the LGN are made onto the basal dendrites (da Costa & Martin, 2009).
Figure 2. Anatomy of feedforward and feedback connections between the LGN and visual cortex (V1).
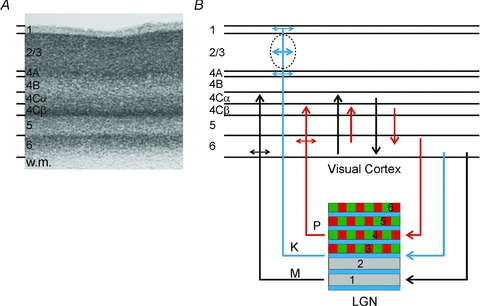
A, Nissl-stained section of V1 from the macaque monkey. Corticogeniculate neurones are located exclusively in layer 6. (w.m., white matter.) B, organization of connections between the LGN and V1. The magnocellular layers of the LGN (1 and 2) are shown in grey, the parvocellular layers (3, 4, 5 and 6) are shown in green and red, the koniocellular layers are located below each of the magnocellular and parvocellular layers and are shown in blue. Magnocellular LGN axons (M) terminate in layers 4Cα and lower layer 6, parvocellular LGN axons (P) terminate in layers 4Cβ and upper layer 6, koniocellular LGN axons (K) terminate in layer 4A, the cytochrome oxidase rich blobs and layer 1. The intrinsic connections in V1 maintain the magno- and parvocellular divisions of layers 4C and 6. Neurones in layer 6 of cortex provide feedback to the LGN. Neurones in the upper third of layer 6 project exclusively to the parvocellular LGN layers. Neurones in the lower third of layer 6 project primarily to the magnocellular layers and perhaps the koniocellular layers.
Evidence for parallel streams of corticogeniculate feedback
Corticogeniculate neurones have a pyramidal morphology and use glutamate for synaptic transmission (reviewed in Briggs & Usrey, 2009b). Their cell bodies are located exclusively in layer 6 of visual cortex and their axons branch to innervate the LGN, the reticular nucleus, and the overlying cortical layers (predominantly specific divisions of layer 4). In addition, a subset of corticogeniculate neurones in the very bottom of layer 6 probably provides weak input to the pulvinar nucleus (Conley & Raczkowski, 1990; Bourassa & Deschenes, 1995; Usrey & Fitzpatrick, 1996; Van Horn & Sherman, 2004). Although corticogeniculate neurones typically make up less than 50% of layer 6 neurones (∼14% in the macaque monkey; Fitzpatrick et al. 1994), their connections are anatomically robust. Indeed, individual LGN neurones receive more synaptic input from corticogeniculate feedback axons than from retinal axons (Guillery, 1969; Erisir et al. 1997a,b;). Similarly, individual layer 4 neurones receive more synaptic input from layer 6 axons than from LGN axons (Ahmed et al. 1994).
In the macaque monkey, layer 6 can be divided into three tiers. The cell bodies of corticogeniculate neurones are restricted to the upper and lower tiers; the middle tier is void of corticogeniculate neurones (Fitzpatrick et al. 1994; see also Lund et al. 1975; Hendrickson et al. 1978). Importantly, neurones in the upper and lower tiers provide anatomically distinct patterns of input to the LGN that follow the organization established by the feedforward parallel processing streams. In particular, corticogeniculate neurones in the upper tier of layer 6 have axons that target the parvocellular layers of the LGN, while neurones in the bottom tier have axons that target the magnocellular layers (Fig. 2; Conley & Raczkowski, 1990; Fitzpatrick et al. 1994). In addition, a small percentage of corticogeniculate neurones in the lower tier of layer 6 have axons that probably provide input selective to the koniocellular layers of the LGN or to the koniocellular layers in combination with either the magnocellular or parvocellular layers (Fitzpatrick et al. 1994; Usrey & Fitzpatrick, 1996; Ichida & Casagrande, 2002).
Several lines of evidence indicate that corticogeniculate neurones in the upper and lower tiers of layer 6 are members of distinct processing streams (Fig. 2). First, neurones in the upper and lower tiers of layer 6 receive different patterns of afferent LGN input. Parvocellular LGN axons that project to layer 4Cβ often give rise to collaterals that terminate in the upper part of layer 6. In contrast, magnocellular LGN axons that project to layer 4Cα often give rise to collaterals that terminate in the lower part of layer 6 (Lund, 1988). Second, the axons of layer 6 neurones not only target the LGN, but also branch and terminate locally in layer 4C of visual cortex. Neurones in the upper tier of layer 6 have axons that terminate primarily in layers 4Cβ and 4A (parvocellular targets); neurones in the lower tier of layer 6 have axons that terminate primarily in layer 4Cα (magnocellular targets; Lund & Boothe, 1975; Wiser & Callaway, 1996). Third, while some layer 6 neurones receive similar input from layers 4Cα and 4Cβ, other layer 6 neurones receive a disproportionate amount of input from either layer 4Cα or 4Cβ (Briggs & Callaway, 2001).
Physiological properties of corticogeniculate neurones
An examination of the visual physiology of corticogeniculate neurones in the macaque monkey distinguishes three major groups of neurones (Briggs & Usrey, 2007, 2009c). The first group is composed of complex cells with fast conducting axons (antidromic activation latency: <7 ms) and visual physiology indicative of a strong influence from the magnocellular stream. These neurones respond well to low contrast stimuli and stimuli modulated at high temporal frequencies. (Briggs & Usrey, 2009; see also Hawken et al. 1988) These neurones also exhibit strong surround suppression and have the greatest selectivity for the direction of stimulus motion. Interestingly, many corticogeniculate neurones in this group are also distinct in receiving direct input from the LGN capable of driving suprathreshold spikes (Briggs & Usrey, 2007). As a consequence, there is a fast, disynaptic circuit from the LGN to V1 and back to the LGN that appears specific to the magnocellular stream.
The second group of corticogeniculate neurones is composed of simple cells with moderate conducting axons (antidromic activation latency: 7–15 ms) and visual physiology indicative of strong parvocellular stream input (Briggs & Usrey, 2009c). Compared to the first group of corticogeniculate neurones, these neurones have linear contrast response functions, prefer stimuli drifting at lower temporal frequencies, prefer stimuli with higher spatial frequencies, display less surround suppression, and have lower stimulus-evoked firing rates. Importantly, neurones in this second group are located more superficially in layer 6 compared to those in the first group, consistent with the sublaminar segregation of corticogeniculate neurones described above.
The third group of corticogeniculate neurones is composed of complex cells with slow conducting axons (antidromic activation latency: >15 ms; Briggs & Usrey, 2009c). Neurones in this group are similar to those in the first group in terms of responding well to low contrast stimuli and stimuli modulated at high temporal frequencies. However, these neurones are uniformly not orientation or direction selective. Consistent with the view that these neurones share a relationship with the koniocellular stream, the responses of neurones in this group are more strongly modulated by stimuli selective for S-cone modulation. Although less is known about the visual physiology of corticogeniculate neurones in non-primate species, a comparison of axon conduction latency and simple vs. complex response profiles supports a classification scheme with three groups of neurones (Harvey, 1978; Tsumoto & Suda, 1980; Swadlow & Weyand, 1987; Grieve & Sillito, 1995; Briggs & Usrey, 2005).
Based on an increasing amount of anatomical and physiological data, it appears clear that the feedforward and feedback pathways interconnecting the LGN and V1 are organized with a specificity that aligns well along the axes of the magnocellular, parvocellular and koniocellular streams. As a consequence of this organization, corticogeniculate feedback is well suited to influence feedforward processing of visual information in a stream-specific fashion.
Functional influence of corticogeniculate feedback on visual processing
The majority of corticogeniculate synapses in the LGN are made onto the distal dendrites of neurones. These synapses are smaller and contain fewer vesicles compared to retinogeniculate synapses. For these reasons and others, including the fact that LGN receptive fields resemble those of their retinal inputs and not their cortical inputs, the corticogeniculate pathway is thought to be modulatory rather than driving in nature (Sherman & Guillery, 1998). This modulatory input is likely to be complex, though, as corticogeniculate neurones may differentially activate neurones in the reticular nucleus leading to a variety of combined excitatory/inhibitory effects in the LGN (Landisman & Connors, 2007; Cruikshank et al. 2010; Lam & Sherman, 2010). Moreover, the balance of excitation and inhibition is likely to change with firing rate, as the synapses that provide direct excitation and disynaptic inhibition may experience varying amounts of rate-dependent facilitation (Cudeiro et al. 2000; Granseth et al. 2002; Li et al. 2003; Alexander & Godwin, 2005).
Results from a variety of species support two prominent roles for feedback: (1) feedback sharpens the receptive fields of LGN neurones, and (2) feedback enhances the transmission of signals relayed through the LGN (reviewed in Briggs & Usrey, 2008). Given the evidence for parallel streams of feedback in the primate, it is worth evaluating these roles for feedback in terms of the magnocellular, parvocellular and koniocellular processing streams. Moreover, because feedback projections display a retinotopic and ocular specificity, the effects of corticogeniculate feedback should be spatially restricted to LGN neurones located within the projection field of feedback axons (Murphy & Sillito, 1996; Angelucci & Sainsbury, 2006; Wang et al. 2006; see also Usrey & Fitzpatrick, 1996; Murphy et al. 2000). With respect to the first role for feedback, corticogeniculate projections are believed to sharpen the receptive fields of LGN neurones by contributing to the strength of their extraclassical (suppressive) surround (Murphy & Sillito, 1987; Jones et al. 2000). This role, however, may be restricted to carnivores as recent work demonstrates that extraclassical suppression in the LGN of primates relies on mechanisms established in the retina (Alitto & Usrey, 2008; but see Webb et al. 2002).
The second major role for corticogeniculate feedback – enhancing signal transmission through the LGN – has been suggested to occur by increasing the gain of LGN responses to visual stimuli, improving the reliability of LGN responses, and/or adjusting the temporal patterns of activity among individual neurones and neuronal ensembles. For instance, results from the macaque monkey demonstrate that corticogeniculate feedback can multiplicatively increase the responses of LGN neurones in a contrast-independent fashion (Przybyszewski et al. 2000). With respect to the parallel processing streams, this effect occurs over a much wider range of contrasts for parvocellular neurones than for magnocellular neurones. Corticogeniculate feedback has also been shown to increase the magnitude and high-velocity cutoff of LGN responses to moving patterns and textures (Gulyas et al. 1990; but see Marrocco et al. 1996), as well as increase the reliability and temporal precision of responses among individual LGN neurones and ensembles of neurones (McClurkin et al. 1994; Funke et al. 1996; Sillito & Jones, 2002; Andolina et al. 2007; de Labra et al. 2007).
The augmenting effect that feedback can have on LGN responses has been proposed to increase with directed attention. Attentional modulation of neuronal activity is well documented in V1 (Motter, 1993; Luck et al. 1997; Watanabe et al. 1998; Brefczynski & DeYoe, 1999; Ito & Gilbert, 1999; Somers et al. 1999; Ress et al. 2000; Marcus & Van Essen, 2002; McAdams & Reid, 2005; Chen et al. 2008). Although the effects of attention have not been measured among identified corticogeniculate neurones, it seems likely that these neurones also experience attentional modulation. If so, then the corticogeniculate feedback pathway could provide a route for attention to influence LGN activity. Consistent with this view, attentional modulation of LGN activity has been reported for both human and non-human primates (O’Connor et al. 2002; McAlonan et al. 2008). Moreover, there is evidence that directed attention may be able to selectively modulate LGN activity in a stream-specific fashion (Vanduffel et al. 2000).
Given the influence of activity levels on the strength and efficacy of corticogeniculate synapses (described above), it seems likely that the influence of feedback on LGN responses should be most robust in the alert animal. Along these lines, results from our laboratory indicate a major difference in the visual responsiveness of corticogeniculate neurones in the alert and anaesthetized monkey (Briggs & Usrey, 2007, 2009c). To date, however, most studies of corticogeniculate feedback have relied on measurements collected from animals in the anaesthetized state. While these measurements have certainly advanced our understanding of the functions of corticogeniculate feedback, it seems likely that we will see acceleration in our understanding of the feedback pathway as more studies are performed with alert animals. In addition, while past methods for assessing the function of feedback projections have relied on large-scale inactivation methods (e.g. cortical cooling, cortical aspiration) which are likely to obscure effects dependent on the fine topography of feedback connections, recent and ongoing advances in the development of molecular and optical methods for targeted inactivation of specific cells and cell types will certainly open new doors for determining the functions of this important pathway for vision.
Acknowledgments
This work was supported by NIH grants EY13588, EY12576, EY15580, NSF grant 0727115 and the McKnight Foundation.
References
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol. 1994;341:39–49. doi: 10.1002/cne.903410105. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Godwin DW. Presynaptic inhibition of corticothalamic feedback by metabotropic glutamate receptors. J Neurophysiol. 2005;94:163–175. doi: 10.1152/jn.01198.2004. [DOI] [PubMed] [Google Scholar]
- Alitto HJ, Moore BD, Rathbun DL, Usrey WM. A comparison of visual responses in the lateral geniculate nucleus of alert and anesthetized macaque monkeys. J Physiol. 2011;589:87–99. doi: 10.1113/jphysiol.2010.190538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitto HJ, Usrey WM. Origin and dynamics of extraclassical suppression in the lateral geniculate nucleus of the macaque monkey. Neuron. 2008;57:135–146. doi: 10.1016/j.neuron.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolina IM, Jones HE, Wang W, Sillito AM. Corticothalamic feedback enhances stimulus response precision in the visual system. Proc Natl Acad Sci U S A. 2007;104:1685–1690. doi: 10.1073/pnas.0609318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci A, Sainsbury K. Contribution of feedforward thalamic afferents and corticogeniculate feedback to the spatial summation area of macaque V1 and LGN. J Comp Neurol. 2006;498:330–351. doi: 10.1002/cne.21060. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Vis Neurosci. 1992;8:483–486. doi: 10.1017/s0952523800004995. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Deschenes M. Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA. A physiological correlate of the ‘spotlight’ of visual attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Briggs F, Callaway EM. Layer-specific input to distinct cell types in layer 6 of monkey primary visual cortex. J Neurosci. 2001;21:3600–3608. doi: 10.1523/JNEUROSCI.21-10-03600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Temporal properties of feedforward and feedback pathways between the thalamus and visual cortex in the ferret. Thalamus Relat Syst. 2005;3:133–139. doi: 10.1017/S1472928807000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. A fast, reciprocal pathway between the lateral geniculate nucleus and visual cortex in the macaque monkey. J Neurosci. 2007;27:5431–5466. doi: 10.1523/JNEUROSCI.1035-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Emerging views of corticothalamic function. Curr Opin Neurobiol. 2008;18:403–407. doi: 10.1016/j.conb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Visual system structure. In: Bruce Goldstein E, editor. The SAGE Encyclopedia of Perception. Thousand Oaks, CA, USA: SAGE Publications, Inc.; 2009a. pp. 1130–1134. [Google Scholar]
- Briggs F, Usrey WM. Corticothalamic connections: structure and function. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 3. Oxford: Academic Press; 2009b. pp. 215–219. [Google Scholar]
- Briggs F, Usrey WM. Parallel processing in the corticogeniculate pathway of the macaque monkey. Neuron. 2009c;62:135–146. doi: 10.1016/j.neuron.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande VA. A third parallel pathway to primate area V1. Trends Neurosci. 1994;17:305–310. doi: 10.1016/0166-2236(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland KS, editors. Cerebral Cortex. Primary Visual Cortex in Primates. Vol. 10. New York: Plenum; 1994. pp. 201–259. [Google Scholar]
- Chatterjee S, Callaway EM. Parallel colour-opponent pathways to primary visual cortex. Nature. 2003;426:668–671. doi: 10.1038/nature02167. [DOI] [PubMed] [Google Scholar]
- Chen Y, Martinez-Conde S, Macknik SL, Bereshpolova Y, Swadlow HA, Alonso JM. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat Neurosci. 2008;11:974–982. doi: 10.1038/nn.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M, Raczkowski D. Sublaminar organization within layer VI of the striate cortex in Galago. J Comp Neurol. 1990;302:425–436. doi: 10.1002/cne.903020218. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65:230–245. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudeiro J, Rivadulla C, Grieve KL. Visual response augmentation in cat (and macaque) LGN: potentiation by corticofugally mediated gain control in the temporal domain. Eur J Neurosci. 2000;12:1135–1144. doi: 10.1046/j.1460-9568.2000.00000.x. [DOI] [PubMed] [Google Scholar]
- da Costa NM, Martin KA. Selective targeting of the dendrites of corticothalamic cells by thalamic afferents in area 17 of the cat. J Neurosci. 2009;29:13919–13928. doi: 10.1523/JNEUROSCI.2785-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Labra C, Rivadulla C, Grieve K, Mariño J, Espinosa N, Cudeiro J. Changes in visual responses in the feline dLGN: selective thalamic suppression induced by transcranial magnetic stimulation of V1. Cereb Cortex. 2007;17:1376–1385. doi: 10.1093/cercor/bhl048. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Bickford ME, Sherman SM. Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: a comparison with corticogeniculate terminals. J Comp Neurol. 1997b;377:535–549. [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Sherman SM. Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proc Natl Acad Sci U S A. 1997a;94:1517–1520. doi: 10.1073/pnas.94.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Usrey WM, Schofield BR, Einstein G. The sublaminar organization of neurons in layer 6 of macaque striate cortex. Vis Neurosci. 1994;11:307–315. doi: 10.1017/s0952523800001656. [DOI] [PubMed] [Google Scholar]
- Funke K, Nelle E, Li B, Worgotter F. Corticofugal feedback improves the timing of retino-geniculate signal transmission. Neuroreport. 1996;7:2130–2134. doi: 10.1097/00001756-199609020-00013. [DOI] [PubMed] [Google Scholar]
- Granseth B, Ahlstrand E, Lindström S. Paired pulse facilitation of corticogeniculate EPSCs in the dorsal lateral geniculate nucleus of the rat investigated in vitro. J Physiol. 2002;544:477–486. doi: 10.1113/jphysiol.2002.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve KL, Sillito AM. Differential properties of cells in the feline primary visual cortex providing the corticofugal feedback to the lateral geniculate nucleus and visual claustrum. J Neurosci. 1995;15:4868–4874. doi: 10.1523/JNEUROSCI.15-07-04868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. A quantitative study of synaptic interconnections in the dorsal lateral geniculate nucleus of the cat. Z Zellforsch. 1969;96:39–48. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- Gulyas B, Lagae L, Eysel U, Orban GA. Corticofugal feedback influences the responses of geniculate neurons to moving stimuli. Exp Brain Res. 1990;79:441–446. doi: 10.1007/BF00608257. [DOI] [PubMed] [Google Scholar]
- Harvey AR. Characteristics of corticothalamic neurons in area 17 of the cat. Neurosci Lett. 1978;7:177–181. doi: 10.1016/0304-3940(78)90164-7. [DOI] [PubMed] [Google Scholar]
- Hawken MJ, Parker AJ, Lund JS. Laminar organization and contrast sensitivity of direction-selective cells in the striate cortex of the old world monkey. J Neurosci. 1988;8:3541–3548. doi: 10.1523/JNEUROSCI.08-10-03541.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawken MJ, Shapley RM, Grosof DH. Temporal-frequency selectivity in monkey visual cortex. Vis Neurosci. 1996;13:477–492. doi: 10.1017/s0952523800008154. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Wilson JR, Ogren MP. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in the old world and new world primates. J Comp Neurol. 1978;182:123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Calkins D. Neuronal chemistry and functional organization in the primate visual system. Trends in Neurosci. 1998;21:344–349. doi: 10.1016/s0166-2236(98)01245-4. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Reid RC. The koniocellular pathway in primate vision. Ann Rev Neurosci. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Ichida JM, Casagrande VA. Organization of the feedback pathway from striate cortex (V1) to the lateral geniculate nucleus (LGN) in the owl monkey (Aotus trivirgatus) J Comp Neurol. 2002;454:272–283. doi: 10.1002/cne.10441. [DOI] [PubMed] [Google Scholar]
- Ito M, Gilbert CD. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron. 1999;22:593–604. doi: 10.1016/s0896-6273(00)80713-8. [DOI] [PubMed] [Google Scholar]
- Jones HE, Andolina IM, Oakely NM, Murphy PC, Sillito AM. Spatial summation in lateral geniculate nucleus and visual cortex. Exp Brain Res. 2000;135:279–284. doi: 10.1007/s002210000574. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol. 1982;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci U S A. 1986;83:2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cereb Cortex. 2010;20:13–24. doi: 10.1093/cercor/bhp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. VPM and PoM nuclei of the rat somatosensory thalamus: intrinsic neuronal properties and corticothalamic feedback. Cereb Cortex. 2007;17:2853–2865. doi: 10.1093/cercor/bhm025. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Schumer RA, Sherman SM, Spear PD, Movshon JA. Visual response properties of neurons in the LGN of normally reared and visually deprived macaque monkeys. J Neurophysiol. 2001;85:2111–2129. doi: 10.1152/jn.2001.85.5.2111. [DOI] [PubMed] [Google Scholar]
- Li J, Guido W, Bickford ME. Two distinct types of corticothalamic EPSPs and their contribution to short-term synaptic plasticity. J Neurophysiol. 2003;90:3429–3440. doi: 10.1152/jn.00456.2003. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Lund JS. Anatomical organization of macaque monkey striate visual cortex. Ann Rev Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
- Lund JS, Boothe R. Interlaminar connections and pyramidal neuron organization in the visual cortex, area 17, of the macaque monkey. J Comp Neurol. 1975;159:305–334. [Google Scholar]
- Lund JS, Lund RD, Hendrickson AE, Bunt AH, Fuchs AF. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey. J Comp Neurol. 1975;164:287–304. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClurkin JW, Optican LM, Richmond BJ. Cortical feedback increases visual information transmitted by monkey parvocellular lateral geniculate nucleus neurons. Vis Neurosci. 1994;11:601–617. doi: 10.1017/s0952523800002492. [DOI] [PubMed] [Google Scholar]
- Marcus DS, Van Essen DC. Scene segmentation and attention in primate cortical areas V1 and V2. J Neurophysiol. 2002;8:2648–2658. doi: 10.1152/jn.00916.2001. [DOI] [PubMed] [Google Scholar]
- Marrocco RT, McClurkin JW, Alkire MT. The influence of the visual cortex on the spatiotemporal response properties of lateral geniculate nucleus cells. Brain Res. 1996;737:110–118. doi: 10.1016/0006-8993(96)00660-9. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR, Ghose GM, Assad JA, McAdams CJ, Boudreau CE, Noerager BD. Visual response latencies of magnocellular and parvocellular LGN neurons in macaque monkeys. Vis Neurosci. 1999;16:1–14. doi: 10.1017/s0952523899156177. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Ann Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2 and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Kiorpes L, Hawken MJ, Cavanaugh JR. Functional maturation of the macaque's lateral geniculate nucleus. J Neurosci. 2005;25:2712–2722. doi: 10.1523/JNEUROSCI.2356-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PC, Duckett SG, Sillito AM. Comparison of the laminar distribution of input from areas 17 and 18 of the visual cortex to the lateral geniculate nucleus of the cat. J Neurosci. 2000;20:845–853. doi: 10.1523/JNEUROSCI.20-02-00845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature. 1987;329:727–729. doi: 10.1038/329727a0. [DOI] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. Functional morphology of the feedback pathway from area 17 of the cat visual cortex to the lateral geniculate nucleus. J Neurosci. 1996;16:1180–1192. doi: 10.1523/JNEUROSCI.16-03-01180.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat Rev Neurosci. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Casagrande VA, Irvin GE, Sesma MA, Petry HM. Contrast-sensitivity functions of W-, X-, and Y-like relay cells in the lateral geniculate nucleus of bush baby, Galago crassicaudatus. J Neurophysiol. 1988;59:1639–1656. doi: 10.1152/jn.1988.59.6.1639. [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- O’Keefe LP, Levitt JB, Kiper DC, Shapley RM, Movshon JA. Functional organization of owl monkey lateral geniculate nucleus and visual cortex. J Neurophysiol. 1998;80:594–609. doi: 10.1152/jn.1998.80.2.594. [DOI] [PubMed] [Google Scholar]
- Przybyszewski AW, Gaska JP, Foote W, Pollen DA. Striate cortex increases contrast gain of macaque LGN neurons. Vis Neurosci. 2000;17:485–494. doi: 10.1017/s0952523800174012. [DOI] [PubMed] [Google Scholar]
- Rathbun DL, Usrey WM. The geniculo-striate pathway. In: Binder MD, Hirokawa N, Windhorst U, Hirsch MC, editors. Encyclopedia of Neuroscience. Heidelberg, Germany: Springer-Verlag; 2008. [Google Scholar]
- Reid RC, Shapley RM. Spatial structure of cone inputs to receptive fields in primate lateral geniculate nucleus. Nature. 1992;356:716–718. doi: 10.1038/356716a0. [DOI] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Roy S, Jayakumar J, Martin PR, Dreher B, Saalmann YB, Hu D, Vidyasagar TR. Segregation of short-wavelength-sensitive (S) cone signals in the macaque dorsal lateral geniculate nucleus. Eur J Neurosci. 2009;30:1517–1526. doi: 10.1111/j.1460-9568.2009.06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Logothetis NK. The color-opponent and broad-band channels of the primate visual system. Trends in Neurosci. 1990;13:392–398. doi: 10.1016/0166-2236(90)90117-s. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Malpeli JG. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. J Neurophysiol. 1978;41:788–797. doi: 10.1152/jn.1978.41.3.788. [DOI] [PubMed] [Google Scholar]
- Shapley RM. Parallel retinocortical channels: X and Y and P and M. In: Brannan J, editor. Applications of Parallel Processing in Vision. New York: Elsevier Science Publishers; 1992. pp. 3–36. [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci U S A. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond B Biol Sci. 2002;357:1739–1752. doi: 10.1098/rstb.2002.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SG, White AJ, Martin PR. Temporal contrast sensitivity in the lateral geniculate nucleus of a New World monkey, the marmoset Callithrix jacchus. J Physiol. 1999;517:907–917. doi: 10.1111/j.1469-7793.1999.0907s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PD, Moore RJ, Kim CB, Xue JT, Tumosa N. Effects of aging on the primate visual system: spatial and temporal processing by lateral geniculate neurons in young adult and old rhesus monkeys. J Neurophysiol. 1994;72:402–420. doi: 10.1152/jn.1994.72.1.402. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Weyand TG. Corticogeniculate neurons, corticotectal neurons, and suspected interneurons in visual cortex of awake rabbits: receptive-field properties, axonal properties, and effects of EEG arousal. J Neurophysiol. 1987;57:977–1001. doi: 10.1152/jn.1987.57.4.977. [DOI] [PubMed] [Google Scholar]
- Tailby C, Szmajda BA, Buzás P, Lee BB, Martin PR. Transmission of blue (S) cone signals through the primate lateral geniculate nucleus. J Physiol. 2008;586:5947–5967. doi: 10.1113/jphysiol.2008.161893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T, Suda K. Three groups of cortico-geniculate neurons and their distribution in binocular and monocular segments of cat striate cortex. J Comp Neurol. 1980;193:223–236. doi: 10.1002/cne.901930115. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Fitzpatrick D. Specificity in the axonal connections of layer VI neurons in tree shrew striate cortex: evidence for separate granular and supragranular systems. J Neurosci. 1996;16:1203–1218. doi: 10.1523/JNEUROSCI.16-03-01203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reid RC. Visual physiology of the lateral geniculate nucleus in two new world monkeys: Saimiri sciureus and Aotus trivirgatis. J Physiol. 2000;523:755–769. doi: 10.1111/j.1469-7793.2000.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanduffel W, Tootell RB, Orban GA. Attention-dependent suppression of metabolic activity in the early stages of the macaque visual system. Cereb Cortex. 2000;10:109–126. doi: 10.1093/cercor/10.2.109. [DOI] [PubMed] [Google Scholar]
- Van Horn SC, Sherman SM. Differences in projection patterns between large and small corticothalamic terminals. J Comp Neurol. 2004;475:406–415. doi: 10.1002/cne.20187. [DOI] [PubMed] [Google Scholar]
- Wang W, Jones HE, Andolina IM, Salt TE, Sillito AM. Functional alignment of feedback effects from visual cortex to thalamus. Nat Neurosci. 2006;9:1330–1336. doi: 10.1038/nn1768. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sasaki Y, Miyauchi S, Putz B, Fujimaki N, Nielsen M, Takino R, Miyakawa S. Attention-regulated activity in human primary visual cortex. J Neurophysiol. 1998;79:2218–2221. doi: 10.1152/jn.1998.79.4.2218. [DOI] [PubMed] [Google Scholar]
- Webb BS, Tinsley CJ, Barraclough NE, Easton A, Parker A, Derrington AM. Feedback from V1 and inhibition from beyond the classical receptive field modulates the responses of neurons in the primate lateral geniculate nucleus. Vis Neurosci. 2002;19:583–592. doi: 10.1017/s0952523802195046. [DOI] [PubMed] [Google Scholar]
- White AJ, Solomon SG, Martin PR. Spatial properties of koniocellular cells in the lateral geniculate nucleus of the marmoset Callithrix jacchus. J Physiol. 2001;533:519–535. doi: 10.1111/j.1469-7793.2001.0519a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser AK, Callaway EM. Contributions of individual layer 6 pyramidal neurons to local circuitry in macaque primary visual cortex. J Neurosci. 1996;16:2724–2739. doi: 10.1523/JNEUROSCI.16-08-02724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


