Abstract
Angiogenesis may be induced in skeletal muscle by metabolic or mechanical factors, but whether an in vivo stimulus threshold applies for physiological angiogenesis is unknown. We compared three models of muscle overload inducing varying degrees of stretch on angiogenesis. Rat extensor digitorum longus (EDL) was overloaded by (a) extirpation of the synergist tibialis anterior (TA), (b) sectioning the distal tendon of the TA, or (c) release of the TA tendon by sectioning the retaining ligament. EDL samples were taken after 4, 7, 14 and 28 days to quantify capillary supply (alkaline phosphatase staining), and co-labelling for cell proliferation (using PCNA). The gradation of overload was confirmed by Western analysis of SERCA and CPT expression (1.6- to 7.2-fold and 8.3- to 33.9-fold changes, respectively), and the force characteristics of EDL. There was a significant increase in the number of new myonuclei only in the extirpated group after 7 days, while there was a graded increase in capillary-linked PCNA density (PCNAcap) among groups compared to controls. However, extirpation caused significant increase in PCNAcap after 7 days, whereas tenotomy showed a more modest and delayed increase at 14 days, and ligament transection induced no significant change. Muscle capillary supply followed a similar trend to that of PCNA, whereas the pro-angiogenic VEGF and Flk-1 protein levels were both up-regulated to a similar extent in all three experimental models 7–14 days after surgery. These results are consistent with the hypothesis that overload-induced angiogenesis is primarily a mechanical response, and that it is graded according to stimulus intensity.
Non-technical summary
The formation of new blood vessels (angiogenesis) is important during development and tissue repair. In many diseases the biggest drive for this process clearly comes from chemical signals. However, normal physiological angiogenesis, such as seen with increased muscle activity, appears to be more driven by mechanical signals including increased friction on the inside of blood vessels, and stretch of vessels caused by the surrounding muscle fibres. It is unclear whether the signals required to stimulate capillary growth act in an all-or-none manner. When muscles were subjected to varying degrees of stretch, angiogenesis was recruited in a graded fashion, although chemical signals were increased to a similar extent. This may prove to be important in the design of targeted therapies to alleviate problems associated with too many or too few vessels.
Introduction
It has long been known that capillary growth (angiogenesis) can be initiated by exercise (Vanotti & Magiday, 1934), but that small amounts of exercise do not induce widespread capillary proliferation in skeletal muscle (Engerman et al. 1967; Hobson & Denekamp, 1984; Prior et al. 2003). Thus, there is likely to be an activation threshold for angiogenesis. In vitro reports suggest that a stimulus threshold needs to be overcome for angiogenic effects of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)-2 to be observed (Yue & Tomanek, 2001; Xue & Greisler, 2002), although there have been no parallel studies conducted in vivo. The level of stimulation required for in situ growth factor expression and physiological angiogenesis is therefore unknown (Gustafsson & Kraus, 2001), whereas establishing whether angiogenesis is a threshold or graded phenomenon is essential to provide a mechanistic basis for development of effective angiotherapies.
An increased number of capillaries was noted in extensor digitorum longus (EDL) muscles of rat overloaded by removal of the tibialis anterior (TA) for up to 22 weeks (Frischknecht & Vrbova, 1991), although only 2 weeks was sufficient to demonstrate overload-induced angiogenesis in a similar model (Egginton et al. 1998). The mechanisms of capillary growth in muscles subjected to compensatory overload are not known. It has been postulated that mechanical factors such as higher luminal shear stress and capillary wall tension, associated with a sustained increase in blood flow, represent an important stimulus for capillary growth in vivo (Hudlická et al. 1992). However, capillary growth during compensatory overload is independent of any alteration in blood flow, and presumably results from mechanotransduction of muscle stretch, i.e. local tensile and shear strains of the muscle fibres and surrounding endothelium (Egginton et al. 1998), leading to the sprouting form of angiogenesis (Egginton et al. 2001). We previously showed that extirpation of TA caused an increase in sarcomere strain in the middle of the m. extensor hallucius proprius (a synergist of the EDL) by ∼ 20% after 2 weeks, which had normalised after 8 weeks (Egginton et al. 1998). Despite widespread claims that the extent of in vivo angiogenesis is determined by the magnitude of any acute inflammatory response (Armstrong et al. 1979), we could find no supportive evidence based on histological phenotype (e.g. tissue oedema) or cellular response (e.g. macrophage infiltration) (Egginton et al. 2001).
Previous work has shown that angiogenesis in response to muscle overload is dependent on VEGF (Williams et al. 2006a) and involves up-regulation of both VEGF and its main signalling receptor Flk-1 (VEGF-R2), but with little involvement of FGF-2 (Egginton et al. 1998; Williams et al. 2006b). The purpose of this study was to test the hypothesis that varying the degree of overload would modulate the extent of capillary growth. Angiogenesis, cellular proliferation, and protein expression of VEGF and Flk-1 were therefore studied in three different models to determine whether the magnitude of the overload stimulus, the extent of VEGF signalling, and/or the amount of cell proliferation could be correlated with the resultant angiogenesis.
Methods
Animals
Experiments were performed on adult male Sprague–Dawley rats, body mass ∼280 g (Table 1), in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986. Three models of overload were used. Hindlimb muscles, such as the m. extensor digitorum longus (EDL), can be chronically overloaded in a graded manner using different approaches: (a) Ext – unilateral extirpation of the synergist m. tibialis anterior (TA) overloads the EDL to a large extent, a surgical procedure that invokes a transient inflammation but subsequent overload-induced hypertrophy (Egginton et al. 1998); (b) Ten – tenotomy (unilateral section of the distal tendon of the TA) involves less surgical trauma, although connective tissue may grow back and form a pseudo-tendon, which subjects the EDL to a lower degree of overload; (c) Lig – tendon release by unilateral transection of the extensor retinaculum ligament that holds the TA tendon in place at the ankle causes the muscle to become mechanically disadvantaged, which increases the range over which the muscle has to work (Koh & Herzog, 1998), and induces a mild form of overload in the EDL. All surgery was performed under aseptic conditions and Fluothane anaesthesia with post-operative analgesia (Temgesic, National Veterinary services, Stoke-on-trent, Staffs, UK). The three groups were taken into final experiment 4, 7, 14 and 28 days after surgery, when body masses were between 250 and 300 g. A fourth group of similar age and mass were taken as controls (Cont, Table 1). Animals were killed by an i.p. injection of 2 ml sodium pentobarbital (200 mg ml−1).
Table 1.
Body and muscle mass response to graded overload
| Body mass (g) | EDL mass (mg) | EDL/body mass (mg/g 10−4) | TA/body mass (mg/g 10−4) | |
|---|---|---|---|---|
| Control (8) | 284 ± 10 | 127 ± 3 | 0.447 ± 0.011 | 1.744 ± 0.037 |
| 4d Lig (4) | 279 ± 9 | 127 ± 3 | 0.455 ± 0.011 | 1.426 ± 0.022* |
| 7d Lig (7) | 261 ± 2 | 120 ± 3 | 0.464 ± 0.017 | 1.584 ± 0.012 |
| 14d Lig (4) | 245 ± 6 | 108 ± 3** | 0.440 ± 0.010 | 1.467 ± 0.056* |
| 28d Lig (4) | 291 ± 2 | 139 ± 5 | 0.477 ± 0.015 | 1.658 ± 0.043 |
| 4d Ten(4) | 281 ± 9 | 132 ± 6 | 0.469 ± 0.009 | 1.586 ± 0.086 |
| 7d Ten(7) | 260 ± 5 | 124 ± 5 | 0.466 ± 0.009 | 1.281 ± 0.119** |
| 14d Ten (4) | 255 ± 6 | 122 ± 3 | 0.482 ± 0.088* | 1.159 ± 0.078** |
| 28d Ten (4) | 269 ± 7 | 134 ± 3 | 0.498 ± 0.011* | 1.515 ± 0.037* |
| 4d Ext (4) | 311 ± 8 | 172 ± 3** | 0.554 ± 0.030** | — |
| 7d Ext (7) | 273 ± 4 | 141 ± 4** | 0.518 ± 0.020* | — |
| 14d Ext (4) | 297 ± 17 | 167 ± 3** | 0.549 ± 0.016** | — |
| 28d Ext (4) | 268 ± 5 | 145 ± 3** | 0.542 ± 0.014** | — |
Means ± s.e.m. (number of animals).
P < 0.05,
P < 0.001 vs. control.
Histology
For all muscles, the mid portion of each EDL was snap frozen in isopentane pre-cooled in liquid nitrogen to be used for histochemical assessment. Serial cryostat sections (8 μm) were stained for alkaline phosphatase (ALP) activity to visualise the location of capillaries. The extent of cell proliferation was quantified by labelling with antibodies to proliferating cell nuclear antigen (PCNA; clone pc10, Dako, UK Ltd, Ely, Cambs, UK), using standard immunohistochemical techniques (with diaminobenzoate as the substrate). Counterstaining with haematoxylin was carried out to visualise all nuclei. Counting of capillaries and of PCNA-positive nuclei co-localised with the ALP staining was performed (×400 magnification, Olympus BH2 microscope equipped with a drawing arm) using four random fields of 0.065 mm2 each. This enabled calculation of the density of proliferating nuclei associated with capillaries. PCNA-positive cells within the interstitium and muscle fibres were also counted; interstitial and myonuclear labelling indices were calculated as the ratio of PCNA-positive nuclei to haematoxylin stained nuclei in the same field. ALP was under-stained to make the colour differentiation from PCNA clearer, but as the capillary densities and C:F were comparable to those of other studies from this laboratory using ALP, it is unlikely capillarity was under-estimated as a result.
Protein measurements
Pooled samples from EDL muscle were homogenised on ice, then protein extracted on ice with radioimmuno precipitation assay (RIPA) buffer containing 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS in phosphate buffered saline (Sigma) with broad spectrum protease inhibition (Protease Inhibitor Cocktail, Sigma) by standard methods. Protein levels were assayed using a detergent compatible protein assay (DC Protein Assay, Bio-Rad, Hemel Hempstead, Herts, UK). Western blots were run on a 7.5% polyacrylamide gel with 50 μg of protein loaded per gel, using a non-reducing sample buffer for VEGF and standard reducing conditions for other proteins, transferred to a PVDF membrane, and blocked with 5% non-fat milk powder in Tris-buffered saline (TBS)/Tween buffer (20 mm Tris base, 137 mm NaCl, 0.1% Tween 20, pH 7.6) for 1 h at room temperature (RT). Estimation of protein levels was undertaken using SDS-PAGE under reducing conditions using standard techniques. Primary antibodies (VEGF A-20, dilution 1:500; Flk-1, at 1:500; SERCA-2a, at 1:1500; muscle-CPT1, at 1:1000; all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were incubated for 1 h at room temperature or overnight at 4°C, followed by 1 × 15 min and 2 × 5 min washes with TBS/Tween. Appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies were incubated and washed similarly, then chemiluminescent substrate applied (SuperSignal Femto, Pierce Biotechnology/Thermo Fisher Scientific, Rockford, IL, USA) and exposures made on photographic film (CL-Xposure, Pierce Biotechnology). Membranes were stripped (0.1 m KOH, 15 min) and re-probed with antibodies against actin (T-20, Santa Cruz) or tubulin (Sigma, UK), primary antibody dilution 1:2500. Data were then normalised to the actin/tubulin levels, and expressed as a percentage of control tissue. Densitometric analysis was performed using Quantity One software (Bio-Rad). VEGF levels were also quantified using an ELISA (mouse VEGF, R&D Systems, Abingdon, Oxon, UK) according to the manufacturer's instructions to validate the Western analysis.
Muscle function
The interventions applied will reduce the contribution of the TA to the dorsiflexion moment by its force exertion onto its origin. However, as the TA and EDL are mechanically connected via their epimysium, force generated by the TA could be transmitted to the EDL and vice versa. As ligament release and tenotomy of TA cause a shortening with respect to the ankle angle, this must also change the length and relative position of the TA with respect to the EDL. As a consequence, shear forces will be applied to the epimysium and a shear load will be applied to the EDL. Alternatively, due to surgical interventions, the forces exerted by TA on its origin are substantially reduced. As TA and EDL are both plantar flexors, a reduction in the ability of TA to contribute to the dorsiflexion moment will increase the demands applied to the EDL to contribute to the dorsiflexion moment. Consequently, the EDL will be relatively more activated than before the surgery and hence mechanically more loaded. To assess the effects of epimuscular force transmission and the reductions in TA force on its origin, the acute effects of the different surgical interventions on the force–length relations of TA and EDL were determine on TA and EDL distal tendons in situ. A fifth group of male Wistar rats (n = 4, 307.5 ± 5.8 g) were therefore used to investigate the effects of interventions on the force–length characteristics of the EDL and TA, in agreement with guidelines under Dutch law and approved by the Animal Care and Use Committee of the VU University, Amsterdam. Rats were anaesthetised using i.p. urethane (initial dose 1.2 ml (100 g body mass)−1, 12.5% solution), supplemental doses given as needed (max 1.5 ml) using pedal reflex as a guide to the plane of surgical anaesthesia, and placed on a heated water pad (37°C). The foot was attached firmly to a plastic plate, and skin and subcutaneous tissues removed over the left foot to ∼1 cm below the extensor retinaculum ligament. The four distal EDL tendons were cut, tied together with polyester thread and attached to a Kevlar® thread (diameter 0.5 mm, 4% breaking load at a load of 800 N) attached to metal rods connected to force transducers (Hottinger Baldwin; maximal drift <0.1%, compliance 0.0048 mm N−1). The TA tendon was removed from its insertion by cutting a small piece of metatarsal bone and similarly attached. Care was taken to align the rods with the muscle axis and pass the foot freely. A metal clamp was secured to the femur and electrodes placed along the tibial branch of the sciatic nerve, cut distally from the electrode. Figure 1 shows a schematic diagram of the arrangement. Both the metal clamp and the foot plate were rigidly fixed within the apparatus such that the knee and ankle angle were both 90 deg, and experiments performed in a controlled environment (22°C, humidity 80%).
Figure 1. Schematic diagram of the rat hindlimb muscles and experimental situation for testing overload effects on contractile characteristics of m. tibialis anterior (TA) and m. extensor digitorum longus (EDL).
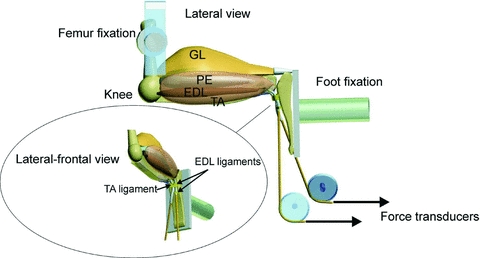
The lateral view shows hindlimb muscles after removal of the m. biceps femoris. Abbreviations: GL, m. gastrocnemius lateralis; TA, m. tibialis anterior; PE, peroneal muscles; EDL, m. extensor digitorum longus. Force–length characteristics of the TA and EDL were determined at 90 deg flexion of knee and foot. While the proximal insertions were left intact, tendons of TA and EDL were distally attached to force transducers via Kevlar® threads and aligned with their lines of pull in vivo. EDL was overloaded by (1) extirpation of TA, (2) tenotomy of TA and (3) TA ligament transection. Note that the retinaculum covering the tendons of the EDL and TA consists of two separate ligaments.
Both EDL and TA tendons were adjusted to their reference length (corresponding to the in vivo situation with the knee and foot at 90 deg), and muscles preconditioned by isometric contractions alternatively at different lengths until forces at short lengths were reproducible (i.e. no effects of previous activity at greater lengths were seen) (Huijing & Baan, 2001). Isometric contractions were generated by supramaximal stimulation (2 mA, 0.1 ms square wave pulse) via electrodes connected to a constant current source, shortened and lengthened by steps of 1 mm to determine active slack and optimum length of the muscle. Two twitches were evoked followed by a tetanic contraction (pulse train 400 ms at 100 Hz), after which the muscles were allowed to recover for 2 min at low length. The force–length characteristics were determined for a range of lengths between active slack and 2 mm over optimum length. After this, control measurements were performed at low and high muscle length to determine if previous activity affected force generation. After determining the force–length relationship of the intact condition (control), the animal was taken out of the experimental apparatus, the extensor retinaculum ligament was transected and the animal was repositioned in the apparatus. Force characteristics were again determined to evaluate the effects (post-ligament transection). Subsequently, the connection of TA with the force transducer was transected and the force–length relationship of the EDL was determined (post-tenotomy). Finally, the anterior crural compartment was opened, the m. biceps femoris was transected and the myofascial connections of the TA with its surrounding were dissected from its distal tendon up to its proximal insertion leaving the blood supply to the muscle intact (post-extirpation).
Statistical analysis
Data are presented as means ± s.e.m. and were analysed using ANOVA, with Fishers protected least-significant difference (PLSD) post hoc test for estimates of inter-group significance. The effects of the interventions on the length–force curves of muscles were tested using a two-way ANOVA for repeated measures. Post hoc tests were performed using the Bonferroni procedure to locate significant differences (P < 0.05).
Results
The EDL muscle underwent significant increase in muscle mass to body mass ratio (Table 1) after only 4 days of extirpation (Ext), likely to be a residue of post-surgical oedema that was apparently resolved by day 7. This transient pseudo-hypertrophy increased muscle mass to a similar extent as the subsequent muscle hypertrophy, which was kept deliberately modest in order to avoid pathophysiological changes. The transient changes in EDL mass suggest a more acute affect, which is followed by adaptation (i.e. true hypertrophy). The initial atrophy of TA, followed by an increase in mass, may suggest that the tenotomised tendon is re-attached or that the tendon length was reduced such that the muscle belly length of TA was increased, and hence provide a greater force contribution to the dorsiflexion moment. There was no difference between the subsequent stages, whereas the tenotomised group (Ten) did not show any increase until 14 days overload, and ligament transection (Lig) produced similar values at all time points, suggesting that the intensity of overloading stimulus was lower in Ten and Lig than that with Ext. Contralateral muscle mass was not significantly different from controls for any group (data not shown). The ipsilateral TA underwent significant atrophy in response to Ten, and to a lesser extent Lig (Table 1), as seen for other muscles (Jamali et al. 2000). That the level of overload was indeed graded among groups was suggested by the response of key indicators involved in Ca2+ handling and muscle metabolism (Fig. 1). SERCA2a increased 1.6-fold (Lig), 3.5-fold (Ten) and 7.2-fold (Ext), while m-CPT1 increased 8.3, 15.2 and 33.9-fold, respectively (Fig. 2).
Figure 2. Relative protein concentration of SERCA2a and m-CPT-1 in EDL subjected to three degrees of overload.
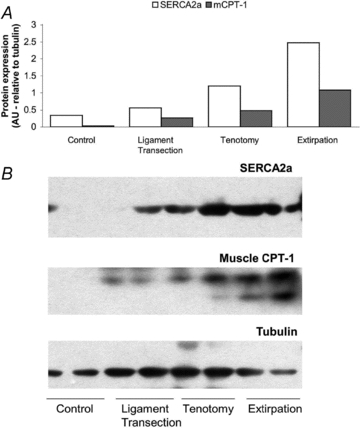
A, relative protein content in pooled muscle samples, normalised to tubulin content, run in duplicate for SERCA2a (open bars) and m-CPT1 (shaded bars) 7 days after intervention. Note the graded increase in both indices of muscle activation with degree of overload. B, representative Western blots; densitometry was performed on the upper band of m-CPT-1, and the lower band is likely to represent another CPT isoform.
The increase in density of PCNA-positive myonuclei (PCNAmyo) occurred at the earliest time point sampled in Ext, but marked heterogeneity in response meant that no significant difference was noted in the other two groups (Table 2). The density of PCNA-positive interstitial nuclei (PCNAint) showed a significant increase at 7 days Ext compared to controls, with a delayed elevation seen at 14 days Ten, whereas Lig showed little change. PCNAmyo and PCNAint followed a broadly similar pattern in Ext, but not Lig or Ten. The density of PCNA-positive capillaries (PCNAcap) was increased in both extirpated and tenotomised groups, closely paralleling the pattern seen for PCNAint (Table 2). The increase is significant after 7 days Ext, consistent with previous studies (Egginton et al. 2001), and was accompanied by a progressive increase in total capillary density after 14 days, reflecting the translation of cell turnover into new vessels. In contrast, the Ten group showed a milder and delayed response which was significant after only 14 days, while Lig showed no discernable response. This demonstrates that the higher the intensity of overload, the earlier is the stimulation of cell proliferation associated with capillaries, suggesting a more intense angiogenic response. Consistent with these findings, both capillary to fibre ratio (C:F) and capillary density (CD) were increased in Ext muscle, a trend that did not reach statistical significance in Ten, and showed little or no change following Lig (Table 2). Expressing proliferation as labelling indices for the three anatomical locations showed a broadly similar pattern of cell turnover associated with angiogenesis, extracellular matrix modification and muscle remodelling in the order Ext > Ten > Lig (Fig. 3 and online Supplemental Material, Supplementary Table 1).
Table 2.
Capillary supply and indices of cellular proliferation
| C:F | CD (mm−2) | PCNAcap (mm−2) | PCNAint (mm−2) | PCNAmyo (mm−2) | |
|---|---|---|---|---|---|
| Control (8) | 1.40 ± 0.09 | 827 ± 79 | 9.2 ± 2.1 | 5.4 ± 1.2 | 3.1 ± 0.8 |
| 4d Lig (4) | 1.49 ± 0.05 | 835 ± 19 | 11.2 ± 4.2 | 5.8 ± 0.4 | 12.3 ± 3.1 |
| 7d Lig (7) | 1.50 ± 0.06 | 853 ± 46 | 9.6 ± 4.9 | 9.6 ± 1.9 | 8.5 ± 3.0 |
| 14d Lig (4) | 1.47 ± 0.06 | 774 ± 34 | 8.1 ± 1.5 | 8.1 ± 1.9 | 5.6 ± 1.4 |
| 4d Ten(4) | 1.37 ± 0.05 | 875 ± 47 | 18.7 ± 4.6 | 18.9 ± 4.9 | 15.1 ± 9.4 |
| 7d Ten(7) | 1.46 ± 0.05 | 843 ± 38 | 19.4 ± 3.4 | 18.8 ± 4.2 | 8.6 ± 2.4 |
| 14d Ten (4) | 1.52 ± 0.02 | 869 ± 54 | 38.4 ± 5.9* | 36.2 ± 7.5** | 5.9 ± 1.0 |
| 4d Ext (4) | 1.47 ± 0.04 | 844 ± 28 | 20.1 ± 5.0 | 19.2 ± 6.5 | 18.9 ± 4.4* |
| 7d Ext (7) | 1.40 ± 0.05 | 949 ± 51 | 62.4 ± 7.3** | 62.7 ± 13.1** | 33.6 ± 8.7** |
| 14d Ext (4) | 2.11 ± 0.07** | 1038 ± 60* | 16.1 ± 3.6 | 16.5 ± 3.2 | 18.3 ± 6.2* |
Means ± s.e.m. (number of animals).
P < 0.05
P < 0.001 vs. Control.
Abbreviations: C:F, capillary to fibre ratio; CD, capillary density; PCNA, profiling cell nuclear antigen; caps, capillaries; int, interstitium; myo, myonuclear.
Figure 3. Cellular proliferation associated with capillaries, interstitium and muscle fibres following graded overload.
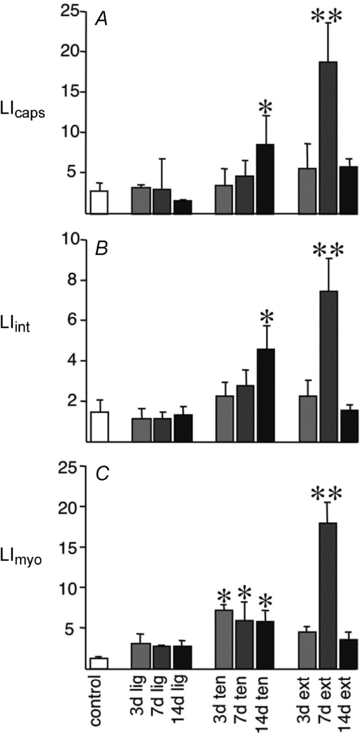
Labelling index (LI) is shown for ligament transection (Lig), tenotomy (Ten) and extirpation (Ext), with bar shading depicting the duration of response. Data are means ± s.e.m. *P < 0.05, **P < 0.001 vs. control.
VEGF and Flk-1 protein levels were both up-regulated to a similar extent in all three models 7 days after surgery, compared to control rats (Fig. 4A). There was a gradual increase in VEGF concentration up to a peak occurring at about 14 days (Fig. 4B). Flk-1 was up-regulated to a similar extent as VEGF by all three treatments, with a peak expression of roughly twofold increase also seen at 14 days, although dropping quickly back to approximately control levels by 28 days after surgery (Fig. 4C). ELISA analysis confirmed up-regulation of VEGF in all three treatments, peaking at around 14 days with a threefold increase over control levels (Fig. 5). No increase in VEGF was seen in Lig at 3 days, though both Ten and Ext showed early increases, and all three models showed VEGF levels returning to control values by 28 days.
Figure 4. VEGF and Flk-1 expression in three models of muscle overload.
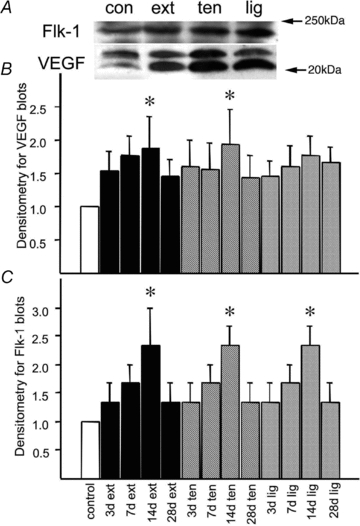
A, Flk-1 and VEGF expression levels are shown for control (Con), 7 days after extirpation (Ext), 7 days after tenotomy (Ten) and 7 days after ligament transection (Lig). Denistometric analysis of both bands provided an estimate of total VEGF content. B, densitometry data for VEGF blots are shown for control rats (1st column), extirpated (columns 2–5), tenotomised (columns 6–9) and ligament transection (columns 10–13) for 3, 7, 14 and 28 days. Data are means ± s.e.m., normalised to control. *P < 0.05 vs. control (n = 3 per group). C, densitometry data for Flk-1 blots are shown for control rats (1st column), extirpated (columns 2–5), tenotomised (columns 6–9) and ligament transection (columns 10–13) for 3, 7, 14 and 28 days. Data are means ± s.e.m., normalised to control. *P < 0.05 vs. control (n = 3 per group). N.B., error bars were generated from replicates of pooled samples to determine the variability among treatments.
Figure 5. Time course of VEGF expression in different models of overload.
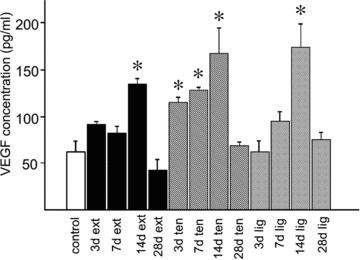
VEGF concentration is given as pg per ml of total protein extracted by detergents (see Methods), using samples pooled from 3 animals measured on the ELISA at three different dilutions. Data are shown for control rats (1st column), extirpated (columns 2–5), tenotomised (columns 6–9) and ligament transection (columns 10–13) for 3, 7, 14 and 28 days.
After the interventions (ligament transection and tenotomy) on the TA muscle, some acute changes to the length–tension relation of the EDL were expected, as force generation at a given ankle angle may be affected in two ways. (a) As the TA and EDL are mechanically connected via their epimysium, the force generated by the former could be transmitted to the latter and vice versa. As ligament release and tenotomy of TA cause a shortening of TA with respect to the ankle angle, this also would change the length and relative position of the TA with respect to the EDL. As a consequence the epimysium is shearing and stiffening, such that forces of TA are transmitted to the EDL. (b) Due to the interventions, the force exerted by TA onto its origin is substantially reduced. As TA and EDL are both plantar flexors, a reduction in the ability of TA to contribute to the dorsiflexion moment will increase the contribution of the EDL. Consequently, the EDL will be relatively more activated than before surgery. Acutely, the length–force relation of the EDL is not changed, but in the longer term maximum force is expected to increase because of the hypertrophy seen as the increase in EDL/body mass ratio. As the EDL is a muscle with a low degree of pennation, hypertrophy will change the length of the muscle little, and thus the joint angle at which the muscle attains its maximum force (Huijing et al. 2005).
Figure 6 shows the active and passive force–length curves of the EDL and TA before and after different interventions, muscle lengths being expressed relative to the reference length (i.e. that of the muscle at knee and ankle angle of 90 deg). For the control condition the length range of force exertion differs between EDL and TA. Although active slack lengths of both muscles were about 5 mm below the reference length, the optimum length of EDL was attained 2–3 mm over reference length, while the TA was still on the ascending limb of its force–length curve. The force characteristics of EDL and TA were significantly changed by the interventions. Ligament transection caused substantial reductions in force (40–60%) at TA lengths higher than the reference length, for EDL this intervention increased force development due to a slight shift of the force–length curve to the right. Logically, TA forces were zero post-Ten, and significantly lower than after Lig. After Ten the force–length curve of EDL were not different from that after Lig. Passive forces were not affected by the interventions.
Figure 6. Cumulative effects of the overload interventions on active and passive force–length characteristics of rat TA and EDL.
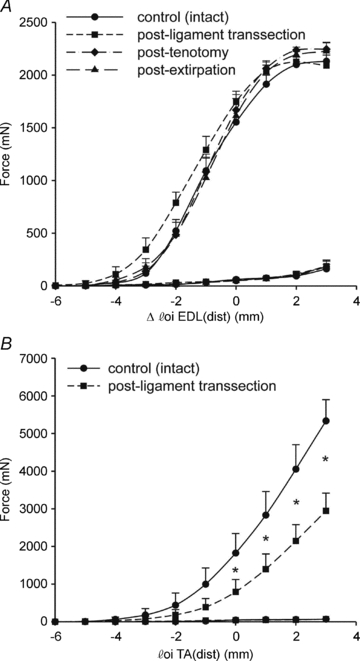
The interventions were performed in the order of TA ligament transection, tenotomy of TA, then extirpation of TA. These data show that the different models of overload did not cause major shifts in the force–length relation of the EDL. A, active and passive forces exerted at the distal tendons of EDL. Muscle tendon complex length is expressed as a deviation (Δlm+t) of the reference length, which is the length of the muscle corresponding to the in vivo situation with the knee and foot in 90 deg. B, active and passive forces exerted at the distal tendon of TA. Note that after tenotomy TA did not exert force anymore. *P < 0.05 between interventions.
Discussion
Growth of capillaries (angiogenesis) may be induced in skeletal muscle by chemical or mechanical factors (Hudlická et al. 1992), an adaptive response that is essential for maintaining adequate tissue function in the face of chronic increases in metabolic demand (Skalak & Price, 1996). Activity-induced angiogenesis occurs in adult skeletal and cardiac muscles, but the mechanism of capillary growth in muscles subjected to compensatory overload is not known. Exercise training in rat results in widespread adaptations in the properties of the skeletal muscle that are specific to the type and amount of activity (Ishihara et al. 1998). Similarly, an increase in muscle mass is a characteristic response of skeletal muscle to a functional overload (Plyley et al. 1998), which can be either compensatory due to ablation or denervation of synergistic muscles (Frischknecht & Vrbova, 1991), or passive stretch by fixation of muscles in lengthened position (Goldspink et al. 1992). Previous studies have reported proliferation of capillaries in such hypertrophied muscle (Degens et al. 1994; Egginton et al. 1998; Degens et al. 2003). Although the hyperaemia following prazosin (an α1 blocker) administration stimulated angiogenesis via increased luminal shear stress, capillary growth induced by skeletal muscle hypertrophy was independent of any increase in blood flow (Egginton et al. 2001). It therefore appears that shear stress does not change during compensatory overload, and that angiogenesis is likely to be initiated by the local tensile and/or shear strains introduced to the capillary endothelium by contraction induced local deformation of skeletal muscle fibres acting via the interstitial filament network. If this were the case, and angiogenesis was not a threshold response, we hypothesised that varying the degree of overload would determine the extent of capillary growth. Thus, we used three surgical interventions to impose an increasing overload of the EDL – ligament transection, tenotomy and extirpation – to examine the role overload and stretch play in initiation of angiogenesis.
The relative muscle mass showed an increase in Ext, indicative of muscle hypertrophy, a modest increase in Ten, while no significant change was observed in Lig, suggesting that the intensity of overloading stimulus was progressively lower. A graded extent of overload is also indicated by protein markers of processes known to be sensitive to mechanical strain (SERCA2a; Talmadge et al. 1996) and mitochondrial β-oxidation (m-CPT-1; Tunstall et al. 2002). A previous report (Egginton et al. 1998) showed a greater compensatory hypertrophy after 2 weeks of synergist removal (∼20%), but this was with larger Wistar rats. Tenotomy and ligament transection produced more modest changes in muscle mass, presumably due to the lower intensity of overload, and hence a longer duration of loading was required to initiate a proliferative response. Mature skeletal muscle is a post-mitotic tissue, so its nuclei are unable to divide after differentiation, and hence any increase in myonuclear proliferation is indicative of satellite cell activation (Zammit, 2008). Although satellite cells are normally quiescent in adult muscle, they are responsible for muscle regeneration following injury and are involved in work-induced hypertrophy of muscle fibres (Bischoff & Heintz, 1994; Petrella et al. 2008). Indeed, compensatory hypertrophy of mature rat EDL cannot occur unless satellite cells are able to reproduce and contribute nuclei to the overloaded muscle fibres, ensuring a constant myonuclear-to-myoplasmic volume ratio (Rosenblatt et al. 1994). Consistent with the differing extent of overload among the interventions, we also found a significant increase in myonuclear PCNA density and myonuclear labelling index at 7 days in Ext, whereas Ten and Lig showed much less or no significant change, respectively.
Synergist extirpation (Ext), which is a strong overload stimulus, increased the density of capillaries and capillary labelling index, indicative of endothelial and perivascular cell proliferation (Egginton et al. 2001), in the EDL muscle. The increase was significant after 7 days of Ext, which is consistent with previous studies (Egginton et al. 1998; Rivilis et al. 2002), and was accompanied by a progressive increase in capillary density after 14 days, reflecting the translation of cell turnover into new vessels. In contrast, Ten showed a more mild (at 7 days the response was significantly lower than that of Ext) and delayed response (peaking at 14 days). This indicates that the greater the intensity of overload, the earlier is the stimulation of cell proliferation associated with capillaries, consistent with a more intense angiogenic response. At 14 days the Ten response was significantly higher than Ext at the same time point, suggesting that delayed cellular proliferation in the former group coincided with reduced proliferation in the latter group, and that an adequate stimulus represents the product of intensity and duration. Interestingly, changes in response to the mildest intervention (Lig) never reached statistical significance, suggesting that muscle has some scope for deformation or metabolic reserve, possibly involving compensatory adjustments e.g. in blood flow. The apparent discrepancy between LIcap and [VEGF] may at first seem confusing, perhaps due to the dogma that VEGF has a simple relationship with angiogenic outcome. We have shown previously that EC proliferation may increase the numbers of EC profiles found within individual capillaries (Zhou et al. 1998), but endothelial proliferation may also occur in the absence of overt capillary growth (Rivilis et al. 2002), while levels of Fik-1 rather that VEGF per se may offer a better correlation with angiogenesis (Milkiewicz et al. 2004). These data show a variation on this pattern where angiogenesis is induced as a result of both synergist extirpation and tenotomy, but the extent of angiogenic response is greater in the former than the latter, with little or no angiogenesis in response to ligament transection. Thus, the angiogenic response is likely to be graded with strength of the mechanical stimulus.
It could be argued that it is the extent of muscle activity, not stretch, that determines the angiogenic response to overload. If Ext induces a higher muscle activity compared to Ten or Lig (see Fig. 1), then metabolic activity may be the key parameter that drives angiogenesis, rather than muscle stretch. To examine how mechanical overload of the EDL was altered by the interventions, force–length characteristics of both EDL and TA were investigated. Ligament transection caused a reduction in the force generated by the TA, which was to be expected as the distance between origin and insertion is decreased by this intervention (Burkholder & Lieber, 1998). EDL force generation at short lengths was substantially (∼1.5- to 3-fold) increased, likely to be explained by transmission of force from TA onto its distal tendon (Maas et al. 2003). As TA and EDL, together with the m. extensor hallucis longus (EHL), are mostly responsible for dorsiflexion movements, a reduction in TA force would require a greater activity of EDL and/or EHL to maintain a similar dorsiflexion moment. The maximum force of EHL is very low (Yucesoy et al. 2005), and its relative contribution is therefore only minor. Tenotomy of TA prevented its direct contribution to dorsiflexion moment around the ankle, and therefore probably increased the activity of EDL and/or EHL further compared to ligament transection. After TA extirpation, the EDL force–length curve did not differ from that of the control and post-tenotomy conditions. This suggests that the force generating potential of EDL was unaltered. Why, then, does this condition cause the greater angiogenic effect? After tenotomy, TA may have contributed to the dorsiflexion moment by transmitting its force onto EDL or EHL. As EDL forces were not increased, and TA is connected to EHL by epimuscular connections (Huijing et al. 2003), forces generated by the TA are likely to be transmitted to the EHL. After TA extirpation, this muscle no longer contributes to the dorsiflexion moment, and as a consequence activation of EDL will be enhanced. In addition, removal of the epimuscular connections between EDL and TA may alter the local tensile and shear strain distributions, which may enhance a local adaptive (angiogenic) response (cf. Huijing & Jaspers, 2005). Taken together, these data show that after the different interventions EDL was gradually overloaded, paralleled by a gradual increased angiogenic signalling, indicating that the overload-induced cell proliferation and angiogenesis are graded according to stimulus intensity. Whether the stimulus is solely mechanical warrants further investigation, as the enhanced intensity of contractile activity may also have metabolic effects.
The proximate stimulus for angiogenesis is often taken to be an acute inflammatory response as a result of surgical trauma (Armstrong et al. 1979), which may be particularly relevant following extirpation, while tenotomy and ligament release would be expected to cause substantially less trauma. We have conducted extensive investigations in order to examine this possibility but ultrastructure, histological and immunohistochemical analyses (e.g. Zhou et al. 1998; Egginton et al. 2001) have provided no evidence of significant macrophage infiltration 2 days post-surgery. We therefore conclude that such additional angiogenic stimulation would at best be a minor component of the observed physiological response.
The mechanism by which mechanical loading of muscle stimulates angiogenesis is therefore still not defined, involving mechanical factors possibly with a metabolic component at higher degrees of overload (above), and whether non-endothelial (e.g. satellite) cell activation has any role is still to be investigated. Angiogenesis stimulated by muscle overload requires VEGF expression (Williams et al. 2006a), and VEGF protein is up-regulated in response to muscle extirpation (Rivilis et al. 2002; Williams et al. 2006b). Hepatocyte growth factor (HGF) is abundant in the extracellular matrix of muscle fibres (Tatsumi et al. 1998), and is released from satellite cells in response to muscle activity (O’Reilly et al. 2008). HGF acts in synergy with VEGF (Xin et al. 2001), potentially amplifying the angiogenic response to a potent endothelial mitogen. It would therefore be reasonable to assume that VEGF is the principle growth factor controlling these pathways, and a graded expression of VEGF might be expected to correlate with the graded angiogenesis observed. However, VEGF protein is up-regulated to a similar extent in all three of the overload models. This means that an increase in VEGF is seen both in the presence of a large angiogenic response to Ext and also where little or no angiogenesis occurs with Lig. This is surprising, as exogenously applied VEGF is a potent stimulator of angiogenesis (Ferrara, 2004), and the presence of similar levels in different models would suggest a similar angiogenic potential. The lack of a correlation between angiogenic outcome and VEGF levels therefore points to a stringent control of angiogenesis to accentuate or ameliorate the pro-angiogenic effects of VEGF. This may parallel the changes in VEGF expression seen in response to ischaemia, where an increase in VEGF can be seen without angiogenesis if there is no parallel increase in Flk-1 (Milkiewicz et al. 2004), suggesting that receptor expression is one mechanism by which VEGF-mediated angiogenesis is regulated. However, this is not what is seen in response to graded overload, as Flk-1 is increased in parallel with VEGF expression, suggesting that the overall balance of pro- and anti-angiogenic molecules is more important than the expression of a single growth factor. While VEGF is essential for angiogenesis in Ext, and probably also in Ten and Lig due to similarity of the mechanical stimulus, it would seem likely that while VEGF may provide a permissive, pro-angiogenic environment the realisation of in vivo angiogenesis may be controlled by other factors.
Interestingly, while there is little angiogenesis in the ligament transection model, the proliferation response is much closer to the other models. Cellular proliferation is therefore closely tied to VEGF/Flk-1 expression, consistent with the role of VEGF acting as an endothelial mitogen (Ferrara, 2004). This may explain why VEGF is necessary for angiogenesis, but not sufficient to elicit a full angiogenic response in skeletal muscle without the co-expression of ancillary molecules. However, exogenously applied VEGF is capable of inducing a full angiogenic response on its own (Phillips et al. 1994), albeit at a supra-physiological dose. VEGF is also capable of inducing release of a large range of pro-angiogenic molecules (Ferrara, 2004), but again it may be that this only occurs at high concentrations. Interestingly, viral transfections generate lower concentrations of VEGF, and these have been shown to promote angiogenesis in the presence of another pro-angiogenic stimulus, such as ischaemia (Takeshita et al. 1996; Shimamura et al. 2006), but have not been reported to stimulate angiogenesis in a stable, quiescent vasculature. Thus, angiogenesis caused by high concentrations of VEGF alone may model pathological angiogenesis, which is borne out by the morphological similarities of the chaotic vessel pattern seen in transgenic mice over-expressing VEGF (Dor et al. 2002) with those in tumours (Papetti & Herman, 2002).
Conclusions
This study examines the extent to which an angiogenic response is proportional to the severity of muscle overload. The stimuli for the EDL will be (a) changes in the mechanical loading, (b) changes in the animals’ use of their limbs or activity level (a mechanical stimulus), (c) physical compression or decompression of the EDL during the duty cycle (also a mechanical stimulus), and (d) inflammation from the surgery (a potential chemical stimulus, which will mainly affect the earlier time points). We cannot claim that the nature of the mechanical stimulus is identical and differs only in magnitude, but we have shown that it does differ in magnitude. The observation of a graded hypertrophic response suggests graded differences in mechanical overload between the different interventions, although effects of surgery cannot be excluded, leading to higher expression of muscle proteins important in metabolism and capillary growth. The lack of graded expression of VEGF and its receptor Flk-1 suggests that if a certain threshold is exceeded, physiological angiogenesis becomes graded, but requires a relatively low growth factor response that correlates with cellular proliferation. The role of VEGF in this case may be primarily to act as an endothelial mitogen, with the control of overt angiogenesis occurring through a balance of other factors, including mechanotransduction by the endothelium. These data suggest that an initial, low-level overload produces a primarily mechanical angiogenic response, while an additional metabolic component may be evident at higher levels of muscle activity. This is consistent with the finding that ‘passive’ exercise may also induce an apparent angiogenic response, in the absence of overt muscle activity (Hellsten et al. 2008).
Acknowledgments
This work was supported by the British Heart Foundation.
Glossary
Abbreviations
- CPT
Carnitine palmitoyl transferase
- EDL
extensor digitorum longus
- FGF
fibroblast growth factor
- Flk-1
fetal liver kinase -1 (or VEGF-R2)
- PCNA
proliferating cell nuclear antigen
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
- TA
tibialis anterior
- VEGF
vascular endothelial growth factor
Author contributions
S.E.: concept and design, data analysis and interpretation, drafting the article; I.B.: data analysis and interpretation; J.W.: data analysis and interpretation, revision of article; D.H. data analysis and interpretation, revision of article; C.B. data analysis and interpretation; R.J. data analysis and interpretation, revision of article. All authors approved the final version for publication. Experiments were conducted in Birmingham and Amsterdam.
Supporting information
Supplementary Table S1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Armstrong RB, Marum P, Tullson P, Saubert CW., 4th Acute hypertrophic response of skeletal muscle to removal of synergists. J Appl Physiol. 1979;46:835–842. doi: 10.1152/jappl.1979.46.4.835. [DOI] [PubMed] [Google Scholar]
- Bischoff R, Heintz C. Enhancement of skeletal muscle regeneration. Dev Dyn. 1994;201:41–54. doi: 10.1002/aja.1002010105. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Lieber RL. Sarcomere number adaptation after retinaculum transection in adult mice. J Exp Biol. 1998;201:309–316. [PubMed] [Google Scholar]
- Degens H, Anderson RK, Alway SE. Capillarization and vascular endothelial growth factor expression in hypertrophying anterior latissimus dorsi muscle of the Japanese quail. Adv Exp Med Biol. 2003;530:577–585. doi: 10.1007/978-1-4615-0075-9_56. [DOI] [PubMed] [Google Scholar]
- Degens H, Turek Z, Hoofd LJ, Binkhorst RA. Capillary proliferation related to fibre types in hypertrophied aging rat M. plantaris. Adv Exp Med Biol. 1994;345:669–676. doi: 10.1007/978-1-4615-2468-7_88. [DOI] [PubMed] [Google Scholar]
- Dor Y, Djonov V, Abramovitch R, Itin A, Fishman GI, Carmeliet P, Goelman G, Keshet E. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21:1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egginton S, Hudlická O, Brown MD, Walter H, Weiss JB, Bate A. Capillary growth in relation to blood flow and performance in overloaded rat skeletal muscle. J Appl Physiol. 1998;85:2025–2032. doi: 10.1152/jappl.1998.85.6.2025. [DOI] [PubMed] [Google Scholar]
- Egginton S, Zhou AL, Brown MD, Hudlická O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;49:634–646. doi: 10.1016/s0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- Engerman RL, Pfaffenbach D, Davis MD. Cell turnover of capillaries. Lab Invest. 1967;17:738–743. [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Vrbova G. Adaptation of rat extensor digitorum longus to overload and increased activity. Pflügers Arch. 1991;419:319–326. doi: 10.1007/BF00371113. [DOI] [PubMed] [Google Scholar]
- Goldspink G, Scutt A, Loughna PT, Wells DJ, Jaenicke T, Gerlach GF. Gene expression in skeletal muscle in response to stretch and force generation. Am J Physiol Regul Integr Comp Physiol. 1992;262:R356–363. doi: 10.1152/ajpregu.1992.262.3.R356. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Kraus WE. Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front Biosci. 2001;6:D75–89. doi: 10.2741/gustafss. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Rufener N, Nielsen JJ, Høier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R975–982. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984;49:405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudlická O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Baan GC. Extramuscular myofascial force transmission within the rat anterior tibial compartment: proximo-distal differences in muscle force. Acta Physiol Scand. 2001;173:297–311. doi: 10.1046/j.1365-201X.2001.00911.x. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Jaspers RT. Adaptation of muscle size and myofascial force transmission: a review and some new experimental results. Scand J Med Sci Sports. 2005;15:349–380. doi: 10.1111/j.1600-0838.2005.00457.x. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Maas H, Baan GC. Compartmental fasciotomy and isolating a muscle from neighboring muscles interfere with myofascial force transmission within the rat anterior crural compartment. J Morphol. 2003;256:306–321. doi: 10.1002/jmor.10097. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Roy RR, Ohira Y, Ibata Y, Edgerton VR. Hypertrophy of rat plantaris muscle fibers after voluntary running with increasing loads. J Appl Physiol. 1998;84:2183–2189. doi: 10.1152/jappl.1998.84.6.2183. [DOI] [PubMed] [Google Scholar]
- Jamali AA, Afshar P, Abrams RA, Lieber RL. Skeletal muscle response to tenotomy. Muscle Nerve. 2000;23:851–862. doi: 10.1002/(sici)1097-4598(200006)23:6<851::aid-mus3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Herzog W. Excursion is important in regulating sarcomere number in the growing rabbit tibialis anterior. J Physiol. 1998;508:267–280. doi: 10.1111/j.1469-7793.1998.267br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Jaspers RT, Baan GC, Huijing PA. Myofascial force transmission between a single head of multi-tendoned muscle and adjacent tissues: length effects of head III of rat EDL muscle. J Appl Physiol. 2003;95:2004–2013. doi: 10.1152/japplphysiol.00220.2003. [DOI] [PubMed] [Google Scholar]
- Milkiewicz M, Pugh CW, Egginton S. Inhibition of endogenous HIF inactivation induces angiogenesis in ischaemic skeletal muscles of mice. J Physiol. 2004;560:21–26. doi: 10.1113/jphysiol.2004.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve. 2008;38:1434–1442. doi: 10.1002/mus.21146. [DOI] [PubMed] [Google Scholar]
- Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Stone AM, Jones BD, Schultz JC, Whitehead RA, Knighton DR. Vascular endothelial growth factor (rhVEGF165) stimulates direct angiogenesis in the rabbit cornea. In Vivo. 1994;8:961–965. [PubMed] [Google Scholar]
- Plyley MJ, Olmstead BJ, Noble EG. Time course of changes in capillarization in hypertrophied rat plantaris muscle. J Appl Physiol. 1998;84:902–907. doi: 10.1152/jappl.1998.84.3.902. [DOI] [PubMed] [Google Scholar]
- Prior BM, Lloyd PG, Yang HT, Terjung RL. Exercise-induced vascular remodeling. Exerc Sport Sci Rev. 2003;31:26–33. doi: 10.1097/00003677-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlická O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H1430–1438. doi: 10.1152/ajpheart.00082.2002. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- Shimamura M, Sato N, Yoshimura S, Kaneda Y, Morishita R. HVJ-based non-viral gene transfer method: successful gene therapy using HGF and VEGF genes in experimental ischemia. Front Biosci. 2006;11:753–759. doi: 10.2741/1833. [DOI] [PubMed] [Google Scholar]
- Skalak TC, Price RJ. The role of mechanical stresses in microvascular remodeling. Microcirculation. 1996;3:143–165. doi: 10.3109/10739689609148284. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Tsurumi Y, Couffinahl T, Asahara T, Bauters C, Symes J, Ferrara N, Isner JM. Gene transfer of naked DNA encoding for three isoforms of vascular endothelial growth factor stimulates collateral development in vivo. Lab Invest. 1996;75:487–501. [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Chalmers GR, Edgerton VR. MHC and sarcoplasmic reticulum protein isoforms in functionally overloaded cat plantaris muscle fibers. J Appl Physiol. 1996;80:1296–1303. doi: 10.1152/jappl.1996.80.4.1296. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Tunstall RJ, Mehan KA, Wadley GD, Collier GR, Bonen A, Hargreaves M, Cameron-Smith D. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E66–72. doi: 10.1152/ajpendo.00475.2001. [DOI] [PubMed] [Google Scholar]
- Vanotti A, Magiday M. Untersuchungen zum studium des trainiertsein über die capillarisierung der trainierten muskulaturen. Arbeitsphysiologie. 1934;7:615–622. [Google Scholar]
- Williams JL, Cartland D, Rudge JS, Egginton S. VEGF trap abolishes shear stress- and overload-dependent angiogenesis in skeletal muscle. Microcirculation. 2006a;13:499–509. doi: 10.1080/10739680600785717. [DOI] [PubMed] [Google Scholar]
- Williams JL, Weichert A, Zakrzewicz A, Da Silva-Azevedo L, Pries AR, Baum O, Egginton S. Differential gene and protein expression in abluminal sprouting and intraluminal splitting forms of angiogenesis. Clin Sci (Lond) 2006b;110:587–595. doi: 10.1042/CS20050185. [DOI] [PubMed] [Google Scholar]
- Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, Schwall R, Ferrara N, Gerritsen ME. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol. 2001;158:1111–1120. doi: 10.1016/S0002-9440(10)64058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Greisler HP. Angiogenic effect of fibroblast growth factor-1 and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin-based 3-dimensional angiogenesis system. Surgery. 2002;132:259–267. doi: 10.1067/msy.2002.125720. [DOI] [PubMed] [Google Scholar]
- Yucesoy CA, Baan GC, Koopman BH, Grootenboer HJ, Huijing PA. Pre-strained epimuscular connections cause muscular myofascial force transmission to affect properties of synergistic EHL and EDL muscles of the rat. J Biomech Eng. 2005;127:819–828. doi: 10.1115/1.1992523. [DOI] [PubMed] [Google Scholar]
- Yue X, Tomanek RJ. Effects of VEGF165 and VEGF121 on vasculogenesis and angiogenesis in cultured embryonic quail hearts. Am J Physiol Heart Circ Physiol. 2001;280:H2240–2247. doi: 10.1152/ajpheart.2001.280.5.H2240. [DOI] [PubMed] [Google Scholar]
- Zammit PS. All muscle satellite cells are equal, but are some more equal than others? J Cell Sci. 2008;121:2975–2982. doi: 10.1242/jcs.019661. [DOI] [PubMed] [Google Scholar]
- Zhou A-L, Egginton S, Brown MD, Hudlická O. Capillary growth in overloaded, hypertrophic adult rat skeletal muscle: an ultrastructural study. Anat Rec. 1998;252:49–63. doi: 10.1002/(SICI)1097-0185(199809)252:1<49::AID-AR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


