Abstract
PURPOSE
The goal of this study was to determine whether the medial rectus muscles of patients with a history of medial rectus underaction or overaction show alterations in the process of satellite cell activation when compared with normal age-matched control muscles.
METHODS
Medial rectus muscles were obtained with consent from adult patients undergoing surgical resection due to medial rectus underaction or overaction and were prepared for histologic examination by fixation and paraffin embedding. Control muscles were obtained from cornea donor eyes of adults who had no history of strabismus or neuromuscular disease. Cross sections were obtained and stained immunohistochemically for the presence of activated satellite cells, as identified by MyoD immunoreactivity, and the presence of the total satellite cell population, as identified by Pax7 immunoreactivity. The percentages of MyoD- and Pax7-positive satellite cells per 100 myofibers in cross section were calculated.
RESULTS
As predicted from results in the literature, MyoD-positive satellite cells, indicative of activation, were present in both the control and resected muscles. In the underacting medial rectus muscles, the percentages of MyoD- and Pax7-positive satellite cells, based on the number of myofibers, were approximately twofold higher than the percentages in the control muscles. In the overacting medial rectus muscles, the percentage of MyoD-positive satellite cells was twofold less than in the control muscles, whereas the percentage of Pax7-positive satellite cells significantly increased compared with that in the control specimens.
CONCLUSIONS
The presence of an increased number of activated satellite cells in the resected underacting medial rectus muscles and the decreased numbers of activated satellite cells in the overacting muscles was unexpected. The upregulation in the number of MyoD-positive satellite cells in underacting muscles suggests that there is potential for successful upregulation of size in these muscles, as the cellular machinery for muscle repair and regeneration, the satellite cells, is retained and active in patients with medial rectus underaction. The decreased number of activated satellite cells in overacting MR muscle suggests that factors as yet unknown in these overacting muscles are able to affect the number of satellite cells and/or their responsiveness compared with normal age-matched control muscles. These hypotheses are currently being tested.
Strabismus is a disorder of ocular alignment of unknown etiology.1,2 The lack of knowledge about its cause may explain, in part, why surgical correction of strabismus and the maintenance of adequate alignment and binocularity are so difficult.3,4 Although recent genetic analyses of incomitant strabismus disorders demonstrated that the genetic causes that underlie specific congenital cranial dysinnervation disorders, such as CFEOM1 and -2, are due to mutations in genes critical to development of ocular motor neurons and their axonal connections,5,6 we are still a long way from understanding the possible genetic factors that underlie the various forms of complex strabismus that occur in the absence of known structural brain anomalies. Recent evidence suggests that comitant horizontal strabismus, such as congenital or infantile esotropias and exotropias, can be found in distantly related members of the same family.7 The exotropias are oculomotor disorders in which there is an imbalance between an underactive medial rectus (MR) muscle and an overactive lateral rectus (LR) muscle. The surgical treatment can be achieved by a recession–resection procedure: the LR is recessed while the MR is resected. Congenital or infantile esotropias are convergent deviations, which usually need surgical procedures if angles of deviation are greater than 30 prism diopters. The realignment is obtained by weakening an “overactive” MR (recess procedure) associated or not with strengthening of an “underactive” LR (resection procedure), depending on the size of the deviation. In some cases, when the deviation is very big, even big recess procedures are not enough to realign the eyes. In these situations, resections or marginal tenotomies are alternative techniques.8 The difficulty in producing a successful surgical outcome supports the idea that changes in the muscles themselves before, during, and after strabismus surgical corrections are performed are not well understood.
Adult mammalian extraocular muscles (EOMs) express several characteristics that are not normally expressed in adult nonocular skeletal muscles. For example, myofibers of adult EOMs continue to express molecules such as the neonatal and developmental myosin heavy-chain isoforms,9 neural cell adhesion molecule,10 and a variety of muscle mitogens and growth factors.11,12 These molecules are normally downregulated in mature limb skeletal muscle, where they are usually associated only with skeletal muscle development or regeneration.
Normal adult EOMs, unlike limb skeletal muscle, continuously remodel individual myofibers throughout life.13,14 In the unperturbed EOMs of adult humans, there is also a small percentage of activated satellite cells, the regenerative cell population found in skeletal muscle, positive for the myogenic lineage-specific marker MyoD.15 This suggests that this process of myofiber remodeling occurs in human EOMs as well. MyoD is expressed in both activated satellite cells and myoblasts but not in myotubes, mature myofibers,16,17 or quiescent satellite cells.18 Based on these data, our objectives were to determine whether the MR muscles of patients with a history of MR underaction or overaction show alterations in the process of satellite cell activation when compared to normal age-matched control muscles.
METHODS
Human MR muscles were obtained from patients undergoing surgery at the University Hospital in Ribeirão Preto (Table 1). All the patients were operated on while under general anesthesia, and they had had no previous EOM surgeries. The control MR muscles were obtained from cornea donor eyes of adults whose deaths were unrelated to muscular disease. Mean time to fixation of the control muscles was 3.3 ± 3.1 hours. MR muscles were obtained from seven patients with a diagnosis of MR underaction and three patients with a diagnosis of MR overaction, and these were fixed immediately after surgical removal. All received resection surgery as part of their normal treatment plan. In the patients with an overacting MR, a small piece near the tendon end was resected. This study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the ethics committee at the University of São Paulo.
TABLE 1.
Clinical Data of Patients Who Provided Specimens
| Patient | Age | Sex | Deviation | Other Clinical Data |
|---|---|---|---|---|
| 1 | 8 | Male | LE XT 40Δ | LE corneal leucoma (opacity) |
| 2 | 9 | Female | LE XT 40Δ | Limited adduction and elevation of LE; incomplete left third cranial congenital nerve paresis; previous operated LE ptosis |
| 3 | 13 | Female | LE XT 32Δ | LE ptosis |
| 4 | 25 | Female | LE XT 40Δ | LE congenital cataract |
| 5 | 27 | Male | RE XT 50Δ | Old RE trauma |
| 6 | 33 | Male | RE XT 50Δ | Limited adduction and depression of RE. incomplete right third cranial nerve paresis. |
| 7 | 36 | Female | RE XT 55Δ | Old RE trauma |
| 8 | 36 | Male | Altern. ET 85Δ | — |
| 9 | 37 | Female | Altern. ET 85Δ | — |
| 10 | 39 | Female | Altern. ET 85Δ | — |
LE, left eye; RE, right eye; XT, exotropia; ET, esotropia.
All muscles were fixed in 4% neutral formalin and embedded in paraffin. Sections were cut at 6 µm and mounted and air dried overnight on subbed glass slides. Paraffin was removed from the sections by incubation in changes of xylene, followed by several washes. Sections were submitted to antigen retrieval by heating them in 1 mM EDTA (pH 8.0) in a laboratory microwave for 24 minutes at 94°. After cooling, the slides were rinsed in PBS, and endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol at room temperature for 30 minutes. After a buffer rinse, nonspecific immunoglobulin binding was blocked for 30 minutes with 10% normal donkey serum in PBS. The cross sections were immunostained overnight for the presence of MyoD at a dilution of 1:100 (Abcam, Cambridge, MA) and for Pax7 at a dilution of 1:20 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). Tissue sections were rinsed with PBS and incubated with biotinylated secondary antibody for 30 minutes followed by incubation with an avidin-biotin complex reagent (Vector Laboratories, Burlingame, CA). The reaction product was visualized using 3′,3′-diaminobenzidine (DAB kit) as the chromogen (Vector Laboratories). To confirm the specificity of the primary antibody, slides were incubated similarly except without incubation in primary antibody. A group of sections from each patient were double stained for MyoD or Pax7 and dystrophin. The dystrophin was visualized using the methods described earlier, with the following changes: The complete dystrophin primary antibody incubation process was performed before the MyoD or Pax7 immunostaining with an antibody concentration of 1:50 (Laboratory Vision, Fremont, CA), and the coloration reaction was performed with an alkaline phosphatase secondary antibody kit (Vectastain) and an alkaline phosphatase black substrate kit (Vector Laboratories).
Four sections from each muscle were prepared for each of the immunostains. The material was analyzed by counting all the MyoD- or Pax7-positive cells and the total number of myofiber cross sections in randomly selected fields. A minimum of 300 myofibers were counted for each control and for each patient MR muscle. For all data reported, the counts were calculated to determine the percentage of MyoD- or Pax7-positive nuclei present per 100 counted myofibers in cross section. At least four cross sections from each control, underacting, and overacting muscle were analyzed for individual myofiber cross-sectional area by manual tracing under bright-field microscopy, with a minimum of 100 myofibers measured for each cross section. Mean myofiber cross-sectional areas were calculated (Nova Prime image analysis system; Bioquant Inc., Nashville, TN). All data are presented as the mean ± SEM. Statistics were analyzed with unpaired t-tests, and differences were considered significant when P < 0.05. An F-test was used to verify that the variances were not significantly different.
RESULTS
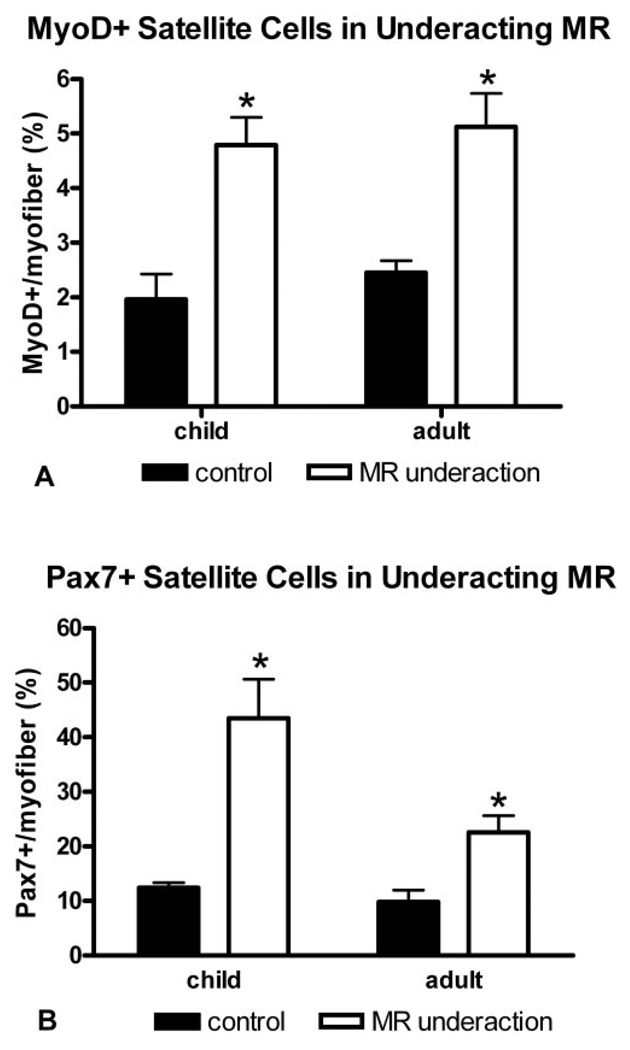
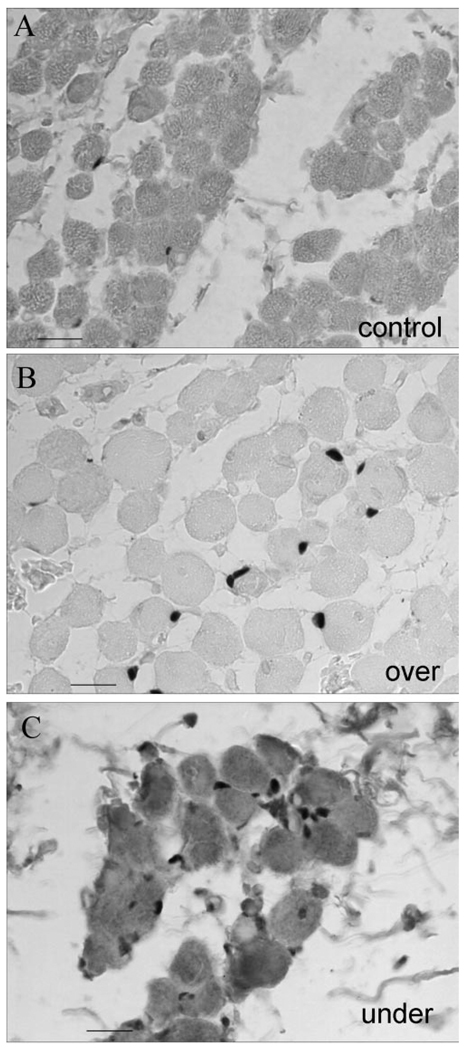
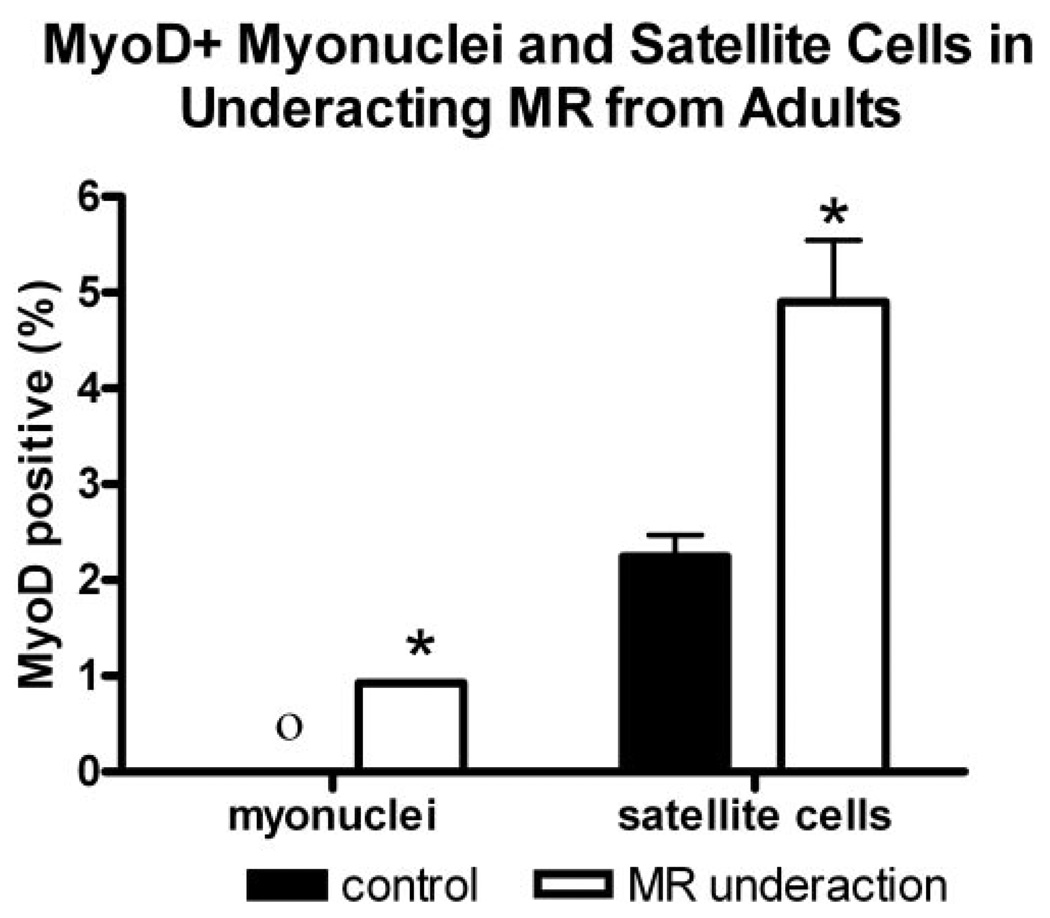
In the muscles of patients with a diagnosis of MR underaction, the percentage of satellite cells positive for MyoD per 100 myofiber cross sections was significantly elevated compared with those in normal age-matched control MR muscle (Figs. 1, 2). In both the child and adult muscles, the percentage of activated satellites positive for MyoD increased twofold over the control. Of interest, these MR muscles also appeared to contain more satellite cells, based on the percentage of satellite cells positive for Pax7 per 100 myofibers (Figs. 2, 3). This increase was again approximately twofold over the control values, which indicates that the proportion of activated satellite cells compared with the total number of satellite cells stayed relatively constant. Although cell size was not measured, the satellite cells were larger in the MR from the patients with strabismus (Figs. 3B, 3C) than in the MR from the normal control muscles (Fig. 3A). This size increase has been associated with satellite cell activation.19 In addition to the significant upregulation of MyoD-positive satellite cells, there are myofibers with MyoD-positive myonuclei (Figs. 1B, 4), extremely rare in normal EOMs.
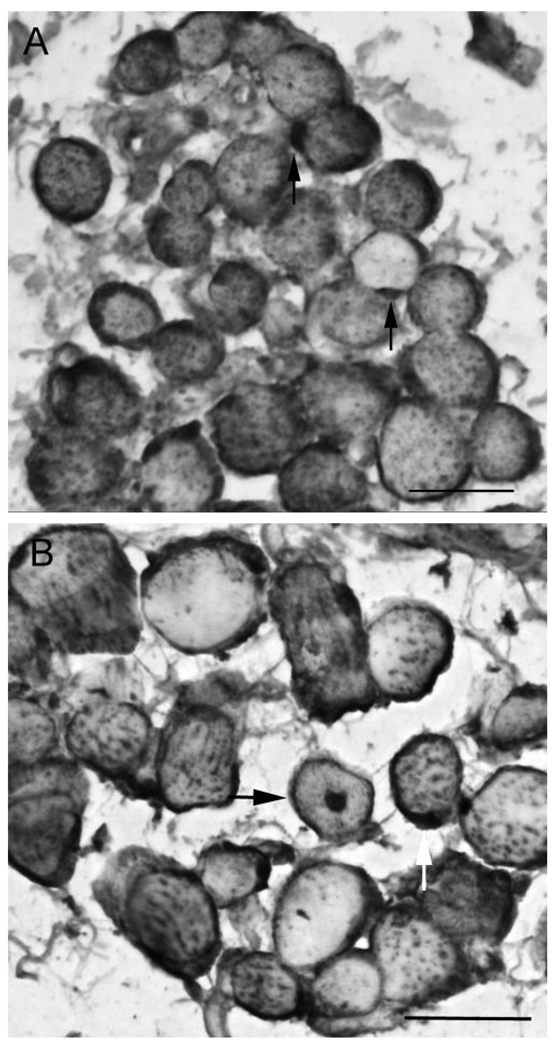
FIGURE 1.
Adult MR muscle from patients with underacting muscles immunostained for MyoD and dystrophin. (A) Arrows: MyoD-positive cells outside the dystrophin-positive sarcolemma. Note that the sarcoplasm of almost every fiber is positive for dystrophin. This is seen in regenerating muscle fibers, but not normal adult limb skeletal muscle myofibers. (B) A centrally located myonucleus (arrow). Bar, 20 µm.
FIGURE 2.
Quantification of the percentage of myofibers with an associated cell positive for MyoD (A) and Pax7 (B) from normal adult and child MR muscles and from patients with MR underaction. *Significant difference from the control.
FIGURE 3.
Adult MR muscle immunostained for Pax7. (A) Adult control MR muscle. Adult MR from patients with MR (B) overaction or (C) underaction. Bar, 10 µm.
FIGURE 4.
Quantification of the percentage of myofibers with associated nuclei or satellite cells positive for MyoD from normal adult MR and MR of patients with MR underaction. *Significant difference from the control. O, no MyoD+ myonuclei in the sections.
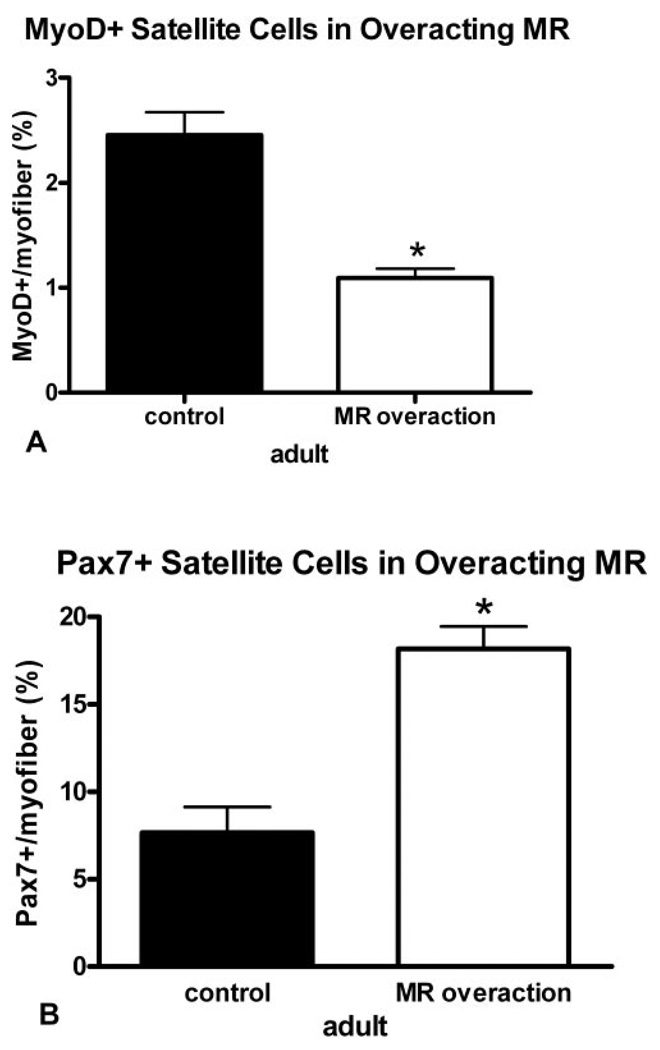
In contrast, in the muscles of patients with MR overaction, there were 50% fewer MyoD-positive cells per myofiber in these muscles, a significant reduction compared with the age-matched control MR muscles (Fig. 5). In contrast, there was a twofold increase in the percentage of Pax7-positive cells per 100 myofibers in the overacting MR muscles, significantly increased compared with the percentage in normal control muscles (Fig. 5). A comparison of the percentage of activated cells in both patient groups showed that 33% in underacting MR muscles and 6% in overacting MR muscles were in the activated state, as defined by the expression of MyoD.
FIGURE 5.
Quantification of the percentage of myofibers with an associated cell positive for MyoD (A) and Pax7 (B) from normal adult MR and MR from patients with MR overaction. *Significant difference from the control.
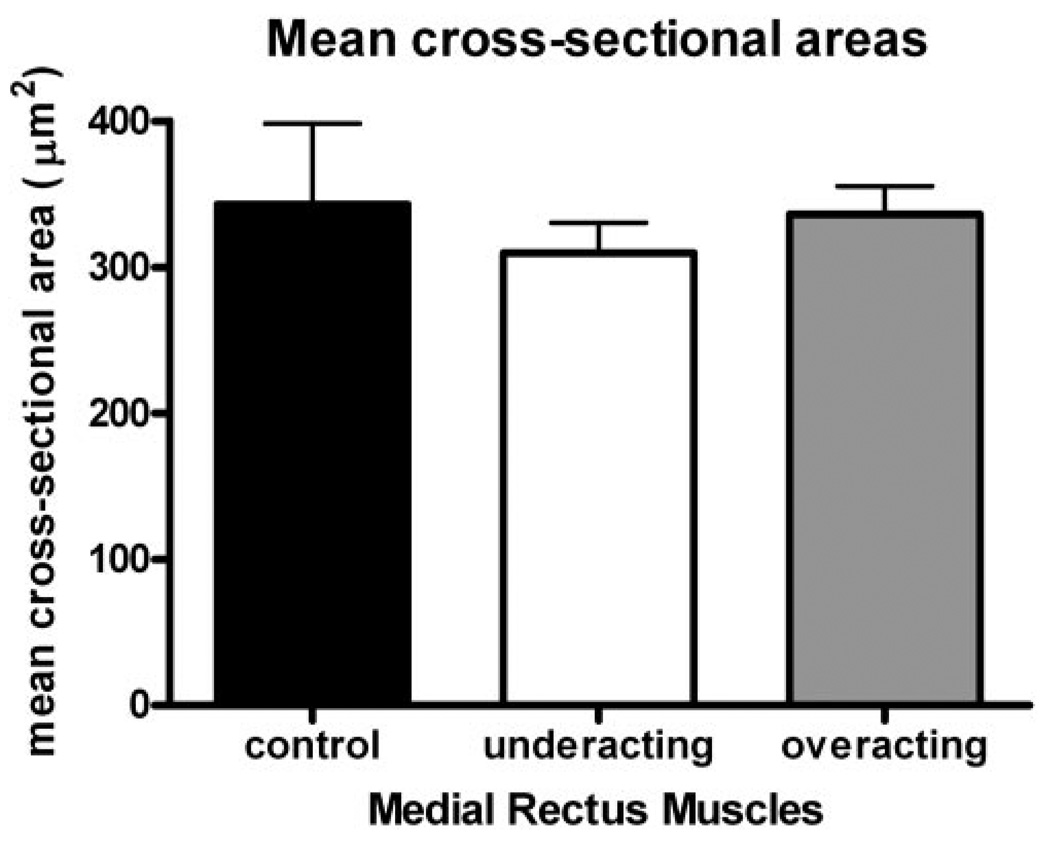
Mean myofiber cross-sectional areas were determined, and no differences were seen between control MR muscles and the muscles of patients with either underacting or overacting muscles (Fig. 6).
FIGURE 6.
Mean cross-sectional areas of myofibers from control, underacting, or overacting MR muscles. No significant difference was found in mean fiber areas between the two patient groups and the control muscle fibers.
DISCUSSION
Little is known about the changes in the EOMs in patients with strabismus. This study was directed at an analysis of the satellite cell complement in MR muscles of patients with strabismus. Although the etiology of most forms of strabismus is unknown, understanding more about muscle cell biology in patients with strabismus may suggest alternative approaches to the treatment of these disorders.
In muscles of patients with strabismus who had underacting MR muscles, there was an upregulation in the number of activated and total satellite cells, based on the number of myofibers in cross sections, compared with the number in normal MR muscles from age-matched control eyes. There are several hypotheses that can be put forward to explain this observation. These changes may be intrinsic to the muscles themselves, or they may be secondary to changes in innervation. There are several studies that support motor innervation as a more likely primary cause of strabismus. Genetic studies of rare congenital cranial dysinnervation disorders that result in congenital incomitant strabismus and fibrosis of the EOM, including CFEOM1 and -2, have demonstrated that these disorders are caused by mutations in two genes, KIF21A and PHOX2A, respectively.5,6 Mutations in either of these genes results in defects in neuronal differentiation and neurite out-growth. 20,21 When the orbit and brain stem of patients with CFEOM1 and -2 were examined by using magnetic resonance imaging, the cranial motor nerves were either absent or showed significant hypoplasia.22,23 The causes of complex strabismus, however, are still unknown.24
There is evidence that also supports the concept that intrinsic properties of the EOMs themselves play a role in either the development or maintenance of strabismus. It should be noted that although dysinnervation has been seen frequently in the magnetic resonance imaging of the orbits of patients with strabismus, the absence of the motor nerve is not always associated with EOM hypoplasia.25 This suggests that the EOMs themselves have the ability for self-maintenance. There is a large number of patients with strabismus who show neither muscle or nerve hypoplasia nor atrophy, and a variety of possible mechanisms have been put forward to explain eye movement disorders, including increased innervation and alterations within the muscles themselves.26 Several studies support the concept that the EOMs have unique responses to alteration in their innervation compared with limb skeletal muscle. For example, functional denervation due to a single injection of botulinum toxin into the EOM results in a significant increase in the number of activated satellite cells compared with normal controls, and this upregulation is maintained for several weeks.27 In muscles that also undergo continuous myofiber remodeling, the laryngeal muscles,28 significant increases in MyoD-positive cells were seen in these muscles, both acutely and chronically, after sectioning of the recurrent laryngeal nerve.29 Although it was not possible to determine whether the increase in satellite cell activation and total number of satellite cells was primary or secondary in the MR of these patients with strabismus, there is no doubt that the EOMs from patients with a diagnosis of MR underaction differed from normal MR in their complement of these cells.
Several hypotheses have been put forward to explain EOM overaction.26 These include one or more of the following: increased innervation; an increase in muscle cross-sectional area, both in individual fibers and total muscle cross sections; and/or changes in expression of individual myosin heavy chain isoforms. However, the actual state of the muscle in these patients is not understood. In the present study, in muscles of patients with strabismus who have overacting MR, there was an increase in the total number of satellite cells, but a decrease in the number of activated satellite cells, based on the number of myofibers. Of interest, in patients with a diagnosis of overacting inferior oblique muscles, there was a significant increase in satellite cells but there were also increases in activated satellite cells, as evidenced by an increase in MyoD-positive cells.30 This difference may be the direct result of the differences in the etiology of inferior oblique and MR overaction.26 In fact, when the eye is in the adducted position, inferior oblique muscle overelevation may be correlated to a possible hypoinnervation of the elevators of the eyes, especially the superior rectus. The inferior oblique, consequently, appears to be overactive when in reality it is not. It has been shown that patterns of nerve activation have significant effects on satellite cells in muscle. Previous studies have demonstrated that alterations in stimulation parameters result in an elevated number of satellite cells in the stimulated muscles.31 In these muscles of patients with strabismus, it is not possible to determine whether these alterations are primary—that is, within the muscle itself— or secondary, due to changes in pattern of stimulation or alterations in innervation. However, the difference in the number of activated satellite cells in the “overacting” MR compared with the normal control muscles suggests several testable hypotheses. One possibility is that the reduction in the number of activated satellite cells may mean that the rate at which these cells fuse into fibers increases, being pushed by their environment into more rapid differentiation. This increase in fusion could be the result of a significant change in electrical stimulation from the oculomotor nerve. Changes in the pattern of electric stimulation is a known determinant of myogenic regulatory factor expression.32,33 It is also possible that there is a reduced number of MyoD-positive satellite cells in the overacting muscles due to increased apoptosis of these cells. Muscle precursor cells often respond to removal of muscle growth factors by serum deprivation in vitro, for example, by either increased differentiation or apoptosis.34 Both of these processes may occur simultaneously. Further studies are ongoing to distinguish between these possibilities.
The goal of strabismus surgery is to alter the motive forces of agonist–antagonist pairs with the goal of sustained changes in the rotational position of the globe. Antagonist-weakening results in reduced tension in the surgically altered muscle for more than 3 weeks,35 while increased muscle tension results in compensatory muscle hypertrophy.36 When recession–resection surgery is performed, no net change in resting tension occurs, and compensatory muscle changes are also minimized. 37 The compensatory changes within the strabismic muscles, as well as in the EOMs after either resection or recession surgery, should be reflected in alterations in the cells that provide the myofibers’ size, the satellite cells. In a study of rabbit EOMs, active and passive stretching of the EOMs as a result of resection surgery caused a significant increase in the number of activated satellite cells and their rapid entry into existing myofibers. This effect was seen in both the actively and passively stretched muscles as they remodeled because of the tension created by the surgery.38 Thus, the recognition that the satellite cell population is altered in human strabismic muscles suggests that pharmacologic approaches would be an important addition to the armamentarium of treatments. Recent work has demonstrated that various myogenic growth factors can modulate muscle mass and force, both acutely and in a sustained fashion in adult rabbits39–41 and in developing chick EOMs.42 This type of pharmacologic approach may be a useful alternative to surgical resection and recession procedures, because it avoids many of the potential long-term biomechanical hazards of surgery and may prevent the compensatory muscle changes that are known to occur with conventional strabismus surgery.
Acknowledgments
Supported by Grant Fapesp 03/07578-6 (RSA-F); National Eye Institute Grants EY015313 (LKM) and EY011375, and an unrestricted grant to the Department of Ophthalmology at the University of Minnesota from Research to Prevent Blindness.
Footnotes
Disclosure: R.S. Antunes-Foschini, None; D. Miyashita, None; H.E.A. Bicas, None; L.K. McLoon, None
References
- 1.Guyton DL. The 10th Bielschowsky Lecture. Changes in strabismus over time: the role of vergence tonus and muscle length adaptation. Binocul Vis Strabismus Q. 2006;21:81–92. [PubMed] [Google Scholar]
- 2.Kushner BJ. Perspective on strabismus. Arch Ophthalmol. 2006;124:1321–1326. doi: 10.1001/archopht.124.9.1321. [DOI] [PubMed] [Google Scholar]
- 3.Livir-Rallatos G, Gunton KB, Calhoun JH. Surgical results in large-angle exotropia. J AAPOS. 2002;6:77–80. doi: 10.1067/mpa.2002.122059. [DOI] [PubMed] [Google Scholar]
- 4.Triger L, Siatkowski RM. Factors associated with horizontal reoperation in infantile esotropia. J AAPOS. 2002;6:15–20. doi: 10.1067/mpa.2002.120644. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Andrews C, Chan WM, et al. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1) Nat Genet. 2003;35:318–321. doi: 10.1038/ng1261. [DOI] [PubMed] [Google Scholar]
- 6.Jen JC, Chan WM, Bosley TM, et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509–1513. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preising M. Towards identification of genes in regionally accumulated strabismus. Strabismus. 2002;10:157–161. doi: 10.1076/stra.10.2.157.8141. [DOI] [PubMed] [Google Scholar]
- 8.Helveston EM, Cofield DD. Indications for marginal myotomy and technique. Am J Ophthalmol. 1970;70:574–578. doi: 10.1016/0002-9394(70)90891-3. [DOI] [PubMed] [Google Scholar]
- 9.Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLoon LK, Wirtschafter JD. N-CAM is expressed in mature extraocular muscles in a pattern conserved between three species. Invest Ophthalmol Vis Sci. 1996;37:318–327. [PubMed] [Google Scholar]
- 11.Junttila T, Rechardt L, Cao Y, Hokfelt T, Pelto-Huikko M. Distribution of acidic fibroblast growth factor-like immunoreactivity in rat skeletal muscle fibers. Brain Res. 1996;22(707):81–87. doi: 10.1016/0006-8993(95)01227-3. [DOI] [PubMed] [Google Scholar]
- 12.Fischer MD, Gorospe JR, Felder E, et al. Expression profiling reveals metabolic and structural components of extraocular muscles. Physiol Genomics. 2002;9:71–84. doi: 10.1152/physiolgenomics.00115.2001. [DOI] [PubMed] [Google Scholar]
- 13.McLoon LK, Wirtschafter JD. Continuous myonuclear addition to single extraocular myofibers in uninjured adult rabbits. Muscle Nerve. 2002;25:348–358. doi: 10.1002/mus.10056. [DOI] [PubMed] [Google Scholar]
- 14.McLoon LK, Rowe J, Wirtschafter JD, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve. 2004;29:707–715. doi: 10.1002/mus.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLoon LK, Wirtschafter JD. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol Vis Sci. 2003;44:1927–1932. doi: 10.1167/iovs.02-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grounds MD, Garrett KL, Wright WE, Beilharz MW. Identification of skeletal muscle precursor cells in vivo by use of MyoD11 and myogenin probes. Cell Tissue Res. 1992;267:99–104. doi: 10.1007/BF00318695. [DOI] [PubMed] [Google Scholar]
- 17.Smith CK, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation and differentiation of rat skeletal muscle satellite cells. J Cell Physiol. 1994;159:379–385. doi: 10.1002/jcp.1041590222. [DOI] [PubMed] [Google Scholar]
- 18.Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morfini G, Quiroga S, Rosa A, Kosik K, Caceres A. Suppression of KIF2 in PC12 cells alters the distribution of a growth cone non-synaptic membrane receptor and inhibits neurite extension. J Cell Biol. 1997;138:657–669. doi: 10.1083/jcb.138.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expressions and interactions of the two closely related homeobox genes Phos2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- 22.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIR21A. Invest Ophthalmol Vis Sci. 2005;46:530–539. doi: 10.1167/iovs.04-1125. [DOI] [PubMed] [Google Scholar]
- 23.Bosley TM, Oystreck DT, Robertson RL, al Awad A, Abu-Amero K, Engle EC. Neurological features of congenital fibrosis of the extraocular muscles type 2 with mutations in PHOX2A. Brain. 2006;129:2363–2374. doi: 10.1093/brain/awl161. [DOI] [PubMed] [Google Scholar]
- 24.Engle EC. The genetic basis of complex strabismus. Pediatr Res. 2006;59:343–348. doi: 10.1203/01.pdr.0000200797.91630.08. [DOI] [PubMed] [Google Scholar]
- 25.Demer JL, Ortube MC, Engle EC, Thacker N. High-resolution magnetic resonance imaging demonstrates abnormalities of motor nerves and extraocular muscles in patients with neuropathic strabismus. J AAPOS. 2006;10:135–142. doi: 10.1016/j.jaapos.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushner BJ. Multiple mechanisms of extraocular muscle “overaction.”. Arch Ophthalmol. 2006;124:680–688. doi: 10.1001/archopht.124.5.680. [DOI] [PubMed] [Google Scholar]
- 27.Ugalde I, Christiansen SP, McLoon LK. Botulinum toxin treatment of extraocular muscles in rabbits results in increased myofiber remodeling. Invest Ophthalmol Vis Sci. 2005;46:4114–4120. doi: 10.1167/iovs.05-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goding GS, Al-Sharif K, McLoon LK. Myonuclear addition to uninjured laryngeal myofibers in adult rabbits. Ann Otol Rhinol Laryngol. 2005;114:552–557. doi: 10.1177/000348940511400711. [DOI] [PubMed] [Google Scholar]
- 29.Shinners MJ, Goding GS, McLoon LK. Effect of recurrent laryngeal nerve section on the laryngeal muscles of adult rabbits. Otolaryngol Head Neck Surg. 2006;134:413–418. doi: 10.1016/j.otohns.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Antunes-Foschini RMS, Ramalho RS, Ramalho LNZ, Bicas HEA. Increased frequency of activated satellite cells in overacting inferior oblique muscles from humans. Invest Ophthalmol Vis Sci. 2006;47:3360–3365. doi: 10.1167/iovs.05-0798. [DOI] [PubMed] [Google Scholar]
- 31.Putman CT, Sultan KR, Wassmer T, Bamford JA, Skorjanc D, Pette D. Fiber-type transitions and satellite cell activation in low-frequency-stimulated muscles of young and old rats. J Gerontol Biol Sci. 2001;56A:B510–B519. doi: 10.1093/gerona/56.12.b510. [DOI] [PubMed] [Google Scholar]
- 32.Eftimie R, Brenner HR, Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci USA. 1991;88:1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes SM, Taylor JM, Tapscott SJ, Gurley CM, Carter MJ, Paterson CA. Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development. 1993;118:1137–1147. doi: 10.1242/dev.118.4.1137. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda R, Yoshida K, Ushiyama M, et al. The small heat shock protein alphaB-crystallin inhibits differentiation-induced caspase 3 activation and myogenic differentiation. Biol Pharm Bull. 2006;29:1815–1819. doi: 10.1248/bpb.29.1815. [DOI] [PubMed] [Google Scholar]
- 35.Christiansen SP, Soulsby ME, Seifen EE. Effect of antagonist weakening on developed tension in cat extraocular muscle. Invest Ophthalmol Vis Sci. 1995;36:2547–2550. [PubMed] [Google Scholar]
- 36.Christiansen SP, Madhat M, Baker L, Baker R. Fiber hypertrophy in rat extraocular muscle following lateral rectus resection. J Pediatr Ophthalmol Strabismus. 1988;25:167–171. doi: 10.3928/0191-3913-19880701-05. [DOI] [PubMed] [Google Scholar]
- 37.Christiansen SP, Harral RL, Brown H. Extraocular muscle fiber morphometry following combined recession-resection procedures in rabbits. J Pediatr Ophthalmol Strabismus. 1996;33:247–250. doi: 10.3928/0191-3913-19960901-09. [DOI] [PubMed] [Google Scholar]
- 38.Christiansen SP, McLoon LK. The effect of resection on satellite cell activity in extraocular muscle. Invest Ophthalmol Vis Sci. 2006;47:605–613. doi: 10.1167/iovs.05-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLoon LK, Christiansen SP. Increasing extraocular muscle strength with insulin-like growth factor II. Invest Ophthalmol Vis Sci. 2003;44:3866–3872. doi: 10.1167/iovs.03-0223. [DOI] [PubMed] [Google Scholar]
- 40.McLoon LK, Anderson BC, Christiansen SP. Increasing muscle strength as a treatment for strabismus: sustained release of insulin growth factor-1 results in stronger extraocular muscle. J AAPOS. 2006;10:424–429. doi: 10.1016/j.jaapos.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson BC, Christiansen SP, Grandt S, Grange RW, McLoon LK. Increased extraocular muscle strength with direct injection of insulin-like growth factor-I. Invest Ophthalmol Vis Sci. 2006;47:2461–2467. doi: 10.1167/iovs.05-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, von Bartheld CS. Role of exogenous and endogenous trophic factors in the regulation of extraocular muscle strength during development. Invest Ophthalmol Vis Sci. 2004;45:3538–3545. doi: 10.1167/iovs.04-0393. [DOI] [PubMed] [Google Scholar]