Abstract
Endocannabinoids (ECs), anandamide (AEA) and 2-arachidonoylglycerol (2-AG), inhibit proliferation of carcinoma cells. Several enzymes hydrolyze ECs to reduce endogenous EC concentrations and produce eicosanoids that promote cell growth. In this study, we determined the effects of EC hydrolysis inhibitors and a putative EC, 2-arachidonylglyceryl ether (noladin ether, NE) on proliferation of prostate carcinoma (PC-3, DU-145, and LNCaP) cells. PC-3 cells had the least specific hydrolysis activity for AEA and administration of AEA effectively inhibited cell proliferation. The proliferation inhibition was blocked by SR141716A (a selective CB1R antagonist) but not SR144528 (a selective CB2R antagonist), suggesting a CB1R-mediated inhibition mechanism. On the other hand, specific hydrolysis activity for 2-AG was high and 2-AG inhibited proliferation only in the presence of EC hydrolysis inhibitors. NE inhibited proliferation in a concentration-dependent manner; however, SR141716A, SR144528 and pertussis toxin did not block the NE-inhibited proliferation, suggesting a CBR-independent mechanism of NE. A peroxisome proliferator-activated receptor gamma (PPARγ) antagonist GW9662 did not block the NE-inhibited proliferation, suggesting that PPARγ was not involved. NE also induced cell cycle arrest in G0/G1 phase in PC-3 cells. NE inhibited the nuclear translocation of nuclear factor-kappa B (NF-κB p65) and down-regulated the expression of cyclin D1 and cyclin E in PC-3 cells, suggesting the NF-κB/cyclin D and cyclin E pathways are involved in the arrest of G1 cell cycle and inhibition of cell growth. These results indicate therapeutic potentials of EC hydrolysis inhibitors and the enzymatically stable NE in prostate cancer.
Keywords: 2-Arachidonylglyceryl ether (noladin ether), cannabinoid receptor, endocannabinoid, proliferation, prostate cancer
Introduction
Well-characterized endocannabinoids (ECs, endogenous ligands of the cannabinoid receptors), anandamide (AEA) [1] and 2-arachidonoylglycerol (2-AG) [2, 3] have therapeutic potential in various types of cancer [4-11]. For example, ECs inhibit the nerve growth factor-induced proliferation of breast carcinoma cells [12], proliferation of colorectal cancer cells [5], and colon [4]and thyroid tumor growth in vivo [7]. Met-F-AEA, an analog of AEA inhibits adhesion and migration of breast carcinoma cells [13, 14].
AEA, through the activation of cannabinoid receptor type-1 (CB1R), inhibits the epidermal growth factor (EGF)-induced proliferation of prostate carcinoma cells by decreasing expression of the epidermal growth factor receptor (EGFR) and increasing ceramide production [15]. Interestingly, among PC-3, DU-145 and LNCaP cells, LNCaP cells are the least sensitive to AEA inhibition. AEA and 2-AG also inhibit prolactin-induced proliferation of DU-145 cells [12]. We previously demonstrated that endocannabinoids such as 2-AG, acting through CB1R, inhibit invasion of PC-3 and DU-145 cells but had little or no effect in LNCaP cells [16] due to its high hydrolysis activity in these cells [17]. AEA is hydrolyzed by fatty acid amide hydrolase (FAAH) [18-20] and 2-AG is hydrolyzed by FAAH and monoacylglycerol lipase (MGL) [21-23] to free arachidonic acid (AA). The hydrolysis has two detrimental effects: it reduces the concentrations of ECs and the free AA is further metabolized to eicosanoids. Free AA and some eicosanoids promote prostate carcinoma cell growth and motility [24-29]. Thus, it is now recognized that the inhibition of EC hydrolysis is a potential therapeutic target for cancer treatment [7, 17, 20, 30-32].
A putative EC, 2-arachidonylglyceryl ether (noladin ether, NE), is chemically similar to 2-arachidonoylglycerol (2-AG) with the glycerol moiety conjugated by ether-linkage to AA [33-35]. NE is more enzymatically stable than 2-AG and AEA [36-38]. NE was first identified in porcine brain [33] and later in rat brain regions [35]; however, other studies did not detect NE in the brains of various mammalian species [39, 40]. The role of NE in cancer, particularly prostate cancer is not well-understood. In this study, we investigated the effects of EC hydrolysis and the enzymatically stable NE on proliferation of prostate carcinoma cells. NE binds to CB1R [33, 34, 41] and has much lower affinity for CB2R [33, 42]; thus, we investigated if the NE inhibited prostate carcinoma cell proliferation is CBR dependent. Since NE activates peroxisome proliferator-activated receptor gamma (PPARγ) [43] and PPARγ mediates proliferation and cell cycle arrest of prostate carcinoma cells [44-47], we investigated if the NE inhibited proliferation by activating PPARγ pathway. Furthermore, we examined the possible mechanism of NE in the regulation of nuclear factor-kappa B (NF-κB) and cell cycle regulatory proteins [48, 49] that lead to the arrest of cell cycle and inhibition of growth of prostate carcinoma cells.
Materials and Methods
Materials
Human prostate carcinoma (PC-3, DU-145, and LNCaP) cells were obtained from the American Type Culture Collection (ATCC, Rockville, Maryland). Thymidine [methyl-3H] (1 μCi/μL) was obtained from Applied Biosystems (Foster City, California). Pertussis toxin (PTX) and methyl thiazol tetrazolium (MTT) were obtained from Sigma–Aldrich Chemical (St. Louis, Missouri). Noladin ether (NE), AEA, 2-AG, AM251, and GW9662 were obtained from Cayman Chemical Co. (Ann Arbor, Michigan). SR141716A and SR144528 were obtained from Research Triangle Institute (Research Triangle Park, North Carolina). 2-Oleoyl-[3H3]-glycerol ([3H3]2-OG, 20 Ci/mmol) and anandamide [ethanolamine 1-3H] ([3H]AEA, 60 Ci/mmol) were obtained from American Radiolabeled Chemical (St Louis, Missouri). SimplyBlue SafeStain and goat anti-mouse IgG-HRP were obtained from Invitrogen (Carlsbad, CA). Tumor necrosis factor-alpha (TNF-α) was obtained from EMD Chemicals, Inc. (Gibbstown, NJ). Rabbit polyclonal IgG against nuclear factor-κB (NF-κB p65) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Goat anti-rabbit IgG (H+L)-FITC was obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Mouse monoclonal antibody to human cyclin D1 was obtained from GenWay Biotech, Inc. (San Diego, CA). Mouse monoclonal antibody to human cyclin E was obtained from Cell Signaling Technology (Danvers, MA). (SDS-PAGE BioRad Ready Gels (10%) were obtained from BioRad (Hercules, CA). ECL Western blot detection kit and BCA protein assay kit were obtained from Thermo Scientific (Rockville, IL). 3-(Octylthio)-1,1,1-trifluoropropan-2-one (OTFP) was generously provided by Dr. Bruce D. Hammock [50, 51]. Diazomethylarachidonyl ketone (DAK) was synthesized in our laboratory [52]. Poly-d-lysine was obtained from BD Biosciences (Bedford, Massachusetts). Other chemicals and reagents were analytical grade or the highest purity available from suppliers and they were used without purification. Distilled, deionized water was used for all experiments.
Cells and cell culture
Prostate carcinoma cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, L-glutamine (2.0 mM), streptomycin (100 μg/mL) and penicillin (100 U/mL). Cells were grown in 75-cm2 polystyrene tissue culture flasks at 37 °C in 5% CO2 to approximately 50-70% confluency before use.
Thymidine incorporation assay
Cell proliferation was determined by thymidine incorporation assay as previously described [53] with minor modifications. PC-3 and DU-145 cells (5.0×104 cells/well) and LNCaP cells (1.0×105 cells/well) were plated in 24-well plates coated with poly-d-lysine and incubated in 5% CO2 for 24 h at 37°C. Cells were washed and incubated in a serum free media for 24 h at 37°C. Then, cells were incubated in the serum free media in the absence or presence of pharmacological agents for 24 h at 37°C. For PTX treatment, cells were incubated with PTX overnight prior to NE treatment [54, 55]. Cells in each well were then incubated with [3H]thymidine (1 μCi) for additional 6 h. Cells were washed with phosphate buffered saline, fixed with cold 5% trichloroacetic acid and solubilized with 0.25 M NaOH. The solution (300 μL) was counted for radioactivity. Each experiment was performed 2-3 times with 6 wells per treatment.
Cell counting
Cell proliferation was also determined by cell counting. Cells were plated at 5×104 cells/well in a 24-well plate and grown to about 60% confluency and treated with pharmacological agents as described above in thymidine incorporation assay. After 24-h incubation, cells were fixed with cold ethanol:methanol on ice and stained with SimplyBlue SafeStain for 20 min to improve cell counting similar to the described protocol [56]. Stained cells were photographed using a microscope with a CCD camera (Photometrics) and counted for number of cells per field. At least 4 fields per treatment were used to average the number of cells and they were normalized to the control cells (as 100%).
MTT assay
Cells were plated in 96-well plates (2.0×104 cells/well) in serum-free and phenol red-free media at 37°C for 48 h. Cell treatment and incubation were performed similar to the thymidine incorporation assay in the serum-free and phenol red-free media for 24 h. Then, methyl thiazol tetrazolium (MTT, 0.25 mg/mL) was added to each well and incubated for 6 h at 37 °C. The media was aspirated and isopropanol containing 0.35% HCl was added to lyse the cells. The absorbance was measured at 570 nm by a microplate reader (Bio-Tek Instruments Inc., Winooski, Vermont).
Determination of endocannabinoid hydrolysis
The hydrolysis of endocannabinoids (2-AG and AEA) by subcellular protein fractions (25 μg) of prostate carcinoma cells was determined by using previously described protocol [16]. 2-Oleoyl-[3H3]-glycerol ([3H3]2-OG) was used as an analog of 2-AG and anandamide [ethanolamine 1-3H] ([3H]AEA) was used for AEA. Incubations were carried out for 30 min at 37 °C, radioactivity in each liquid phases were separated and counted [16], and the specific hydrolysis activity was calculated.
Determination of NE by LC-ESI-MS
Concentrations of exogenously added NE in PC-3 and LNCaP cells were determined by LC-ESI-MS [16]. Cells were grown in T-75 flasks and treated with NE (18.25 μg) for 0 and 2 h at 37 °C. Then, cells were lysed, scraped, and cell lysate and media were collected. [2H8]2-AG (15 ng) was added as an internal standard into the samples and extracted by solid phase extraction. NE was determined by LC-ESI-MS [16] and the detection was made in the positive mode. The m/z 365 and 387 were used for NE and [2H8]2-AG measurements, respectively. The concentrations of NE were calculated by comparing their ratios of peak areas to the standard curves.
Cell cycle measurement
Cells were grown in T-75 flasks to about 50% confluency, changed to media containing 0.5% serum and incubated for 60 h at 37°C. Cells were washed and incubated in the 0.5% serum media in the presence of vehicle or NE (10 μM) for 30 min at 37°C. Then, cells were washed and incubated with vehicle or NE in the media containing either 0.5% serum or 10% serum for 24 h at 37°C. Cells were trypsinized, centrifuged for 5 min at 1500 rpm, washed with PBS, and then treated with 50 μg/mL RNase A. DNA was stained with 50 μg/mL propidium iodide for 10 min at room temperature. Cell cycle analysis was performed on FACS Calibur (Becton Dickinson, San Jose, California) and data were analyzed using Modfit LT from Verity Software (Topsham, Maine).
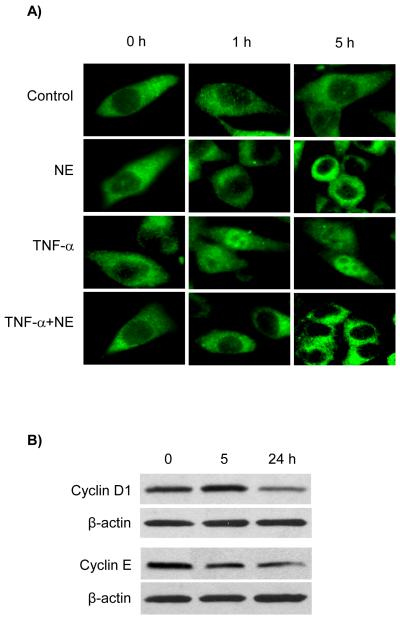
Immunofluorescence staining of nuclear factor-κB (NF-κB) localization in PC-3 cells
Cells were plated in 24-well plates on cover slips in the complete media for 2 days. Then, the media were replaced with the serum free media for 24 h. The cells were treated with serum free media in the absence or presence of NE (10 μM), TNF-α (10 ng/mL), and NE and TNF-α for 1 and 5 h. TNF-α was used as a positive control. Then, cells were fixed and stained with the antibody to NF-κB p65 as described [57]. The cells were examined using a fluorescence microscope (Nikon Eclipse E600).
Western blot analysis of cyclin D1and cyclin E in PC-3 cells
Since cyclin D1 and cyclin E are essential for the transition of cells the G1 to S phase of the cell cycle by selectively binding to and activating cyclin-dependent kinases (CDKs), the effect of NE on the expression of cyclin D1 and cyclin E was examined in PC-3 cells. PC-3 cells were cultured to about 70% and the media were changed to the serum free media for 24 h. Then, the cells were treated with vehicle control or NE (10 μM) for 0, 5 and 24 h. Cells were lyzed and proteins were electrophoretically separated on SDS-PAGE BioRad Ready Gels (10%) and the blots were incubated with the specific antibody to cyclin D1 or cyclin E, followed with the secondary antibody-HRP. Protein amount was used as loading control and β-actin immunoreactive band intensity was used for comparison. The detection was made by using ECL Western Blotting Substrate and immunoreactive bands were captured by Fuji film X-ray (Tokyo, Japan).
Data analysis
Data are calculated as the average and reported as mean ± SEM. The significance of difference among group was calculated by InSat 3 software (GraphPad Software, Inc., San Diego, CA) using unpaired t test for two-tailed P value. P < 0.05 is considered as significantly different.
Results
Specific hydrolysis activity of endocannabinoids in cellular protein fractions of prostate carcinoma cells
EC hydrolysis is an important deactivation pathway for ECs and it contributes to their regulation of cell proliferation. The specific hydrolysis activities for 2-OG (2-AG analog) and AEA in membrane and cytosolic fractions of prostate carcinoma cells were determined. [3H]AEA was used as a substrate for fatty acid amide hydrolase (FAAH) and [3H3]2-OG (commercially available analog of 2-AG) was used as a substrate for monoacylglycerol lipase (MGL), FAAH and possibly other hydrolases. Since FAAH is a membrane-bound enzyme, the hydrolysis activity for AEA was observed in the membrane fractions only. The specific hydrolysis activity of AEA in PC-3 cells was very low and the highest activity was in LNCaP cells (Table 1). Specific hydrolysis activity for 2-OG (2-AG) was observed in both cytosolic and membrane fractions with a higher activity in membrane fractions for all cell lines. Membrane fractions of DU-145 and LNCaP cells had a two-fold higher hydrolysis activity for 2-OG (2-AG) than membrane fractions of PC-3 cells.
Table 1.
Specific hydrolysis activity for 2-OG and AEA in subcellular protein fractions of prostate carcinoma cells
|
Specific Hydrolysis Activity (pmol/min/mg protein) |
|||
|---|---|---|---|
| Cells | Subcellular Fraction | [3H3]2-OG | [3H]AEA |
| PC-3 | Cytosolic Fraction | 37.43±6.51 | 0.79±3.43 |
| Membrane Fraction | 88.78±2.50 | 9.68±2.03 | |
| DU-145 | Cytosolic Fraction | 51.78±4.23 | 0.40±4.35 |
| Membrane Fraction | 162.97±6.09 | 21.39±1.60 | |
| LNCaP | Cytosolic Fraction | 22.90±2.99 | 1.31±3.10 |
| Membrane Fraction | 174.65±2.92 | 92.91±4.43 | |
2-OG (2-AG) is the substrate for FAAH, MGL and other hydrolases ,whereas AEA is the substrate for FAAH (membrane bound enzyme) only.
The results are mean ± SEM (n = 6).
Effects of AEA and 2-AG on proliferation of PC-3 cells
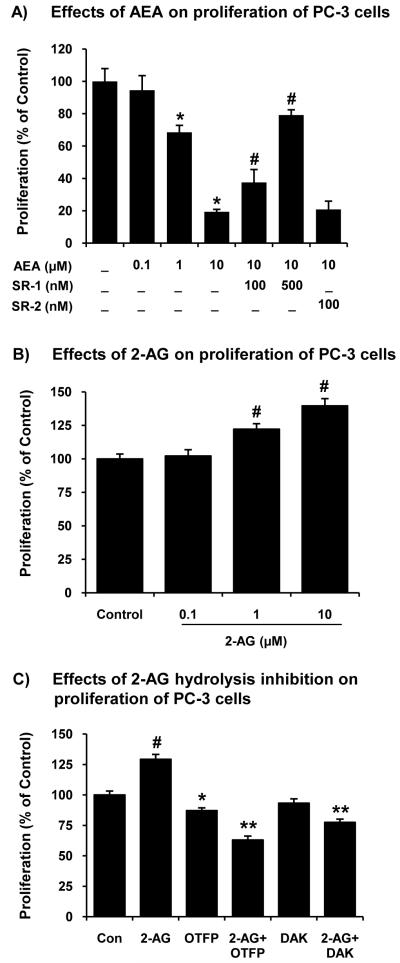
PC-3 cells exhibited the lowest specific hydrolysis activity for ECs, particularly a very low AEA hydrolysis activity (Table 1). The effects of ECs on proliferation in PC-3 cells were determined. Exogenously added AEA significantly decreased proliferation of PC-3 cells in a concentration-dependent manner (Fig. 1A). In addition, SR141716A (SR-1) (100, 500 nM) blocked the AEA-inhibited proliferation while SR144528 (SR-2) did not significantly reverse the inhibition of proliferation by AEA. These results suggest that AEA inhibits proliferation of PC-3 cells through a CB1R-dependent mechanism.
Figure 1.
Effects of ECs and hydrolysis inhibitors on prostate carcinoma cell proliferation. (A) Effects of AEA (0.1, 1.0 and 10 μM), SR141716A (SR-1, a selective CB1R antagonist) and SR144528 (SR-2, a selective CB2R antagonist) on proliferation (thymidine incorporation assay) of PC-3 cells. Values are mean ± SEM (n = 12). *, significantly lower than the control cells with p < 0.05; #, significantly higher than AEA-treated cells. (B) Effects of 2-AG (0.1, 1.0 and 10 μM) on proliferation (thymidine incorporation assay) of PC-3 cells. Values are mean ± SEM (n = 12). #, significantly higher than the control cells with p < 0.05. (C) Effects of hydrolase inhibitors (OTFP and DAK, 10 μM) and a combination of OTFP or DAK and 2-AG (10 μM) on proliferation (thymidine incorporation assay) of PC-3 cells. Values are mean ± SEM (n = 6-12). *, significantly lower than the control cells with p < 0.05; #, significantly higher than the control cells with p < 0.05; **, significantly lower than the OTFP-treated or DAK-treated cells with p < 0.05.
On the other hand, exogenously added 2-AG increased instead of decreased proliferation of PC-3 cells (Fig. 1B). PC-3 cells rapidly hydrolyze 2-AG to free AA and AA is further metabolized to eicosanoids [25]. To test whether the 2-AG hydrolysis increased cell proliferation, PC-3 cells were treated with hydrolase inhibitors such as OTFP [32] and DAK [16] prior to the treatment with 2-AG (1.0 μM). OTFP (10 μM) inhibited cell proliferation while DAK (10 μM) did not significantly inhibit cell proliferation. The combined treatments of exogenous 2-AG and hydrolase inhibitors further decreased cell proliferation from inhibitors alone (Fig. 1C). These results indicate that 2-AG, when protected from hydrolysis by MGL and FAAH, inhibited cell proliferation.
Effects of noladin ether and CBR antagonists on proliferation of prostate carcinoma cells
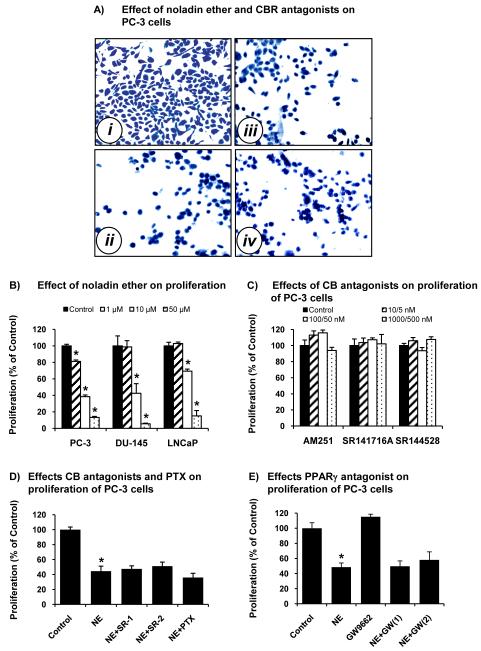
NE is known to be more enzymatically resistant to hydrolysis than 2-AG and AEA. NE (10 μM) induced PC-3 cells to have a rounded shape and decreased cell counts (Fig. 2A, ii) as compared with control cells (Fig. 2A, i). SR-141716A (500 nM) and SR144528 (500 nM) did not block the decrease of cell counts by NE (Fig. 2A, iii & iv). Treatment of PC-3, DU-145, and LNCaP cells with NE (1.0, 10, 50 μM) decreased proliferation of these cells in a concentration-dependent manner (Fig. 2B). In some experiments, the MTT assay was used to determine viable cells, and the results were similar to the thymidine incorporation assay (data not shown). Interestingly, NE (1.0 and 10 μM) less effectively inhibited proliferation in LNCaP cells than in PC-3 cells.
Figure 2.
Effects of NE and selective CBR antagonists on cell counts and proliferation of prostate carcinoma cells. (A) Examples of PC-3 cells treated with vehicle control (i), NE (10 μM) (ii), NE (10 μM) and SR141716A (SR-1, 500 nM) (iii), and NE (10 μM) and SR144528 (SR-2, 500 nM) (iv) at 37°C for 24 h. Then, the cells were stained with SimplyBlue SafeStain and counted. (B) Proliferation (thymidine incorporation assay) of PC-3, DU-145 and LNCaP cells treated with NE (1.0, 10 and 50 μM). Proliferation was normalized to the control cells (100%). Values are mean ± SEM (n = 12). *, significantly lower than the control cells with p < 0.05. (C) Proliferation (thymidine incorporation assay) of PC-3 cells treated with vehicle control, AM251 (0.01, 0.1, and 1.0 μM), SR141716A (5, 50, and 500 nM), and SR144528 (5, 50, and 500 nM). Proliferation was normalized to the control cells (100%). The results for DU-145 and LNCaP cells treated with AM251, SR141716A, and SR144528 are similar to PC-3 cells (data not shown). Values are mean ± SEM (n = 12). (D) Proliferation (thymidine incorporation assay) of PC-3 cells treated with vehicle control, NE (10 μM), NE (10 μM) and SR141716A (500 nM), NE (10 μM) and SR144528 (500 nM), and pretreatment with PTX (100 ng/mL) for 18 h and NE (10 μM). Proliferation was normalized to the control cells (100%). Values are mean ± SEM (n = 12). *, significantly lower than the control cells with p < 0.05. (E) Proliferation (thymidine incorporation assay) of PC-3 cells treated with vehicle control, NE (10 μM), GW9662 (10 μM), NE (10 μM) and GW9662 (1 μM = GW(1)), and NE (10 μM) and GW9662 (10 μM = GW(2)). Proliferation was normalized to the control cells (100%). Values are mean ± SEM (n = 6-12). *, significantly lower than the control cells with p < 0.05.
Since NE is a ligand for CB1R ≫ CB2R [33, 34, 41, 42], the effects of CBR antagonists on cell proliferation were determined. AM251, a selective CB1R antagonist, SR141716A (SR-1), a selective CB1R antagonist, and SR144528 (SR-2), a selective CB2R antagonist, did not significantly change proliferation of prostate carcinoma cells (Fig. 2C). SR-1 and SR-2 (100 and 500 nM) did not significantly change NE-inhibited proliferation of PC-3 cells (Fig. 2D). Furthermore, pretreatment of PC-3 cells with PTX (100 ng/mL) for 18 h did not significantly change the NE-inhibited cell proliferation (Fig. 2D).
Effects of noladin ether and a PPARγ antagonist on proliferation of prostate carcinoma cells
Since NE activates PPARγ [43] and agonist-activated PPARγ inhibits proliferation and induces cell cycle arrest in prostate carcinoma cells [44, 58], the effects of a PPARγ antagonist on cell proliferation were determined. GW9962, a selective PPARγ antagonist (1 and 10 μM) did not significantly change NE-inhibited proliferation of PC-3 cells (Fig. 2E).
Effects of hydrolase inhibitors and noladin ether on proliferation of LNCaP cells
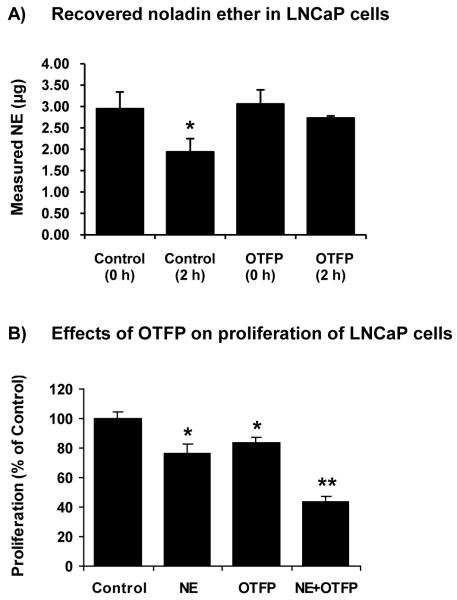
Since LNCaP cells had very high EC specific hydrolysis activity (Table 1) and NE less effectively inhibited proliferation in LNCaP cells (Fig. 2B), we investigated the stability of NE and whether OFTP (a hydrolase inhibitor) increases NE stability. LNCaP cells were incubated with NE (18.25 μg) in the absence or presence of OTFP (10 M) for 0 and 2 h. Then, NE concentrations were determined by LC-ESI-MS. Only 2.95±0.39 μg (16.2%) of NE was recovered at 0-h incubation and only 1.94±0.31 μg (10.6%) was recovered after 2-h incubation (Fig. 3A). OTFP did not improve the recovery of NE at 0-h incubation (3.06±0.33 μg) but it protected the loss of NE at 2-h incubation (2.73±0.05 μg). The low recovery of NE from the samples at 0 h is probably due to the very high hydrophobic nature of the compound and it may be highly adsorbed to plastic surfaces. This was further supported by the 25% recovery of NE in cell-free media.
Figure 3.
Recovery of NE and the effects of hydrolase inhibitor, OTFP, on proliferation in LNCaP cells. (A) Recovered NE in LNCaP cells in the absence and presence of OTFP (10 μM) at 0-h and 2-h incubation. The amount of added NE was 18.25 μg. Values are mean ± SD (n = 3). *, significantly lower than the control cells with p < 0.05. (B) Proliferation (thymidine incorporation assay) of LNCaP cells treated with NE (10 μM), OFTP (10 μM), and a combination of NE and OTFP. Values are mean ± SEM (n = 12-18). *, significantly lower than the control cells with p < 0.05; **, significantly lower than each treatment alone with p < 0.05.
Interestingly, OTFP (10 μM) inhibited proliferation of LNCaP cells (Fig. 3B). The proliferation was further reduced in LNCaP cells treated with a combination of NE and OTFP (Fig. 3B).
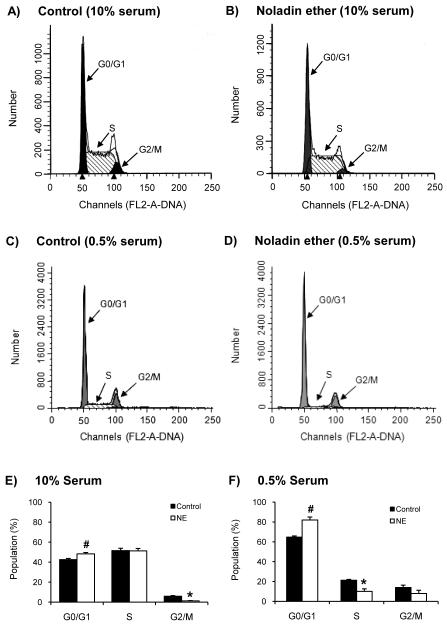
Effects of noladin ether on cell cycle arrest of PC-3 cells
PC-3 cells were treated with NE (10 μM) in the media containing 0.5% or 10% serum. Interestingly, NE increased cell population in G0/G1 phase in both conditions. In the media containing 10% serum, NE increased the cell population in G0/G1 phase and decreased the population in G2/M phase as compared with the control (Fig. 4A, B, and E). However, PC-3 cells in the media containing 0.5% serum, the cell population increased in G0/G1 phase and the population decreased in S phase (Fig. 4C, D, F). Comparing the two conditions of serum concentrations, the increase of the population of cells in G0/G1 phase in the media containing 0.5% serum was greater than the population in the media containing 10% serum. The population in S phase was lower in the media containing 0.5% serum than 10% serum, indicating that serum shifted the cell growth cycle. NE blocked the cell growth in media containing different serum concentrations.
Figure 4.
Effects of NE on cell cycle of PC-3 cells. Examples of cell cycle measurement of PC-3 cells treated with vehicle control (A) and NE (10 μM) (B) in the medium containing 10% serum for 24 h as described in the text. Examples of cell cycle measurement of PC-3 cells treated with vehicle (C) and NE (10 μM) (D) in the medium containing 0.5% serum for 24 h. (E) Population of PC-3 cells in different cell phases following treatment with NE (10 μM) and incubated in the medium containing 10% serum for 24 h. (F) Population of PC-3 cells in different cell phases following treatment with NE (10 μM) and incubated in the medium containing 0.5% serum for 24 h. Values are mean ± SEM (n = 8). #, significantly higher than the control cells with p < 0.05; *, significantly lower than the control cells with p < 0.05.
Effects of noladin ether on NF-κB translocation in PC-3 cells
Immunofluorescence images of PC-3 cells indicated that NF-κB p65 mainly located in the cytosol of the cells. NE slightly induced PC-3 cells to become rounded and decreased the NF-κB p65 in the nucleus at 1 h and the effects of NE were more apparent at 5 h. TNF-α treatment (as a positive control) significantly increased the translocation of NF-κB p65 from the cytoplasm to the nucleus. Interestingly, NE abolished the TNF-α-induced nuclear translocation of NF-κB p65 in PC-3 cells (Fig. 5A).
Figure 5.
Effects of NE on the nuclear translocation of NF-κB and expression of cyclin D1 and cyclin E in PC-3 cells. (A) Example of fluorescence images of NF-κB p65 immunostaining in PC-3 cells treated with vehicle control, NE (10 μM), TNF-α (10 ng/mL), and NE and TNF-α at 37°C for 1 and 5 h. (B) Examples of immunoreactive bands of cyclin D1 and cyclin E in PC-3 cells treated with NE (10 μM) at 37°C for 0, 5, and 24 h. Protein concentrations and β-actin were used as loading controls. These Western blots are representatives of identical results from three separate experiments.
Effects of noladin ether on the expression of cyclin D1 and cyclin E in PC-3 cells
Since NE arrested cell cycle in the G0/G1 phase, the expression of cyclin D1 and cyclin E was determined in PC-3 cells. Western blot analysis indicated that the immunoreactive bands of cyclin D1 markedly decreased in PC-3 cells treated with NE at 24 h, indicating the loss of cyclin D1 expression (Fig. 5B). Immunoreactive bands of cyclin E also decreased in PC-3 cells treated with NE at 5 h of treatment and further decreased at 24 h (Fig. 5B), indicating the loss of cyclin E expression with NE treatment.
Discussion
The AEA specific hydrolysis activity in the membrane fractions of prostate carcinoma cells correlated well with the FAAH expression in these cells [17]. PC-3 cells have very low FAAH expression and have a very low AEA specific hydrolysis activity. LNCaP cells express high level of FAAH and have a very high AEA specific hydrolysis activity. As the result, AEA inhibited proliferation of PC-3 cells but not in LNCaP cells [15]. On the other hand, 2-AG increased proliferation of all cell lines in the absence of hydrolase inhibitors, similar to the effects of ECs on cell invasion/migration [16, 17, 25]. Previously, we demonstrated that prostate carcinoma cells readily hydrolyze 2-AG to free arachidonic acid (AA) [25], and AA induces proliferation of prostate carcinoma cells [24, 59]. Taken together, these results suggest that enzymes involved in EC hydrolysis are critical for the EC signaling in cancer and potentially are therapeutic targets for cancer treatment.
Noladin ether, a stable analog of 2-AG, also effectively caused prostate carcinoma cells to change shape to assume a rounded morphology, suggesting a diminished cell motility and/or proliferation. NE decreased proliferation of PC-3, DU-145, and LNCaP cells in a concentration-related manner. Interestingly, proliferation of LNCaP cells was not as sensitive to NE (at low concentrations) as PC-3 cells. LNCaP cells exhibited a much higher hydrolysis of the ECs than PC-3 cells, which led us to question whether NE is stable in these cells. LC-ESI-MS analysis indicated that only about 16% of NE was recovered from the cell lysate and media at 0 h, suggesting that NE is very hydrophobic and is likely taken into the cells and/or adsorbs to the wells of tissue culture plates. At 2-h incubation, a further loss of NE was observed. This further loss of NE was inhibited by the treatment of cells with OTFP. At present, it is not known whether OTFP really inhibited the degradation of NE or it inhibited the uptake and/or possible incorporation of NE into cell membranes. The cellular uptake of NE by the anandamide transporter into rat C6 glioma cells and a partial incorporation into phospholipids and possibly diacylglycerols and triacylglycerols have been demonstrated [35].
AM251 and SR141716A (selective CB1R antagonists) and SR144528 (a selective CB2R antagonist) did not alter cell counts and proliferation of prostate carcinoma cells. Furthermore, SR141716A, SR144528, and PTX did not reverse the NE-inhibited cell proliferation. These results suggest that NE inhibits proliferation by a non-CB1R/CB2R-mediated mechanism and Go/i linked receptors are not involved. The NE activity by a CB1R-independent, CB2R-independent pathway has been previously demonstrated in vascular beds and various cells [60].
Since agonist-activated PPARγ inhibits proliferation of prostate carcinoma cells and NE can activate PPARγ, the inhibition of proliferation by NE through the PPARγ pathway was determined. GW9662 did not significantly block the NE-inhibited proliferation, suggesting that PPARγ is not the major pathway for NE inhibition.
Noladin ether caused a cell cycle arrest of PC-3 cells in the G0/G1 phase in the low and high serum conditions as indicated by the higher population in the G0/G1 phase of NE-treated cells compared to control cells. However, the medium containing 10% serum markedly shifted the population of cells to S phase and G2/M phase as compared with the medium containing 0.5% serum, indicating the effect of serum on the cell growth. In 0.5% serum, NE increased the population in the G0/G1 phase and decreased the population in the S phase as compared with the control cells. These results indicate that NE can arrest cell cycle of PC-3 cells in the presence of serum and the reduced serum conditions. The arrest of cell cycle of PC-3 cells in G1 phase (10 - 20%) by NE is similar to the results from the treatment of PC-3 cells with other pharmacological agents [49, 61-63].
The treatment of PC-3 cells with NE inhibited the nuclear translocation of NF-κB, decreased the expression of cyclin D1 and cyclin E, the major regulators of transition of cells from the G1 to S phase in the cell cycle. These results indicate that the NF-κB/cyclin D1 and cyclin E pathways are involved in the NE-induced G1 cell cycle arrest.
This study demonstrated that EC hydrolysis inhibitors and the putative EC, NE are anti-proliferative in prostate carcinoma cells. Although AEA inhibits cell proliferation via a CB1R-dependent mechanism, the putative endocannabinoid NE inhibits proliferation by a CBR-independent pathway that involves the inhibition of NF-κB nuclear translocation and the expression of cyclin D1 and cyclin E.
Conclusion
ECs inhibit proliferation of prostate carcinoma cells. However, their susceptibility to enzymatic hydrolysis reduces the inhibition efficiency and complicates their functions in cells. A more enzymatically stable compound such as NE may serve as a model for drug development. Attempts have been made for development of more stable and water soluble ECs [64], particularly water-soluble prodrugs of NE [65]. These developed compounds are less hydrophobic and will be practical and useful for therapeutic studies in prostate cancer.
Acknowledgments
This work was supported by the Wisconsin Breast Cancer Showhouse for a Cure, the Cancer Center of the Medical College of Wisconsin, and the National Institute of Health (DA 09155). The authors thank Dr. Cecilia Hillard for providing SR141716A and SR144528 in this study, and thank Adam Pfeiffer for his technical assistance.
Abbreviations
- AA
arachidonic acid
- AEA
anandamide (arachidonylethanolamide)
- 2-AG
2-arachidonoylglycerol
- CBR
cannabinoid receptor
- DAK
diazomethylarachidonyl ketone
- EC
endocannabinoids
- FAAH
fatty acid amide hydrolase
- MGL
monoacylglycerol lipase
- NE
2-arachinoylglyceryl ether (noladin ether)
- NF-κB
nuclear factor-kappa B
- 2-OG
2-oleoylglycerol
- OTFP
3-(octylthio)-1,1,1-trifluoropropan-2-one
- PPARγ
peroxisome proliferator-activated receptor gamma
- TNF-α
tumor necrosis factor-alpha
Footnotes
Conflict of Interests
The authors have declared that no conflict of interests.
References
- [1].Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- [2].Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- [3].Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- [4].Izzo AA, Aviello G, Petrosino S, Orlando P, Marsicano G, Lutz B, et al. Increased endocannabinoid levels reduce the development of precancerous lesions in the mouse colon. J Mol Med. 2008;86(1):89–98. doi: 10.1007/s00109-007-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ligresti A, Bisogno T, Matias I, De Petrocellis L, Cascio MG, Cosenza V, et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology. 2003;125(3):677–87. doi: 10.1016/s0016-5085(03)00881-3. [DOI] [PubMed] [Google Scholar]
- [6].Parolaro D, Massi P, Rubino T, Monti E. Endocannabinoids in the immune system and cancer. Prostaglandins Leukot Essent Fatty Acids. 2002;66(2-3):319–32. doi: 10.1054/plef.2001.0355. [DOI] [PubMed] [Google Scholar]
- [7].Bifulco M, Laezza C, Valenti M, Ligresti A, Portella G, D.M. V. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. Faseb J. 2004;18(13):1606–8. doi: 10.1096/fj.04-1754fje. [DOI] [PubMed] [Google Scholar]
- [8].Bifulco M, Laezza C, Gazzerro P, Pentimalli F. Endocannabinoids as emerging suppressors of angiogenesis and tumor invasion (review) Oncol Rep. 2007;17(4):813–6. [PubMed] [Google Scholar]
- [9].Bifulco M, Di Marzo V. Targeting the endocannabinoid system in cancer therapy: a call for further research. Nat Med. 2002;8(6):547–50. doi: 10.1038/nm0602-547. [DOI] [PubMed] [Google Scholar]
- [10].Bifulco M, Malfitano AM, Pisanti S, Laezza C. Endocannabinoids in endocrine and related tumours. Endocr Relat Cancer. 2008;15(2):391–408. doi: 10.1677/ERC-07-0258. [DOI] [PubMed] [Google Scholar]
- [11].Flygare J, Sander B. The endocannabinoid system in cancer-potential therapeutic target? Semin Cancer Biol. 2008;18(3):176–89. doi: 10.1016/j.semcancer.2007.12.008. [DOI] [PubMed] [Google Scholar]
- [12].Melck D, De Petrocellis L, Orlando P, Bisogno T, Laezza C, Bifulco M, et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141(1):118–26. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- [13].Grimaldi C, Pisanti S, Laezza C, Malfitano AM, Santoro A, Vitale M, et al. Anandamide inhibits adhesion and migration of breast cancer cells. Exp Cell Res. 2006;312(4):363–73. doi: 10.1016/j.yexcr.2005.10.024. [DOI] [PubMed] [Google Scholar]
- [14].Laezza C, Pisanti S, Malfitano AM, Bifulco M. The anandamide analog, Met-F-AEA, controls human breast cancer cell migration via the RHOA/RHO kinase signaling pathway. Endocr Relat Cancer. 2008;15(4):965–74. doi: 10.1677/ERC-08-0030. [DOI] [PubMed] [Google Scholar]
- [15].Mimeault M, Pommery N, Wattez N, Bailly C, Henichart JP. Anti-proliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: implication of epidermal growth factor receptor down-regulation and ceramide production. Prostate. 2003;56(1):1–12. doi: 10.1002/pros.10190. [DOI] [PubMed] [Google Scholar]
- [16].Nithipatikom K, Endsley MP, Isbell MA, Falck JR, Iwamoto Y, Hillard CJ, et al. 2-Arachidonoylglycerol: A novel inhibitor of androgen-independent prostate cancer cell invasion. Cancer Res. 2004;64(24):8826–30. doi: 10.1158/0008-5472.CAN-04-3136. [DOI] [PubMed] [Google Scholar]
- [17].Endsley MP, Thill R, Choudhry I, Williams CL, Kajdacsy-Balla A, Campbell WB, et al. Expression and function of fatty acid amide hydrolase in prostate cancer. Int J Cancer. 2008;123(6):1318–26. doi: 10.1002/ijc.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ueda N, Puffenbarger RA, Yamamoto S, Deutsch DG. The fatty acid amide hydrolase (FAAH) Chem Phys Lipids. 2000;108(1-2):107–21. doi: 10.1016/s0009-3084(00)00190-0. [DOI] [PubMed] [Google Scholar]
- [19].Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, et al. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem. 1999;264(1):258–67. doi: 10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- [20].Bari M, Battista N, Fezza F, Gasperi V, Maccarrone M. New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev Med Chem. 2006;6(3):257–68. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- [21].Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99(16):10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121(1-2):149–58. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- [23].Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66(5):1260–4. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- [24].Hughes-Fulford M, Li CF, Boonyaratanakornkit J, Sayyah S. Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Res. 2006;66(3):1427–33. doi: 10.1158/0008-5472.CAN-05-0914. [DOI] [PubMed] [Google Scholar]
- [25].Endsley MP, Aggarwal N, Isbell MA, Wheelock CE, Hammock BD, Falck JR, et al. Diverse roles of 2-arachidonoylglycerol in invasion of prostate carcinoma cells: Location, hydrolysis and 12-lipoxygenase metabolism. Int J Cancer. 2007;121(5):984–91. doi: 10.1002/ijc.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191(2):125–35. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- [27].Fujita H, Koshida K, Keller ET, Takahashi Y, Yoshimito T, Namiki M, et al. Cyclooxygenase-2 promotes prostate cancer progression. Prostate. 2002;53(3):232–40. doi: 10.1002/pros.10152. [DOI] [PubMed] [Google Scholar]
- [28].Nie D, Che M, Grignon D, Tang K, Honn KV. Role of eicosanoids in prostate cancer progression. Cancer Metastasis Rev. 2001;20(3-4):195–206. doi: 10.1023/a:1015579209850. [DOI] [PubMed] [Google Scholar]
- [29].Pommery N, Taverne T, Telliez A, Goossens L, Charlier C, Pommery J, et al. New COX-2/5-LOX inhibitors: apoptosis-inducing agents potentially useful in prostate cancer chemotherapy. J Med Chem. 2004;47(25):6195–206. doi: 10.1021/jm0407761. [DOI] [PubMed] [Google Scholar]
- [30].Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs. 2010;11(1):51–62. [PubMed] [Google Scholar]
- [31].Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nithipatikom K, Endsley MP, Isbell MA, Wheelock CE, Hammock BD, Campbell WB. A new class of inhibitors of 2-arachidonoylglycerol hydrolysis and invasion of prostate cancer cells. Biochem Biophys Res Commun. 2005;332(4):1028–33. doi: 10.1016/j.bbrc.2005.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A. 2001;98(7):3662–5. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Appendino G, Ligresti A, Minassi A, Daddario N, Bisogno T, Di Marzo V. Homologues and isomers of noladin ether, a putative novel endocannabinoid: interaction with rat cannabinoid CB(1) receptors. Bioorg Med Chem Lett. 2003;13(1):43–6. doi: 10.1016/s0960-894x(02)00839-9. [DOI] [PubMed] [Google Scholar]
- [35].Fezza F, Bisogno T, Minassi A, Appendino G, Mechoulam R, Di Marzo V. Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002;513(2-3):294–8. doi: 10.1016/s0014-5793(02)02341-4. [DOI] [PubMed] [Google Scholar]
- [36].Laine K, Jarvinen K, Mechoulam R, Breuer A, Jarvinen T. Comparison of the enzymatic stability and intraocular pressure effects of 2-arachidonylglycerol and noladin ether, a novel putative endocannabinoid. Invest Ophthalmol Vis Sci. 2002;43(10):3216–22. [PubMed] [Google Scholar]
- [37].Suhara Y, Takayama H, Nakane S, Miyashita T, Waku K, Sugiura T. Synthesis and biological activities of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, and its metabolically stable ether-linked analogues. Chem Pharm Bull (Tokyo) 2000;48(7):903–7. doi: 10.1248/cpb.48.903. [DOI] [PubMed] [Google Scholar]
- [38].Steffens M, Schulze-Bonhage A, Surges R, Feuerstein TJ. Fatty acid amidohydrolase in human neocortex-activity in epileptic and non-epileptic brain tissue and inhibition by putative endocannabinoids. Neurosci Lett. 2005;385(1):13–7. doi: 10.1016/j.neulet.2005.05.019. [DOI] [PubMed] [Google Scholar]
- [39].Oka S, Tsuchie A, Tokumura A, Muramatsu M, Suhara Y, Takayama H, et al. Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J Neurochem. 2003;85(6):1374–81. doi: 10.1046/j.1471-4159.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- [40].Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem. 2007;360(2):216–26. doi: 10.1016/j.ab.2006.10.039. [DOI] [PubMed] [Google Scholar]
- [41].Steffens M, Zentner J, Honegger J, Feuerstein TJ. Binding affinity and agonist activity of putative endogenous cannabinoids at the human neocortical CB1 receptor. Biochem Pharmacol. 2005;69(1):169–78. doi: 10.1016/j.bcp.2004.08.033. [DOI] [PubMed] [Google Scholar]
- [42].Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J Pharmacol Exp Ther. 2005;314(2):868–75. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- [43].Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70(1):101–11. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- [44].Lea MA, Sura M, Desbordes C. Inhibition of cell proliferation by potential peroxisome proliferator-activated receptor (PPAR) gamma agonists and antagonists. Anticancer Res. 2004;24(5A):2765–71. [PubMed] [Google Scholar]
- [45].Annicotte JS, Iankova I, Miard S, Fritz V, Sarruf D, Abella A, et al. Peroxisome proliferator-activated receptor gamma regulates E-cadherin expression and inhibits growth and invasion of prostate cancer. Mol Cell Biol. 2006;26(20):7561–74. doi: 10.1128/MCB.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pitulis N, Papageorgiou E, Tenta R, Lembessis P, Koutsilieris M. IL-6 and PPARgamma signalling in human PC-3 prostate cancer cells. Anticancer Res. 2009;29(6):2331–7. [PubMed] [Google Scholar]
- [47].Campbell SE, Musich PR, Whaley SG, Stimmel JB, Leesnitzer LM, Dessus-Babus S, et al. Gamma tocopherol upregulates the expression of 15-S-HETE and induces growth arrest through a PPAR gamma-dependent mechanism in PC-3 human prostate cancer cells. Nutr Cancer. 2009;61(5):649–62. doi: 10.1080/01635580902825654. [DOI] [PubMed] [Google Scholar]
- [48].Dahlman JM, Wang J, Bakkar N, Guttridge DC. The RelA/p65 subunit of NF-kappaB specifically regulates cyclin D1 protein stability: implications for cell cycle withdrawal and skeletal myogenesis. J Cell Biochem. 2009;106(1):42–51. doi: 10.1002/jcb.21976. [DOI] [PubMed] [Google Scholar]
- [49].Son DJ, Park MH, Chae SJ, Moon SO, Lee JW, Song HS, et al. Inhibitory effect of snake venom toxin from Vipera lebetina turanica on hormone-refractory human prostate cancer cell growth: induction of apoptosis through inactivation of nuclear factor kappaB. Mol Cancer Ther. 2007;6(2):675–83. doi: 10.1158/1535-7163.MCT-06-0328. [DOI] [PubMed] [Google Scholar]
- [50].Wheelock CE, Severson TF, Hammock BD. Synthesis of new carboxylesterase inhibitors and evaluation of potency and water solubility. Chem Res Toxicol. 2001;14(12):1563–72. doi: 10.1021/tx015508+. [DOI] [PubMed] [Google Scholar]
- [51].Wheelock CE, Colvin ME, Uemura I, Olmstead MM, Sanborn JR, Nakagawa Y, et al. Use of ab initio calculations to predict the biological potency of carboxylesterase inhibitors. J Med Chem. 2002;45(25):5576–93. doi: 10.1021/jm020072w. [DOI] [PubMed] [Google Scholar]
- [52].Edgemond WS, Greenberg MJ, McGinley PJ, Muthian S, Campbell WB, Hillard CJ. Synthesis and characterization of diazomethylarachidonyl ketone: an irreversible inhibitor of N-arachidonylethanolamine amidohydrolase. J Pharmacol Exp Ther. 1998;286(1):184–90. [PubMed] [Google Scholar]
- [53].Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem. 2003;278(43):42660–7. doi: 10.1074/jbc.M304984200. [DOI] [PubMed] [Google Scholar]
- [54].Kue PF, Daaka Y. Essential role for G proteins in prostate cancer cell growth and signaling. J Urol. 2000;164(6):2162–7. [PubMed] [Google Scholar]
- [55].Lyons-Darden T, Daaka Y. Requirement for G proteins in insulin-like growth factor-I-induced growth of prostate cells. J Mol Endocrinol. 2004;33(1):165–73. doi: 10.1677/jme.0.0330165. [DOI] [PubMed] [Google Scholar]
- [56].Price RD, Oe T, Yamaji T, Matsuoka N. A simple, flexible, nonfluorescent system for the automated screening of neurite outgrowth. J Biomol Screen. 2006;11(2):155–64. doi: 10.1177/1087057105283344. [DOI] [PubMed] [Google Scholar]
- [57].Tew GW, Lorimer EL, Berg TJ, Zhi H, Li R, Williams CL. SmgGDS Regulates Cell Proliferation, Migration, and NF-{kappa}B Transcriptional Activity in Non-small Cell Lung Carcinoma. J Biol Chem. 2008;283(2):963–76. doi: 10.1074/jbc.M707526200. [DOI] [PubMed] [Google Scholar]
- [58].Nagata D, Yoshihiro H, Nakanishi M, Naruyama H, Okada S, Ando R, et al. Peroxisome proliferator-activated receptor-gamma and growth inhibition by its ligands in prostate cancer. Cancer Detect Prev. 2008;32(3):259–66. doi: 10.1016/j.cdp.2008.05.008. [DOI] [PubMed] [Google Scholar]
- [59].Hughes-Fulford M, Chen Y, Tjandrawinata RR. Fatty acid regulates gene expression and growth of human prostate cancer PC-3 cells. Carcinogenesis. 2001;22(5):701–7. doi: 10.1093/carcin/22.5.701. [DOI] [PubMed] [Google Scholar]
- [60].Duncan M, Millns P, Smart D, Wright JE, Kendall DA, Ralevic V. Noladin ether, a putative endocannabinoid, attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed via a non-CB1/CB2 G(i/o) linked receptor. Br J Pharmacol. 2004;142(3):509–18. doi: 10.1038/sj.bjp.0705789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1) Cell Cycle. 2007;6(23):2953–61. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- [62].Kanda H, Ishii K, Ogura Y, Imamura T, Kanai M, Arima K, et al. Naftopidil, a selective alpha-1 adrenoceptor antagonist, inhibits growth of human prostate cancer cells by G1 cell cycle arrest. Int J Cancer. 2008;122(2):444–51. doi: 10.1002/ijc.23095. [DOI] [PubMed] [Google Scholar]
- [63].Takahashi N, Watanabe Y, Maitani Y, Yamauchi T, Higashiyama K, Ohba T. p-Dodecylaminophenol derived from the synthetic retinoid, fenretinide: antitumor efficacy in vitro and in vivo against human prostate cancer and mechanism of action. Int J Cancer. 2008;122(3):689–98. doi: 10.1002/ijc.23154. [DOI] [PubMed] [Google Scholar]
- [64].Mahadevan A, Razdan RK. Further advances in the synthesis of endocannabinoid-related ligands. Aaps J. 2005;7(2):E496–502. doi: 10.1208/aapsj070250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Juntunen J, Vepsalainen J, Niemi R, Laine K, Jarvinen T. Synthesis, in vitro evaluation, and intraocular pressure effects of water-soluble prodrugs of endocannabinoid noladin ether. J Med Chem. 2003;46(23):5083–6. doi: 10.1021/jm030877j. [DOI] [PubMed] [Google Scholar]