Abstract
OBJECTIVE
We examined how women with osteoarthritis naturally use activity pacing and how pacing relates to symptoms and physical activity within daily routines.
METHOD
Thirty women with knee or hip osteoarthritis (mean age = 63.8 ± 6.9) wore an actigraph accelerometer and repeatedly reported activity pacing, pain, and fatigue. Using the median split, symptom patterns were compared for low and high pacers. The relationship between activity pacing and physical activity was also examined.
RESULTS
Activity pacing was low (1.4 ± 0.9); pain and fatigue were mild (1.0 ± 0.7 and 1.1 ± 0.7, respectively). When compared with low pacers, high pacers had more severe, escalating symptoms. Activity pacing was related to lower physical activity (β = −28.14, SE = 6.24), t (586) = −4.51, p = .0001.
CONCLUSION
Pain, fatigue, and activity pacing use varied depending on average activity pacing level. High pacers may benefit from interventions to manage daily symptoms.
Keywords: knee and hip osteoarthritis, measurement, older adults, symptoms
Osteoarthritis is a leading cause of disability in older adults. Older adults with lower-extremity osteoarthritis (LE–OA) commonly attribute problems with their mobility and daily activity performance to symptoms such as pain (Levielle, Fried, & Guralnik, 2002). In response to symptoms, older adults with LE–OA report making many behavioral adaptations (such as resting or slowing down) to perform daily activities (Gignac, Cott, & Badley, 2000; Klinger, Spaulding, Polatajko, MacKinnon, & Miller, 1999). Despite the association between reported adaptations and OA pain (Fried, Young, Rubin, & Bandeen-Roche, 2001; Klinger et al., 1999), little is known about how adaptations and symptoms occur within the context of daily life.
Studies that tap into daily life experience use a “daily process” approach in which people respond to questions up to several times a day over a set time period. Participants may keep a daily diary and are often prompted by a device (e.g., Palm Pilot, watch with audible timer) to respond at a particular time. This method of prompting, called ecological momentary assessment (EMA), is more advantageous than self-report measures, which are burdensome and involve recall (over days, weeks, or months) that likely lead to underreporting (Gignac et al., 2000) and biased symptom reports (Stone et al., 1999). Results of daily process studies show many individual differences in the experience of symptoms (Affleck, Urrows, Tennen, Higgins, & Abeles, 1996) as well as factors that influence symptoms such as gender (Affleck et al., 1999), pain-related fear (Roelefs, Peters, Patijn, Schouten, & Vlaeyen, 2004), and sleep quality (Affleck et al., 1996).
Understanding behavioral adaptations and symptoms within daily routines is particularly relevant to occupational therapy and can help refine treatment strategies for people with problematic OA symptoms. Only one daily process study on the use of behavioral adaptations among people with OA was found. In that study, women attempted to reduce their pain 80% of the days examined and used techniques such as distraction and relaxation (Affleck et al., 1999). No studies could be found that examined the use of activity pacing in daily life.
Activity Pacing
Activity pacing, defined as going slower and taking breaks or breaking activities up into smaller pieces, is a key adaptation strategy taught in many chronic pain programs and one of the most highly endorsed pain management strategies among occupational therapists (Brown, 2002). In a recent survey, 98% of occupational therapists working in the area of chronic pain reported teaching activity pacing (Birkholz, Aylwin, & Harman, 2004b). Instruction in activity pacing is thought to help alter inefficient activity patterns such as being overactive, resulting in prolonged inactive periods, or being underactive, which can lead to reduced physical capacities that increase disability (Birkholz, Aylwin, & Harman, 2004a). Little is known about the activity patterns of adults with OA or how they naturally pace activities within their daily routines.
We examined the use of activity pacing and symptoms of pain and fatigue over time and measured physical activity continuously using an EMA device (e.g., actigraph watch). The purpose of this study was to examine the use of activity pacing among women with symptomatic LE–OA and then to determine whether there was a relationship between the use of activity pacing and physical activity within daily routines. We hypothesized that increased use of activity pacing would be related to a decrease in objective physical activity.
Methods
Participants
Potential participants either responded to fliers or were contacted as members of the Research Participant Registry at the Claude D. Pepper Center at the University of Michigan. Adults determined to be eligible from telephone screening signed informed consent forms approved by the University of Michigan Institutional Review Board and underwent x-rays of both hips and knees to determine the presence and severity of OA. Costs for the x-ray procedure were covered by grant sponsorship, and participants were compensated for their time involved in the study. Participants were included if they were female, ages 55 to 80, had a score of ≥24 on the Mini-Mental Status Exam (Folstein, Folstein, & McHugh, 1975), were English speaking, and could operate the acti-graph watch used in the study. Participants needed to have pain in at least one hip or knee joint for 3 months or more that interfered with daily activities and that was of at least mild severity as demonstrated by a score of 5 or greater on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC; Topp, Woolley, Khuder, & Kahaleh, 2002). They also needed to have radiographic evidence of OA (Kellgren Lawrence score of 2 or greater) in that joint (Kellgren & Lawrence, 1957). Participants were excluded if they were nonambulatory, had medical conditions or problems (other than OA) that interfered with daily activities (such as cardiopulmonary problems, neurological conditions, autoimmune diseases), or had a joint replacement or hip or knee surgery in the previous 6 months. Of 68 participants screened, 15 were excluded by telephone for not having enough OA pain or having other conditions that interfered with activity performance; 18 were excluded by x-ray (i.e., not having a Kellgren Lawrence score of ≥2); and 5 chose not to participate before the x-ray screening. The remaining sample had 30 women.
Procedure
Data were obtained from two laboratory visits and at home. During the first visit, a trained research assistant administered self-report measures and physical performance tests. The participant was then instructed in the use of the acti-graph watch and how to enter data for the home period. Participants wore the watch for 1 week (from Sunday evening to Saturday morning) and were instructed to keep the watch on at all times except when bathing, showering, or swimming. During weekdays, participants entered responses into the watch about activity pacing, pain, and fatigue at prespecified time points throughout the day. They also kept a log book to record their daily activities as well as when they woke up and went to bed, and any time they took off the watch. The second visit involved discussion with the research assistant about the watch wear period and strategies used to cope with OA symptoms.
Measures
Measures Collected Using the EMA Device
The activity pacing scale is composed of two items and was modified from the activity pacing subscale of the Chronic Pain Coping Inventory (Nielson, Jensen, & Hill, 2001). Because this study involved repeated administration of the scale within a day and over several days, a reduced set of items and a time referent in the item stem were needed. Participants responded to the following questions: During the past few hours … (1) have you gone slower and taken breaks to do activities, and (2) did you break up activities into manageable pieces to do them? These items were rated on a scale in which 0 = not at all and 4 = always. Activity pacing was assessed at five time points: 2 hr after usual wake-up time (mid-morning), 4-hr intervals throughout the day (afternoon, late afternoon, evening), and 30 min before bed. In a subsample of this cohort, internal consistency of this two-item scale was .71, which is considered adequate (Bland & Altman, 1997). Symptoms of pain and fatigue were each rated on a scale ranging from 0 meaning not at all to 4 meaning extremely severe. Participants rated their symptoms at the same time as they rated their level of activity pacing with the addition of a sixth time, when they woke up each day.
Physical activity was measured by the EMA device, an actigraph watch that is a wrist-worn accelerometer (Actiwatch-S, Mini Mitter Co., Bend, OR). The wrist-worn Actiwatch-S was found to discriminate between peak and high-level activity among people with fibromyalgia and controls (Kop et al., 2005). It also has demonstrated excellent interdevice reliability mounted at the wrist (r = .98) and has established preliminary criterion validity among a sample with mild chronic pain (Gironda, Lloyd, Clark, & Walker, 2007). Changes in acceleration were recorded into the watch as activity counts, saved every 15 s, and then averaged each minute. The resulting unit of measurement is activity counts per minute. Higher activity counts reflect participation in higher-intensity activities (Swartz et al., 2000). Activity levels over each day as well as within the day (e.g., midmorning, afternoon, late afternoon, and evening) were calculated first by averaging all activity counts for a given time interval each day and then by averaging the resulting values over the entire period of watch wear (Kop et al., 2005). Because participants wore the watch continuously for 5 days, it was necessary to establish participants’ wake-up and bed times. A previously established algorithm was used to derive actual wake-up and bed time for each participant each day (Kop et al., 2005).
Other Self-Report and Performance Measures
Medication use was assessed repeatedly throughout the study to examine whether it was a strategy used to deal with symptoms and whether this usage affected activity pacing. Daily medication use (type and time taken) for OA symptoms was listed in the daily logs. Medications were tallied, and the summed score was used as a covariate in the multivariate analysis. Pain, stiffness, and physical disability at baseline were assessed using the WOMAC (Bellamy, Buchanan, Goldsmith, Campbell, & Stitt, 1988). Functional mobility was measured using the Timed Up and Go Test (Podsiadlo & Richardson, 1991). In this test, a participant gets up out of a chair, walks 3 m, and returns to a seated position while being timed. An average of three trials was used in the analysis.
Data Analysis
For each participant, aggregate levels of activity pacing, pain, and fatigue were determined by averaging all responses over the 5 days. To examine individual patterns throughout an average day, daily levels of activity pacing, pain, and fatigue were calculated by averaging scores over the 5 days at each time point. Because so little is known about the use of activity pacing and what an appropriate threshold is for low versus high activity pacing, we used a median split of the data to compare low pacers and high pacers. Demographics, physical factors, symptoms, and physical activity of low and high pacers were compared using t tests.
The relation between activity pacing and physical activity over the 5-day period was examined using a hierarchical linear regression model. This method is commonly used with repeated measures data and takes into account different levels of variation such as that which occurs both within and between participants (Singer, 1998). In the model, physical activity was the dependent variable; activity pacing was an independent variable; and the covariates were age, self-reported physical disability (WOMAC sub-scale), functional mobility (Timed Up and Go Test score), and daily pain medication use. A between-subjects factor of activity pacing was included to examine whether the relationship between activity pacing and physical activity varied by participant’s use of pacing (e.g., an aggregate of activity pacing at all time points).
Results
Of the 30 participants (mean age = 63.8 ± 6.9), 90% were White and 10% were African American. Eighty-seven percent of participants had attended at least some college, and 40% of the sample was currently working at least part-time. Twenty-seven participants had knee OA, and three had hip OA. Seventeen percent of participants (n = 5) took regular prescription medication (particularly nonsteroidal anti-inflammatory drugs) for OA symptoms. During the home period, 50% of the sample took over-the-counter medication (such as acetaminophen or ibuprofen) to relieve pain at least once. On the WOMAC, participants reported mild pain, mild-to-moderate stiffness, and mild physical disability because of arthritis (Ms = 5.0, 3.0, and 18.1, SDs = 3.2, 2.1, and 14.1, respectively).
Activity Pacing, Pain, and Fatigue in Daily Routines
Frequency of activity pacing (aggregated time points and averaged across participants) was low (1.4 ± 0.9), and severity of pain and fatigue was mild (1.0 ± 0.7 and 1.1 ± 0.7, respectively). Table 1 shows differences between low and high pacers. Low pacers did not differ significantly from high pacers in age, body mass index, physical disability, or functional mobility. Over the 5-day period, however, high pacers had a higher average level of morning fatigue (p = .05) compared with low pacers. There appeared to be a trend of higher average levels of fatigue and morning pain found among high pacers compared with low pacers; however, the results were not statistically significant (p = .06; see Table 2).
Table 1.
Characteristics of Low Pacers and High Pacers
| Mean (SD) |
||
|---|---|---|
| Low Pacers (n = 15) | High Pacers (n = 15) | |
| Age | 63.7 (6.3) | 63.9 (7.2) |
| Body mass index | 29.1 (7.1) | 31.0 (5.2) |
| WOMAC Physical Disability scale | 15.0 (12.0) | 21.1 (15.0) |
| Timed Up and Go Test | 9.2 (2.1) | 9.5 (1.5) |
Note. Independent sample t tests were performed, and all were nonsignificant (p > .05). WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Table 2.
Aggregate Pain, Fatigue, and Physical Activity Over 5 Days for Low Pacers and High Pacers
| Mean (SD) |
p | ||
|---|---|---|---|
| Low Pacers | High Pacers | ||
| Pain | 0.7 (0.5) | 1.2 (0.8) | .08 |
| a.m. pain | 1.2 (0.7) | 1.7 (0.7) | .06 |
| p.m. pain | 1.0 (0.7) | 1.3 (0.7) | .26 |
| Fatigue | 0.7 (0.5) | 1.2 (0.7) | .06 |
| a.m. fatigue | 0.7 (0.6) | 1.3 (0.9) | .05 |
| p.m. fatigue | 0.7 (0.6) | 0.9 (0.6) | .26 |
| Physical activitya | 377.6 (121.7) | 326.2 (94.1) | .20 |
Note. For all comparisons, independent t tests were performed. The a.m. variables of pain and fatigue were calculated by averaging the first three time points in a day for each symptom and aggregating over the 5 days. For p.m. variables, the last two time points in the day were averaged and aggregated.
Physical activity was measured by the actigraph watch in activity counts per minute. See the “Methods” section for exact calculation.
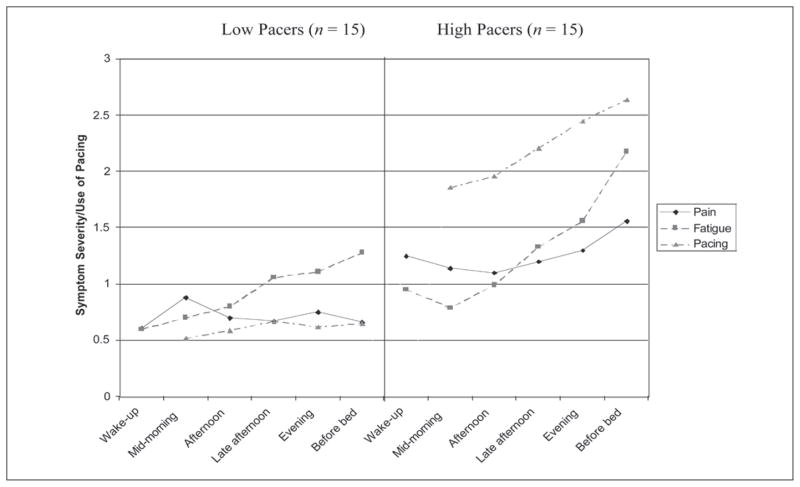
Patterns of pain, fatigue, and activity pacing were analyzed with respect to time of day for low and high pacers. Figure 1 shows differences in pain and fatigue as well as how activity pacing was used throughout the 5-day period. Although low pacers had pain that tended to decrease over the day, high pacers had an increase of pain. For both groups, fatigue tended to increase over the day; however, a more marked increase was seen in high pacers. Increased activity pacing over the day was found among the high pacers, which appears to occur in conjunction with an escalation in pain and fatigue. This pattern was not found among low pacers.
Figure 1.
Pain, fatigue, and activity pacing by time of day in low and high pacers.
Activity Pacing in Relationship to Physical Activity
The relationship between the use of activity pacing and physical activity was examined, controlling for other factors in a hierarchical linear regression model (Table 3). In this model, 41.1% of the variability in physical activity was between subjects, the rest was within-subject variability (58.9%). As expected, a negative relationship between activity pacing and physical activity was found. The increased use of activity pacing was associated with lower physical activity (β = −28.14, SE = 6.24, t[586] = −4.51, p = .0001).
Table 3.
Hierarchical Linear Regression Model Showing the Relationship Between Activity Pacing and Physical Activity
| β | SE | df | t | p | |
|---|---|---|---|---|---|
| Activity pacing scale | −28.14 | 6.24 | 586 | −4.51 | .0001 |
| Between-subjects factor for activity pacinga | −36.45 | 22.92 | 25 | −1.59 | .13 |
| Age | −1.53 | 3.35 | 25 | −.46 | .65 |
| WOMAC Physical Disability scale | 1.22 | 1.69 | 25 | .72 | .48 |
| Timed Up and Go Test | −19.50 | 10.41 | 25 | −1.87 | .07 |
| Daily pain medication use | −0.90 | 18.54 | 586 | −.05 | .96 |
Note. In this model, physical activity was the dependent variable, activity pacing was an independent variable, and the other variables were covariates. WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
This factor was included to determine whether the relationship between activity pacing and physical activity varied by a participant’s use of pacing.
Discussion and Summary
The purpose of this pilot study was to examine how women with LE–OA adapt to pain and fatigue symptoms within their daily routines and how that adaptation affects physical activity. Activity pacing is a core strategy taught by occupational therapists (Birkholz et al., 2004b), and studying the natural use of activity pacing within daily routines was thought to be a necessary step in understanding how to better develop and refine interventions for people with LE–OA.
Comparison of low pacers and high pacers revealed important group differences that could be particularly useful in intervention development. Although no demographic or physical factors differentiated these two groups, high pacers tended to have more severe symptoms. High pacers had an escalation of symptoms throughout a day, whereas low pacers did not have an increase in pain and had a less steep escalation in fatigue. Even though all participants reported problematic pain that interfered with daily activities, the findings suggest that high pacers are more actively dealing with symptoms. Asking about the use of activity pacing may be one way to find the target group who would most benefit from intervention to deal with symptoms.
The use of activity pacing was associated with less physical activity, which supports our hypothesis. We thought that the natural use of activity pacing would be associated with decreased activity, that is, that reports of going slower, taking breaks, and breaking activities into smaller pieces would be reflected in lower levels of physical activity. This relationship remained after controlling for other factors. It is not clear whether this relationship would be found among women with LE–OA who receive instruction in activity pacing. Future studies will need to examine whether formal instruction would lead to increased physical activity.
Among high pacers, the use of activity pacing increased steadily throughout the day, along with pain and fatigue, suggesting that activity pacing may be a reaction to having symptoms. Pain and fatigue might then be more severe without the use of pacing. Activity pacing is typically taught to people with chronic pain as a strategy for activity engagement (Birkholtz et al., 2004a), but it is not known whether activity pacing has a direct effect on pain and fatigue. If instruction in activity pacing has this effect, intervention studies using EMA can help determine whether activity pacing earlier in the day is associated with lower levels of pain and fatigue later in the day.
Because this sample was primarily White and well educated, findings are limited in their generalizability to a more diverse population. Second, this sample mainly reported mild symptoms. The relationship between activity pacing and physical activity may be different for women who typically experience moderate or severe symptoms. Third, the 5-point rating scales of activity pacing, pain, and fatigue may limit comparability across studies that have used scales ranging from 0 to 10. Fourth, although the two-item activity pacing scale used in this study had adequate internal consistency, stronger results may have been achieved by using fewer administrations and having more items on the scale. The choice of more frequent administrations (five times a day) was preferable in this pilot study to more fully examine activity pacing patterns in daily routines.
The sample size in this study was small (N = 30), which limits the ability to obtain precise estimates of model parameters and to know the stability of our internal consistency estimates for the activity pacing scale. On the basis of this pilot study, it would be useful to replicate these analyses in a larger sample. Strengths of the study include the ability to tap into participants’ experiences during their daily routines and the objective assessment of physical activity using actigraphy, which to our knowledge has not yet been done in adults with LE–OA.
In summary, this was a pilot study to examine the use of activity pacing, pain, fatigue, and physical activity in women with LE–OA. Pain, fatigue, and use of activity pacing were low on average but varied depending on level of average activity pacing. High pacers in particular may benefit from targeted interventions to better manage their escalating daily symptoms. Thus, ascertaining the frequency of use of activity pacing may be important to determine a target sample for intervention.
Acknowledgments
The work from this study was supported by the American College of Rheumatology Research and Education Foundation (Health Professional Investigator Award) and in part by the National Center for Research Resources (M01–RR000042). Susan L. Murphy is a recipient of a K01 Mentored Research Scientist Career Development Award from the National Center for Medical Rehabilitation Research (HD045293), and Neil B. Alexander is a recipient of a K24 Mid-Career Investigator Award in Patient-Oriented Research (AG109675) from the National Institute on Aging.
Contributor Information
Susan L. Murphy, Email: sumurphy@umich.edu, Division of Geriatric Medicine and Institute of Gerontology, Department of Internal Medicine, University of Michigan, Ann Arbor, and Research Health Science Specialist, Geriatric Research, Education and Clinical Center, Veterans Affairs Ann Arbor Health Care System, Ann Arbor, MI 48109-2007.
Dylan M. Smith, VA Center for Practice Management and Outcomes Research, Veterans Affairs Ann Arbor Healthcare System, Ann Arbor, MI, and Research Assistant Professor, Department of Internal Medicine, University of Michigan School of Medicine, Ann Arbor.
Neil B. Alexander, Division of Geriatric Medicine and Institute of Gerontology, Department of Internal Medicine, University of Michigan, and Director, Geriatric Research, Education and Clinical Center, Veterans Affairs Ann Arbor Health Care System, Ann Arbor, MI.
References
- Affleck G, Tennen H, Keefe FJ, Lefebvre JC, Kashikar-Zuck S, Wright K, et al. Everyday life with osteoarthritis or rheumatoid arthritis: Independent effects of disease and gender on daily pain, mood, and coping. Pain. 1999;83:601–609. doi: 10.1016/S0304-3959(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Journal of Rheumatology. 1988;15:1833–1840. [PubMed] [Google Scholar]
- Birkholtz M, Aylwin L, Harman RM. Activity pacing in chronic pain management: One aim, but which method? Part one: Introduction and literature review. British Journal of Occupational Therapy. 2004a;67:447–452. [Google Scholar]
- Birkholtz M, Aylwin L, Harman RM. Activity pacing in chronic pain management: One aim, but which method? Part two: National Activity Pacing Survey. British Journal of Occupational Therapy. 2004b;67:481–487. [Google Scholar]
- Bland JM, Altman DG. Statistics notes: Cronbach’s alpha. British Medical Journal. 1997;314:572. [Google Scholar]
- Brown CA. Occupational therapists’ beliefs regarding treatment options for people with chronic pain. British Journal of Occupational Therapy. 2002;65:398–404. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fried LP, Young Y, Rubin G, Bandeen-Roche K. Self-reported preclinical disability identifies older women with early declines in performance and early disease. Journal of Clinical Epidemiology. 2001;54:889–901. doi: 10.1016/s0895-4356(01)00357-2. [DOI] [PubMed] [Google Scholar]
- Gignac MAM, Cott C, Badley EM. Adaptation to chronic illness and disability and its relationship to perceptions of independence and dependence. Journal of Gerontology. 2000;55B:P362–P372. doi: 10.1093/geronb/55.6.p362. [DOI] [PubMed] [Google Scholar]
- Gironda RJ, Lloyd J, Clark ME, Walker RL. Preliminary evaluation of reliability and criterion validity of Actiwatch-Score. Journal of Rehabilitation Research and Development. 2007;44:223–230. doi: 10.1682/jrrd.2006.06.0058. [DOI] [PubMed] [Google Scholar]
- Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Annals of the Rheumatic Diseases. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger L, Spaulding SJ, Polatajko HJ, MacKinnon JR, Miller L. Chronic pain in the elderly: Occupational adaptation as a means of coping with osteoarthritis of the hip and/or knee. Clinical Journal of Pain. 1999;15:275–283. doi: 10.1097/00002508-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis and Rheumatism. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- Levielle SG, Fried LP, Guralnik JM. Disabling symptoms: What do older women report? Journal of General Internal Medicine. 2002;17:766–773. doi: 10.1046/j.1525-1497.2002.20229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson WR, Jensen MP, Hill ML. An activity pacing scale for the Chronic Pain Coping Inventory: Development in a sample of patients with fibromyalgia syndrome. Pain. 2001;89:111–115. doi: 10.1016/s0304-3959(00)00351-1. [DOI] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Roelefs J, Peters ML, Patijn J, Schouten EGW, Vlaeyen JWS. Electronic diary assessment of pain-related fear, attention to pain, and pain intensity in chronic low back pain patients. Pain. 2004;112:335–342. doi: 10.1016/j.pain.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23:323–355. [Google Scholar]
- Stone AA, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS, editors. The science of self-report: Implications for research and practice. New York: Erlbaum; 1999. [Google Scholar]
- Swartz AM, Strath SJ, Bassett DR, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Medicine and Science in Sports and Exercise. 2000;32:S450–S456. doi: 10.1097/00005768-200009001-00003. [DOI] [PubMed] [Google Scholar]
- Topp R, Woolley S, Khuder S, Kahaleh B. The effect of dynamic versus isometric resistance training on pain and functioning among adults with osteoarthritis of the knee. Archives of Physical Medicine and Rehabilitation. 2002;83:1187–1195. doi: 10.1053/apmr.2002.33988. [DOI] [PubMed] [Google Scholar]