Abstract
Many published articles have documented the impact of prostate-cancer treatment on sexual functioning in men treated for localized disease. Surprisingly, the literature on interventions to rehabilitate men’s sexual functioning is much more limited. In this article, we review the sexual-rehabilitation interventions for prostate-cancer patients and identify a number of common themes across interventions. We also identify areas where further research is needed and propose a conceptual model based on psychological and nursing theories and informed by the published research.
Keywords: prostate cancer, symptom management, psychosocial interventions, conceptual model
Prostate cancer is the most common cancer among American men, with an estimated 186,320 new cases expected in 2008. It represents 25% of all new cancer diagnoses in men, has an incidence comparable to that of breast cancer in women, and continues to disproportionately affect minority men.1 Patients with early, localized prostate cancer have a number of treatment options, including surgical removal of the prostate, radiation therapy (external beam or implantation of radioactive “seeds”), hormonal therapy, cryoablation, or expectant monitoring (“active surveillance”).2, 3 However most of these currently available treatments carry the risk of a number of treatment-related side-effects, including urinary incontinence, erectile dysfunction (ED), and others that vary, depending on the treatment received.4 The issue of treatment-related side-effects is particularly important because the prognosis of men with prostate cancer, relative to other cancers, is very good; and potential treatment-related symptoms can have important implications for health-related quality of life (HRQOL). Because early prostate cancer has along natural history, men who develop treatment-related side-effects experience them for years.5
Beginning in 1995 with the publication of the University of California Los Angeles CLA Prostate Cancer Index by Litwin et al, much has been learned about HRQOL in men treated for localized prostate cancer.4 Many papers have been published from large disease registries such as the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE™) study, a 13,000-man national study primarily drawn from community urology practices6 and the Prostate Cancer Outcomes Study (PCOS), a study that obtained follow-up HRQOL data from men who were part of the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program.6, 7 A large number of publications from smaller studies also have documented the changes in HRQOL experienced by men treated for localized prostate cancer.8-10 Recent reviews have summarized the patterns in HRQOL over time.11, 12
The recent Institute of Medicine (IOM) report, From Cancer Patient to Survivor: Lost in Transition,13 highlights the growing number of persons now living far beyond treatment for their cancer and the particular needs of cancer survivors for research and interventions that can improve symptom management and other aspects of quality of life. In particular, the IOM report recommends “intervention for consequences of cancer and its treatment, for example: medical problems such as lymphedema and sexual dysfunction; symptoms, including pain and fatigue; psychological distress experienced by cancer survivors, and their caregivers.”13 Among men with localized prostate cancer, the number of descriptive studies carried out has grown quite large and documents the ongoing burden of treatment-related symptoms; however, the literature contains relatively few intervention studies.14
Early general interventions for men with prostate cancer have included support-group programs,15 diet and lifestyle interventions,16-18 cognitive-behavioral stress-management programs,19 psychoeducational groups,9 nurse case management,20 and uncertainty management.21 In a study of the unmet information needs for men with prostate cancer, Boberg and colleagues found the greatest need for improvement in prostate-cancer education programs related to treatment-related symptoms and cancer recurrence.22 Among the published intervention studies, most have not provided specific information about managing treatment-related symptoms that are an important concern of men treated for localized disease and that address the IOM recommendations. The purpose of this article was to identify and briefly review published reports of sexual-rehabilitation and symptom-management interventions for men with localized prostate cancer, using PubMed and the recently published Cochrane reports on interventions for ED and sexual dysfunction after cancer treatment,23, 24 that sought to manage treatment-related side-effects or reduce the level of sexual-symptom distress or bother.
General interventions that reported sexual rehabilitation outcomes
Uncertainty Management
One of the earliest interventions that included sexual rehabilitation after prostate cancer was conducted by Mishel and colleagues (Table 1) at the University of North Carolina School of Nursing. The program was delivered by trained nurse educators over the phone and focused on psychological outcomes, such as problem-solving, cognitive reframing, cancer knowledge, and patient-provider communications, as well as disease-specific outcomes that included symptom distress, number of symptoms, urinary and sexual functioning, and satisfaction with sexual functioning.21 Participants were assigned to 1 of 3 groups: intervention for prostate-cancer survivor alone, intervention for prostate-cancer survivor alone plus modified intervention for a family member, or a usual-care control condition. The intervention included both techniques to assess and reduce uncertainty about prostate cancer, as well as didactic information about various concerns, including symptom management. The intervention was offered in weekly telephone calls over 8 consecutive weeks. Most intervention effects were from baseline to 4 months. Men in the 2 uncertainty-management arms reported significantly better scores on the cognitive reframing and problem-solving scales at 4 months than men in the control arm, but this effect did not hold up at the 7-month assessment. As would be expected, given what is known in the prostate-cancer-symptom literature, men in all 3 groups reported fewer symptoms over time. A significant difference in urinary incontinence and satisfaction with sexual functioning was seen at 4 months between men in the 2 intervention groups combined vs. control-group men. However, no significant differences between groups were found in cancer knowledge, patient-provider communication, or erectile functioning.21
Table 1.
Psychosocial interventions for sexual rehabilitation after treatment for localized prostate cancer
| Author | Publication year |
Number of participants |
Randomized | Intervention type | Partner included |
Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Mishel et al21 |
2002 | 239 | Yes | Psychoeducational telephone sessions with patient and designated person |
Patient could designate person to be included in intervention (i.e., partner, family member, etc.) |
SDS, SWOG QOL, non- standard urinary function, and sexual function and satisfaction questions |
Significant improvement in sexual satisfaction, trend toward interaction between sexual functioning and ethnicity |
| Lepore et al9 |
2003 | 250 | Yes | 6 educational sessions vs. 6 educational sessions plus discussion group |
No | PCI, SF-36 | Sexual bother reduced for men in the education- plus-discussion arm |
| Maliski et al26 |
2004 | 7 nurse case managers’ notes on 30 patients |
No | Case management | No | Not specified | Not specified |
| Weber et al27 |
2004 | 30 | Yes | In-person peer support |
No | PCI, GDS | Significantly less sexual bother in treatment group participants at follow-up |
| Canada et al30 |
2005 | 51 couples | Yes | 4 sessions of psychoeducation for patient and partner |
Some | IIEF, DAS, FSFI, PCI, SF- 36, BSI |
Significant improvements for treatment men in all IIEF domains and for treatment women in all FSFI domains |
| Giesler et al31 |
2005 | 99 | Yes | Monthly sessions (2 in person, 4 by telephone) with patient and partner |
Some | PC-QoL, DAS, CES-D, SF-36 |
Significant gains in sexual functioning and significant reductions in how much sexual dysfunction limited role activities at 7 and 12 months post-treatment |
| Titta et al32 |
2006 | 57 | Yes | Short-term psychodynamic therapy in person and on the phone |
Yes | IIEF | Significant improvements in erectile functioning over time for both groups but men in the sexual counseling plus injection group reported significantly better erectile functioning at 18 month follow-up |
| Molton et al33 |
2008 | 101 | Yes | 10-week cognitive behavioral stress- management intervention |
No | PCI | Men with greater interpersonal sensitivity reported greater increases in sexual functioning than men lower on this personality characteristic |
This study included information about managing symptoms, such as sexual functioning. However, data were collected before the introduction of sildenafil and similar oral medications and focused on facilitating insertive sexual practices, mainly through the use of mechanical devices that helped participants develop erections. One reason participants who received the intervention did not show significant improvements in sexual functioning is that they also reported being troubled by the intrusiveness of the erectile aids. Another important lesson from the Mishel et al study is that symptom-management education may be more effective if it is available over an extended period (Mishel and her colleagues offered participants telephone sessions over 8 consecutive weeks). This is an important distinction as symptoms vary over time, based on the type of treatment selected.25
Psychoeducational Groups
Another intervention that focused on disease-specific outcomes, as well as general HRQOL, randomized men to 1 of 3 groups: education, education plus discussion, or usual care.9 Education sessions were offered over 6 weeks and consisted of 1-hour lectures on prostate-cancer biology, epidemiology, follow-up treatment, symptom management, and partner issues. Men also received printed materials summarizing the lectures. Men in the education plus discussion group received an additional 45-minute discussion group after each lecture, facilitated by a male clinical psychologist. The study focused on general and disease-specific HRQOL, prostate-cancer knowledge, an index of positive health behaviors mentioned in the lectures, and a standard measure of depression. The education plus discussion intervention was generally more effective than the control condition. Men in the education conditions reported significantly better outcomes for prostate-cancer knowledge, physical functioning, positive health behaviors, and sexual bother. No significant differences were found in sexual or urinary functioning. There was a differential effect by educational level, with less educated men benefiting more from the intervention.9
Nurse Case-Manager Intervention
Maliski and colleagues provided an intervention for low-income men with prostate cancer, using a nurse case-manager format.26 They based their intervention on Self-Efficacy Theory, with the primary goal of empowering patients by increasing self-efficacy. Unlike other studies reviewed in this article, the seven nurse case managers were not providing patients with a standard intervention. As a first step toward developing a standardized intervention, Maliski et al used retrospective record review to examine characteristics of intervention strategies used by nurse case managers. The interventions employed by the nurse case managers included assessment of patient needs and the best strategy to meet those needs, facilitation of successful self-action by patients, advocacy for patients’ needs and concerns, coordination between care providers, teaching new knowledge and skills (including sexual rehabilitation), emotional support, collaborative problem-solving, and tracking patients. While this is an important and promising approach to describe intervention strategies currently in use, it would be difficult to replicate the intervention with other patients until it has been better specified.
Dyadic Support
Another nurse-led intervention study focused on the impact of dyadic support for men who had not attended a prostate-cancer support group. 27 This study is particularly important because many men are unwilling or unable to seek support for psychosocial or physical concerns from a support group.28, 29 Men who provided support to study participants were prostate-cancer survivors who had attended prostate-cancer support groups and were experienced in giving and receiving support about prostate- cancer concerns. The primary purpose of the intervention was to provide social support and lessen depression related to prostate-cancer treatment and side-effects. Results showed improvements in depression at 4 weeks for treatment-group subjects and significantly better self-efficacy at 8 weeks for treatment-group men, who also reported significantly less sexual bother and a trend toward better sexual functioning at 8 weeks than men in the control group.
Interventions that specifically target sexual rehabilitation
Psychoeducational Intervention for Men and Their Partners
Canada, Schover, and colleagues developed a 4-session psychoeducational intervention for men treated for localized prostate cancer with surgery or radiation and their partners.30 Each session included both didactic information about sexual side-effects, as well as behavioral homework, and skill-building exercises to improve couple communication and increase sexual stimulation. Men were randomized to attend either with their partner or alone. Partners in the patient-only condition still completed homework. Results were not affected by the partner’s attendance. Men completing the intervention experienced significant reductions in male overall distress and male global sexual function, and their female partners increased? global sexual function at 3 months. Over time, these improvements lessened. Men who completed the intervention reported increasing use of ED treatments over time.
Nurse-Led Computerized/Telephone Intervention
Another patient-education program led by nurses used a computerized questionnaire to determine which symptoms required intervention.31 Men and their partners were randomized to receive the intervention or standard care. Intervention sessions were scheduled monthly. The first 2 sessions were completed in person, and the 4 remaining sessions were completed by telephone. Sessions included both the patient and his partner. Sexual symptoms and side-effects were the most commonly reported concern among men in the study. Men in the treatment group were offered a videotape that offered models of how to discuss sexual problems with a partner. The nurse interventionist offered further information about communication skills and ED treatments. Treatment-group participants reported significant improvements in sexual functioning and bother at 4, 7, and 12 months post-baseline compared with control-group men.
Sexual Counseling for Erectile Rehabilitation
A unique study by Titta et al focused specifically on facilitating intracavernous injection therapy for ED in men treated for localized prostate cancer or muscle-invasive bladder cancer.32 Participants in this intervention were offered didactic information about how to use injections and randomized to receive either didactic information alone or didactic information plus telephone-based, short-term psychodynamic sexual counseling. Over time, men in the sexual-counseling group were significantly less likely to stop using injection therapy, even though rates of side-effects (e.g., pain or bruising) were similar in the 2 groups. Both groups had good response to injections and significantly improved erectile functioning scores over the 18-month follow-up period.
Group-Based Stress Management
On the basis of their work with patients living with other chronic illnesses, Molton and colleagues adapted their cognitive behavioral stress-management program to assist men treated for localized prostate cancer to improve their sexual functioning, while also improving psychosocial outcomes.33 The intervention included 10 group sessions encompassing both information about the restoration of sexual functioning, as well as relaxation exercises, and cognitive, behavioral, and interpersonal skills necessary to cope with life stressors. Sexual functioning was measured at baseline and post-intervention. Men receiving the intervention reported significantly better sexual functioning than men in the control condition, who completed a 4-hour workshop that taught the same stress- management skills as the longer intervention. Interpersonal sensitivity, described by Molton et al as “a problematic interpersonal style characterized by being ‘too sensitive’ to others, a tendency to perceive and elicit criticism, and a chronic perception of rejection and abandonment,” moderated the intervention effects. Post-hoc analysis showed that men with greater interpersonal sensitivity showed greater pre-post improvement in sexual functioning after completing the 10-week intervention, compared with men in the intervention group with lower interpersonal sensitivity.
Common Themes
Global vs. specific goals
A number of common themes emerged from the studies reviewed here. First, most interventions have focused primarily on psychosocial outcomes. For example, Mishel et al based their intervention on Uncertainty in Illness Theory and emphasized the reduction of uncertainty in patients and their partner or other designated person. Such programs have had mixed effects on sexual outcomes. Mishel et al reported that their intervention reduced sexual bother and improved urinary functioning, but they did not find significant differences in sexual functioning.21 Lepore and colleagues also reported significant decreases in sexual bother but no effect of their intervention on urinary or sexual functioning.9
Interventions involving sexual rehabilitation as the primary goal have had better results than interventions focusing on more general goals. Short-term changes in sexual functioning were reported by Canada et al30 and Molton et al 33. In the intervention by Canada et al, improvements in sexual functioning faded over time. Molton et al did not report long-term results. Both Titta et al32 and Giesler et al31 reported continued improvements in sexual functioning at 12 months or longer after baseline. Giesler at al offered their intervention over a 6-month period. It may be that offering patient education over an extended period increases the efficacy of the intervention.
Treatment implementation characteristics (intensity, frequency, and duration)
Adherence to ED treatment recommendations can be problematic. Many studies have reported the necessity of trying successive treatments to achieve better sexual functioning and the low rates of ED treatment use as time since prostate-cancer treatment increases34, 35 Thus, it was particularly gratifying that Canada et al reported higher rates of ED treatment use 6 months after enrollment in men who completed their intervention.30. In the case of the Titta et al study,32 the investigators focused on intracavernous injection. Most studies and general practice in ED clinics start with less invasive treatments (i.e., oral medications) and progress to move invasive treatments when patients fail oral medications. However, such an approach offers men the negative experiences of either obtaining no response to ED treatment or having a positive response that then diminishes over time. Such experiences could be problematic from the perspective of Self-Efficacy Theory because they may reduce a man’s confidence that ED treatment can be successful, Bandura’s concept of outcome efficacy.36 The intervention reported by Titta et al bypassed treatment approaches that may or may not be effective for men treated for localized prostate cancer, bypassing the possible negative consequences of ED-treatment failure.
Interventions varied in length, from 4 to 10 sessions spread over a month to 6 months. Although the length of the intervention was the same for all participants, Giesler et al described a program that was adaptive in that it focused on the symptoms most problematic for each participant. The particulars are unclear; however, the intervention described by Maliski et al also was tailored to each participant’s concerns. Other studies offered the same interventions to each participant. In times when resources are hard to find, longer interventions that address multiple symptoms (e.g., Molton et al) might be difficult to sustain. In other conditions, a stepped-care approach, motivated in part by cost considerations, has been shown to work.37-39 The approach by Giesler et al could be further adapted to adapt not just intervention content but also amount of intervention received to a patient’s needs for sexual rehabilitation.
Moderators of treatment
A participant’s response to an intervention is based not only on clinical characteristics such as whether he had a nerve-sparing prostatectomy or not but also on sociodemographic and psychological characteristics. Lepore et al found men with little education benefited more from the psychosocial intervention than men with more education. In particular, less-educated men showed significantly greater improvements in physical functioning, positive health behaviors, and sexual bother. Similarly, a study using CaPSURE data from 3 Veterans Affairs (VA) medical centers showed that men with limited formal education had poorer HRQOL over a 2-year period after prostate-cancer treatment, after controlling for other sociodemographic and clinical variables, than men with a high level of education.40 Thus, men with limited education may be in greater need of sexual-rehabilitation interventions than men with a high level of education, but it is important to ensure that patient-education materials are targeted to the appropriate reading level. Readability of materials and health literacy of the target audience are particularly a concern with men treated for prostate cancer. African American men, a group with a significantly high prevalence of prostate cancer, are over-represented among lower-health-literacy men with prostate cancer.41, 42
Low sexual desire may result from some prostate cancer treatments. The level of desire in men treated with surgery generally remains unchanged but is typically lower for men on androgen deprivation therapy.10, 43 Some studies also have reported reductions in level of desire for men treated with radiation.44 Low desire is difficult to treat, particularly for prostate cancer survivors because pharmacological treatment usually involves testosterone, which is controversial because of the possibility of cancer recurrence, and because low desire is sometimes mistaken for erectile dysfunction.45-47
Other personal characteristics also may be important predictors of response to sexual-rehabilitation programs or predictive of the need for intervention. Molton et al showed that men with high levels of interpersonal sensitivity reported greater gains in sexual functioning than men with low levels. Dahn et al reported significantly poorer HRQOL among men treated for prostate cancer who had high sexual desire and low erectile functioning than among men with low desire and low erectile functioning.8 Thus, another feasibility consideration may be targeting sexual-rehabilitation efforts to men who are the most likely to benefit from such an interventions (men with high interpersonal sensitivity) or men who may be most likely to report poor general HRQOL – those who have a substantial mismatch between level of desire and erectile ability. Though other areas of disease management have benefited from research trying to understand how to match patients to the optimal treatment, little has been done in cancer survivorship. The work by Molton et al and Dahn et al provides an excellent start in understanding who might benefit most from sexual-rehabilitation efforts.
Missing voices
Another area of sexual rehabilitation in need of further research and clinical efforts is in helping gay men reestablish their sexual lives after cancer. 48 No published data suggest that gay men are diagnosed with prostate cancer at any different rate than their heterosexual counterparts. Estimates suggest that approximately 5,000 gay men may be diagnosed with prostate cancer in a year and that 50,000 or more gay men are living with prostate cancer and its treatment-related side-effects.48 Prostate cancer affects gay men in many of the same ways as heterosexual men, but some of their concerns may differ. For example, the average of men diagnosed with prostate cancer is 70 years old.49 Thus, gay men with prostate cancer are typically older and may have more concerns about disclosing their sexuality to healthcare providers than younger gay men who may feel more comfortable being open about their sexuality.50 Such reluctance may also preclude gay men diagnosed with prostate cancer from involving their partners in healthcare decisions and treatment planning, in contrast with their heterosexual counterparts. Many gay men report that healthcare providers fail to ask about sexual orientation during initial consultations and assume they are heterosexual.50 Older gay men might be less likely than younger gay men to insist on including partners in the face of opposition or even lack of support for inclusion by healthcare providers.51 Gay men who are not partnered may lack not only a supportive partner but also other family-support systems enjoyed by heterosexual men. Such men also have the same challenges that single heterosexual men face when seeking sexual rehabilitation of not having a primary partner with whom they have long-established trust and affection.52 Support from peers also may be difficult to obtain. The number of support groups specifically for gay men with prostate cancer is limited to half a dozen in large cities. Gay men in other areas are forced to find a support group open to having gay men participate, remain closeted, rely on internet-based support groups, or be socially isolated.51 After treatment, some sexual functioning and dysfunction may be similar for gay and straight men; but gay men have some particular concerns. For example, sexual rehabilitation may be focused on creating erections rigid enough for vaginal penetration. However, anal penetration requires a greater degree of rigidity than vaginal intercourse.51, 53 Moreover, research on communication between gay men with prostate cancer and their partners is lacking to inform whether or not, and if so how, sexual risk taking for HIV infection changes with cancer treatment-related sexual dysfunction.
A Biopsychosocial Model of Cancer-Symptom Management
Keeping in mind the reports of sexual-rehabilitation interventions already in the literature, we now shift our focus to proposing a biopsychosocial model of prostate-cancer symptom management. It is based on two theoretical and conceptual frameworks, the University of California San Francisco Symptom Management Model (SMM) and Self-Efficacy Theory and informed by results from the studies reviewed above36, 54 The original SMM was published in 1994 and revised in 2001.55, 56 The symptom experience includes an individual’s perception of a symptom, evaluation of the meaning of the symptom, and response to a symptom. The symptom-management-strategies dimension includes the specifics of the intervention (i.e., what, when why, where, how much, and to whom). The outcomes dimension specifies that outcomes emerge from the symptom-management strategies, as well as from the symptom experience. The outcome dimension focuses on 8 factors (i.e., functional status, emotional status, self-care, costs, HRQOL, morbidity and co-morbidity, and mortality).
Self-Efficacy Theory has been used in numerous psychosocial interventions for patients with chronic diseases57-59 and is highly compatible with the SMM. It holds that 2 important determinants of behavior are outcome efficacy (confidence that an outcome can be affected) and self-efficacy (confidence that one can personally accomplish an outcome).36 In the SMM, self-efficacy would be considered part of the symptom experience; for example, men who repeatedly fail to have an erection sufficient for intercourse are likely to have low confidence in their ability to have an erection the next time they want to have sex. This experience of erectile dysfunction erodes their confidence and makes it less likely that they will attempt sex in the future. For some men, their erectile dysfunction limits showing any affection to their partner for fear their partner will want intercourse the man cannot provide.34.
Figure 1 shows our adaptation of the SMM and Self-Efficacy Theory to the specific case of prostate-cancer symptom management, including sexual rehabilitation. Interventions derived from this model should provide participants with the knowledge, skills, and resources that will lead to a set of mastery experiences as participants begin to manage their symptoms effectively, leading to stronger beliefs that prostate-cancer symptoms can be managed (Bandura’s concept of outcome efficacy) and that they are capable of managing their symptoms (self-efficacy). Staff providing rehabilitation interventions can help patients increase their sense of a supportive environment by assisting them with their symptom management work (interpersonal outcome expectancies). Like the interventions published by Maliski et al,26 Weber et al,27 and Lepore et al,9 our model views increasing self efficacy as an integral part of symptom management.
Figure 1.
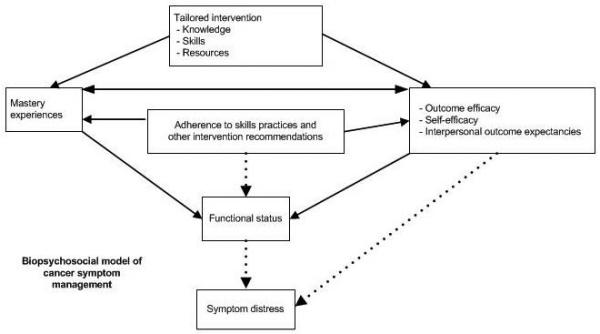
As participants master symptom-management tasks, they improve their functional status. We hypothesize that men who improve their functional status will have reduced symptom distress and that men who are adherent with interventions are more likely to reduce their symptom distress than men who are not adherent with the intervention. Adherence also is likely to increase the number of mastery experiences that a participant has and his sense of outcome and personal efficacy. Previous work in men with advanced cancer suggests that men with a large number of symptoms report more distress than men with fewer symptoms.60 Our model is based on the premise that this relationship between functional status and symptom distress exists and that providing rehabilitation for treatment-related side-effects and other symptoms will reduce both symptom distress and general levels of distress. Similar to the approaches by Mishel et al and Giesler et al, our model is focused on reducing symptom distress.
Conclusions
Many studies have described the substantial impact of prostate-cancer treatment on sexual functioning. In this article, we have summarized the limited number of intervention studies developed sexual rehabilitation of prostate-cancer survivors. A number of common themes emerged. Several interventions have focused primarily on psychosocial symptoms, with sexual rehabilitation as a secondary goal. Interventions that focused on sexual rehabilitation as a primary goal had better results. One intervention was tailored to address the symptoms of greatest concern to the participants. Developing such tailored interventions may be a way to increase “face validity” of the intervention with participants by closely linking the intervention to what they report as their primary concerns and could be linked with a stepped-care approach to increase the likelihood of developing interventions cost-effective that can be sustained past the end of a funded study.
Although clinical characteristics are important, others such as personality traits, literacy level, or the lack of congruence between sexual desire and functioning, may be important determinants of who needs rehabilitation efforts the most and who is most likely to benefit from them. Research is needed to understand how gay men are affected by prostate cancer and its treatment, and how sexual-rehabilitation efforts could be tailored to their particular needs. Finally, we have proposed a conceptual model for prostate–cancer-symptom research informed by psychological and nursing theories and the published research on sexual rehabilitation in prostate-cancer survivors.
Further work is needed to build on the published work reviewed here to encourage sexual-rehabilitation efforts to focus on populations with greatest need because of psychological makeup, low health literacy, or other characteristics that may put a prostate-cancer survivor at risk for low HRQOL. Physicians should recognize the importance of sexual rehabilitation programs and actively refer their patients to such programs. Mental health providers and nurses should provide sexual rehabilitation interventions to patients and work with their physician colleagues to provide effective medical interventions supported by patient education materials at the appropriate reading level. Such efforts will address concerns raised in the IOM report by helping men move smoothly from being a patient to a survivor, armed with the necessary tools and support needed to live well after cancer.
Acknowledgments
Support: Funded by grants R03 CA101586 (DML) and R03 CA128475 (DWC) from the National Cancer Institute. This material is partly the result of work supported with resources and the use of facilities at the Health Services Research & Development Center of Excellence (HFP90-020), Michael E. DeBakey Veterans Affairs Medical Center. Dr. Latini is supported by Mentored Research Scholar Grant 06-083-01-CPPB from the American Cancer Society.
References
- 1.American Cancer Society . Cancer Facts and Figures 2008. Atlanta, GA: 2008. [Google Scholar]
- 2.Lynch JH, Batuello JT, Crawford ED, Gomella LG, Kaufman J, Petrylak DP, et al. Therapeutic strategies for localized prostate cancer. Rev Urol. 2001;3(Suppl 2):S39. [PMC free article] [PubMed] [Google Scholar]
- 3.Speight JL, Roach M. New techniques and management options for localized prostate cancer. Rev Urol. 2006;8(Suppl 2):S22. [PMC free article] [PubMed] [Google Scholar]
- 4.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Leach GE, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 5.Talcott JA. Quality of life in early prostate cancer: Do we know enough to treat? Hematology/Oncology Clinics of North America. 1996;10:691. doi: 10.1016/s0889-8588(05)70361-0. [DOI] [PubMed] [Google Scholar]
- 6.Lubeck DP, Litwin MS, Henning JM, Stier DM, Mazonson P, Fisk R, et al. CaPSURE Research Panel The CaPSURE database: a methodology for clinical practice and research in prostate cancer. Urology. 1996;48:773. doi: 10.1016/s0090-4295(96)00226-9. Cancer of the Prostate Strategic Urologic Research Endeavor. [DOI] [PubMed] [Google Scholar]
- 7.Potosky AL, Harlan LC, Stanford JL, Gilliland FD, Hamilton AS, Albertsen PC, et al. Prostate cancer practice patterns and quality of life: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 1999;91:1719. doi: 10.1093/jnci/91.20.1719. [DOI] [PubMed] [Google Scholar]
- 8.Dahn JR, Penedo FJ, Gonzalez JS, Esquiabro M, Antoni MH, Roos BA, et al. Sexual functioning and quality of life after prostate cancer treatment: considering sexual desire. Urology. 2004;63:273. doi: 10.1016/j.urology.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Lepore SJ, Helgeson VS, Eton DT, Schulz R. Improving quality of life in men with prostate cancer: a randomized controlled trial of group education interventions. Health Psychol. 2003;22:443. doi: 10.1037/0278-6133.22.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schover LR. Sexual rehabilitation after treatment for prostate cancer. Cancer. 1993;71:1024. doi: 10.1002/1097-0142(19930201)71:3+<1024::aid-cncr2820711421>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Eton DT, Lepore SJ. Prostate cancer and health-related quality of life: a review of the literature. Psychooncology. 2002;11:307. doi: 10.1002/pon.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penson DF. Quality of life after therapy for localized prostate cancer. Cancer J. 2007;13:318. doi: 10.1097/PPO.0b013e3181570121. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine . From Cancer Patient to Cancer Survivor: Lost in Transition. National Academies Press; Washington, DC: 2005. [Google Scholar]
- 14.Visser A, van Andel G. Psychosocial and educational aspects in prostate cancer patients. Patient Educ Couns. 2003;49:203. doi: 10.1016/s0738-3991(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society . Man to Man. Vol. 2001. American Cancer Society; 1999. http://www3.cancer.org/cancerinfo/Documents/cancer_36/m2m_background.asp. [Google Scholar]
- 16.Demark-Wahnefried W, Morey MC, Clipp EC, Pieper CF, Snyder DC, Sloane R, et al. Leading the Way in Exercise and Diet (Project LEAD): intervening to improve function among older breast and prostate cancer survivors. Control Clin Trials. 2003;24:206. doi: 10.1016/s0197-2456(02)00266-0. [DOI] [PubMed] [Google Scholar]
- 17.Carmack Taylor CL, Smith MA, De Moor C, Dunn AL, Pettaway C, Sellin R, et al. Quality of life intervention for prostate cancer patients: design and baseline characteristics of the active for life after cancer trial. Control Clin Trials. 2004;25:265. doi: 10.1016/j.cct.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Berglund G, Petersson LM, Eriksson KR, Haggman M. “Between men”: patient perceptions and priorities in a rehabilitation program for men with prostate cancer. Patient Educ Couns. 2003;49:285. doi: 10.1016/s0738-3991(02)00186-6. [DOI] [PubMed] [Google Scholar]
- 19.Penedo FJ, Dahn JR, Molton I, Gonzalez JS, Kinsinger D, Roos BA, et al. Cognitive-behavioral stress management improves stress-management skills and quality of life in men recovering from treatment of prostate carcinoma. Cancer. 2004;100:192. doi: 10.1002/cncr.11894. [DOI] [PubMed] [Google Scholar]
- 20.Maliski SL, Kwan L, Krupski T, Fink A, Orecklin JR, Litwin MS. Confidence in the ability to communicate with physicians among low-income patients with prostate cancer. Urology. 2004;64:329. doi: 10.1016/j.urology.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Mishel MH, Belyea M, Germino BB, Stewart JL, Bailey DE, Jr., Robertson C, et al. Helping patients with localized prostate carcinoma manage uncertainty and treatment side effects: nurse-delivered psychoeducational intervention over the telephone. Cancer. 2002;94:1854. doi: 10.1002/cncr.10390. [DOI] [PubMed] [Google Scholar]
- 22.Boberg EW, Gustafson DH, Hawkins RP, Offord KP, Koch C, Wen KY, et al. Assessing the unmet information, support and care delivery needs of men with prostate cancer. Patient Educ Couns. 2003;49:233. doi: 10.1016/s0738-3991(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 23.Miles CL, Candy B, Jones L, Williams R, Tookman A, King M. Interventions for sexual dysfunction following treatments for cancer. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005540.pub2. CD005540. [DOI] [PubMed] [Google Scholar]
- 24.Melnik T, Soares BG, Nasselo AG. Psychosocial interventions for erectile dysfunction. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004825.pub2. CD004825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology. 1999;53:180. doi: 10.1016/s0090-4295(98)00408-7. [DOI] [PubMed] [Google Scholar]
- 26.Maliski SL, Clerkin B, Letwin MS. Describing a nurse case manager intervention to empower low-income men with prostate cancer. Oncol Nurs Forum. 2004;31:57. doi: 10.1188/04.ONF.57-64. [DOI] [PubMed] [Google Scholar]
- 27.Weber BA, Roberts BL, Resnick M, Deimling G, Zauszniewski JA, Musil C, et al. The effect of dyadic intervention on self-efficacy, social support, and depression for men with prostate cancer. Psychooncology. 2004;13:47. doi: 10.1002/pon.718. [DOI] [PubMed] [Google Scholar]
- 28.Deans G, Bennett-Emslie GB, Weir J, Smith DC, Kaye SB. Cancer support groups--who joins and why? Br J Cancer. 1988;58:670. doi: 10.1038/bjc.1988.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz D, Koppie TM, Wu D, Meng MV, Grossfeld GD, Sadesky N, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092. doi: 10.1016/S0022-5347(05)64303-0. [DOI] [PubMed] [Google Scholar]
- 30.Canada AL, Neese LE, Sui D, Schover LR. Pilot intervention to enhance sexual rehabilitation for couples after treatment for localized prostate carcinoma. Cancer. 2005;104:2689. doi: 10.1002/cncr.21537. [DOI] [PubMed] [Google Scholar]
- 31.Giesler RB, Given B, Given CW, Rawl S, Monahan P, Burns D, et al. Improving the quality of life of patients with prostate carcinoma: a randomized trial testing the efficacy of a nurse-driven intervention. Cancer. 2005;104:752. doi: 10.1002/cncr.21231. [DOI] [PubMed] [Google Scholar]
- 32.Titta M, Tavolini IM, Moro FD, Cisternino A, Bassi P. Sexual counseling improved erectile rehabilitation after non-nerve-sparing radical retropubic prostatectomy or cystectomy--results of a randomized prospective study. J Sex Med. 2006;3:267. doi: 10.1111/j.1743-6109.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- 33.Molton IR, Siegel SD, Penedo FJ, Dahn JR, Kinsinger D, Traeger LN, et al. Promoting recovery of sexual functioning after radical prostatectomy with group-based stress management: the role of interpersonal sensitivity. J Psychosom Res. 2008;64:527. doi: 10.1016/j.jpsychores.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latini DM, Penson DF, Colwell HH, Lubeck DP, Mehta SS, Henning JM, et al. Psychological impact of erectile dysfunction: validation of a new health related quality of life measure for patients with erectile dysfunction. J Urol. 2002;168:2086. doi: 10.1016/S0022-5347(05)64302-9. [DOI] [PubMed] [Google Scholar]
- 35.Schover LR, Fouladi RT, Warneke CL, Neese L, Klein EA, Zippe C, et al. The use of treatments for erectile dysfunction among survivors of prostate carcinoma. Cancer. 2002;95:2397. doi: 10.1002/cncr.10970. [DOI] [PubMed] [Google Scholar]
- 36.Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice-Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- 37.Newman MG. Recommendations for a cost-offset model of psychotherapy allocation using generalized anxiety disorder as an example. Journal of Consulting and Clinical Psychology. 2000;68:549. [PubMed] [Google Scholar]
- 38.Otto MW, Pollack MH, Maki KM. Empirically supported treatments for panic disorder: Costs, benefits, and stepped care. Journal of Consulting and Clinical Psychology. 2000;68:556. [PubMed] [Google Scholar]
- 39.Wilson GT, Vitousek KM, Loeb KL. Stepped care treatment for eating disorders. Journal of Consulting and Clinical Psychology. 2000;68:564. [PubMed] [Google Scholar]
- 40.Knight SJ, Latini DM, Hart SL, Sadetsky N, Kane CJ, DuChane J, et al. Education predicts quality of life among men with prostate cancer cared for in the Department of Veterans Affairs: a longitudinal quality of life analysis from CaPSURE. Cancer. 2007;109:1769. doi: 10.1002/cncr.22597. [DOI] [PubMed] [Google Scholar]
- 41.Bennett CL, Ferreira MR, Davis TC, Kaplan J, Weinberger M, Kuzel T, et al. Relation between literacy, race, and stage of presentation among low-income patients with prostate cancer. J Clin Oncol. 1998;16:3101. doi: 10.1200/JCO.1998.16.9.3101. [DOI] [PubMed] [Google Scholar]
- 42.Wolf MS, Knight SJ, Lyons EA, Durazo-Arvizu R, Pickard SA, Arseven A, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006;68:89. doi: 10.1016/j.urology.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 43.Schover LR. Sexuality and fertility in urologic cancer patients. Cancer. 1987;60:553. doi: 10.1002/1097-0142(19870801)60:3+<553::aid-cncr2820601519>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Helgason AR, Fredrikson M, Adolfsson J, Steineck G. Decreased sexual capacity after external radiation therapy for prostate cancer impairs quality of life. Int J Radiat Oncol Biol Phys. 1995;32:33. doi: 10.1016/0360-3016(95)00542-7. [DOI] [PubMed] [Google Scholar]
- 45.Meuleman EJ, van Lankveld JJ. Hypoactive sexual desire disorder: an underestimated condition in men. BJU Int. 2005;95:291. doi: 10.1111/j.1464-410X.2005.05285.x. [DOI] [PubMed] [Google Scholar]
- 46.Khera M, Lipshultz LI. The role of testosterone replacement therapy following radical prostatectomy. Urol Clin North Am. 2007;34:549. doi: 10.1016/j.ucl.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Brand TC, Canby-Hagino E, Thompson IM. Testosterone replacement therapy and prostate cancer: a word of caution. Curr Urol Rep. 2007;8:185. doi: 10.1007/s11934-007-0004-x. [DOI] [PubMed] [Google Scholar]
- 48.Blank TO. Gay men and prostate cancer: invisible diversity. J Clin Oncol. 2005;23:2593. doi: 10.1200/JCO.2005.00.968. [DOI] [PubMed] [Google Scholar]
- 49.Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, et al. Cancer surveillance series: interpreting trends in prostate cancer--part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91:1017. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 50.Mitteldorf D. Psychotherapy with gay prostate cancer patients. In: Perlman G, Drescher J, editors. A gay man’s guide to prostate cancer. The Haworth Medical Press; Binghamton, NY: 2005. pp. 57–67. [Google Scholar]
- 51.Cornell D. A gay urologist’s changing views of prostate cancer. In: Perlman G, Drescher J, editors. A gay man’s guide to prostate cancer. The Haworth Medical Press; Binghamton, NY: 2005. pp. 29–41. [Google Scholar]
- 52.McCarthy BW. Treatment of erectile dysfunction with single men. In: Rosen RC, Leiblum SC, editors. Erectile disorders: Assessment and treatment. Guilford Press; New York: 1992. pp. 313–340. [Google Scholar]
- 53.Goldstone SE. The ups and downs of gay sex after prostate cancer treatment. In: Perlman G, Drescher J, editors. A Gay Man’s Guide to Prostate Cancer. The Haworth Medical Press; Binghamton, NY: 2005. pp. 43–55. [Google Scholar]
- 54.Bandura A. Self-efficacy: The exercise of control. W. H. Freeman & Co.; New York: 1997. [Google Scholar]
- 55.UCSF Faculty Group in Symptom Management A model for symptom management. Image J Nurs Sch. 1994;26:272. [PubMed] [Google Scholar]
- 56.Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33:668. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 57.Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Jr., Bandura A, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39:1217. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Lorig KR, Sobel DS, Stewart AL, Brown BW, Jr., Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37:5. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Wilson SR, Latini D, Starr NJ, Fish L, Loes LM, Page A, et al. Education of parents of infants and very young children with asthma: a developmental evaluation of the Wee Wheezers program. J Asthma. 1996;33:239. doi: 10.3109/02770909609055365. [DOI] [PubMed] [Google Scholar]
- 60.Ullrich PM, Carson MR, Lutgendorf SK, Williams RD. Cancer fear and mood disturbance after radical prostatectomy: consequences of biochemical evidence of recurrence. J Urol. 2003;169:1449. doi: 10.1097/01.ju.0000053243.87457.60. [DOI] [PubMed] [Google Scholar]


