Abstract
Associations between drugs of abuse and cues facilitate the acquisition and maintenance of addictive behaviors. Although significant research has been done to elucidate the role that simple discriminative or discrete conditioned stimuli (e.g., a tone or a light) play in addiction, less is known about complex environmental cues. The purpose of the present study was to examine the role of a musical conditioned stimulus by assessing locomotor activity and in vivo microdialysis. Two groups of rats were given non-contingent injections of methamphetamine (1.0 mg/kg) or vehicle and placed in standard conditioning chambers. During these conditioning sessions both groups were exposed to a continuous conditioned stimulus, in the form of a musical selection (“Four” by Miles Davis) played repeatedly for ninety minutes. After seven consecutive conditioning days subjects were given one day of rest, and subsequently tested for locomotor activity or dopamine release in the absence of drug while the musical conditioned stimulus was continually present. The brain regions examined included the basolateral amygdala, nucleus accumbens, and prefrontal cortex. The results show that music is an effective contextual conditioned stimulus, significantly increasing locomotor activity after repeated association with methamphetamine. Furthermore, this musical conditioned stimulus significantly increased extracellular dopamine levels in the basolateral amygdala and nucleus accumbens. These findings support other evidence showing the importance of these brain regions in conditioned learning paradigms, and demonstrate that music is an effective conditioned stimulus warranting further investigation.
Keywords: Conditioning, Music, Cues, Methamphetamine, Reward, Addiction
1. Introduction
Drug addiction is a chronic relapsing disease characterized by neurobiological changes that lead to compulsive drug taking (Koob 2000). Exposure to cues previously paired with drug reward have been shown to elicit craving and cause drug seeking behavior in both human and animal models of relapse (Childress et al. 1999; Di Ciano and Everitt 2003; Fuchs et al. 2008; O’Brien et al. 1998). These drug-paired conditioned stimuli (CS) ostensibly hijack normal learning and memory processes, allowing maladaptive addictive behaviors to acquire increased salience. It is therefore of great importance to elucidate the behavioral and biochemical mechanisms through which drug-associated stimuli exert their effects.
The basolateral amygdala (BLA) encodes the motivating and reinforcing properties of CS via populations of cue-responsive neurons. This neuronal activation supplies cognitive systems with information regarding the motivational and reinforcing qualities of an associated cue that help determine, plan, and execute the appropriate behavioral response (Tye and Janak 2007). Alterations of the cortico-limbic-striatal circuitry may influence associative learning mediated by the BLA, the brain area ultimately responsible for cue-induced reinstatement of drug-seeking behavior (McLaughlin and Floresco 2007). Repeated psychostimulant exposure may affect reciprocal, functional connections between the BLA and other reward-related structures, signaling changes in incentive salience and influencing behavioral and neurochemical responses.
The functional relationship between the BLA and the nucleus accumbens (NAcc) promotes goal-directed behavior based on learned associations related to reward. Exposure to reward predictive stimuli has been shown to increase dopamine (DA) efflux in the NAcc and BLA (Weiss et al. 2000). Furthermore, several studies using conditioning paradigms have documented the complex role of the NAcc with regard to psychostimulant seeking and goal directed behavior (Bossert et al. 2005; Everitt and Robbins 2005; See 2005). The persistent craving induced by a drug-paired CS has been proposed to be mediated by drug-induced maladaptive encoding of memory via an amygdala-accumbens interaction (Di Ciano and Everitt 2004).
Involvement of the prefrontal cortex (PFC) in addictive behaviors can be attributed to processes that coordinate sensory and cognitive information, allowing selection of the goal-directed behavior that will likely produce the most advantageous outcome. The BLA provides the PFC with information regarding the motivational significance of the stimuli, and the PFC processes and integrates the affective afferent projections to determine the salient relevance of the stimulus, in order to guide behavior appropriately and purposefully (McLaughlin and See 2003).
Previous studies that have examined the role of psychostimulant-paired CS in these brain regions have almost all used simple discrete or discriminative CS (e.g., a tone or light). While meeting the criteria of neutrality prior to conditioning, these CS fail to simulate the complexity of contextual cues present in human drug experiences. Conditioned place preference (CPP) paradigms have been used extensively to investigate more complex contextual cues (Bardo et al. 1995; Tzschentke 2007). However, the effect seen with CPP relies upon CS-reward interactions that take place within the same apparatus. One could seemingly make a stronger case for the influence of a reward-paired contextual CS if the conditioning and test environments were not similar. If a contextual CS was able to influence drug seeking behavior in a different environment, then there would be more certainty that the CS was the determining factor, rather than place serving as a potential confound. The ultimate goal of any animal model is to experimentally replicate conditions present in human pathologies, in order to generalize findings to the human population. Therefore, it would be beneficial if animal models of drug seeking were able to incorporate the same kinds of environmental stimuli that are salient in the human addiction cycle.
Recently, it has been reported that music can enhance MDMA-conditioned reward in rats (Feduccia and Duvauchelle 2008). This study showed increases in both locomotor behavior and extracellular DA in the NAcc when music was paired with MDMA during operant self-administration. Another recent report demonstrated that rats have the ability to discriminate between music by Bach and Stravinsky, and even transfer this discrimination to novel musical selections by the same artists (Otsuka et al. 2009). Furthermore, both of these studies found that music evoked no primary reinforcing properties by itself. Taken together, these reports indicate that music could serve as an effective contextual conditioned stimulus in rats.
Pleasurable music evokes neurological responses in humans that are similar to the effects induced by drugs of abuse. Specifically, highly enjoyable music has been shown to activate reward-related brain regions such as the striatum, amygdala, and PFC (Blood and Zatorre 2001). Music has also been shown to increase dopaminergic neurotransmission (Sutoo and Akiyama 2004). More advanced techniques such as functional magnetic resonance imaging (fMRI) have shown that pleasurable music is strongly correlated with activation in reward-related brain regions such as the NAcc and ventral tegmental area (VTA); enhanced functional connectivity between brain regions that mediate reward may help explain why listening to music is regarded as a highly pleasurable human experience (Menon and Levitin 2005). Recent clinical reports also indicate that music can be an effective treatment for a multitude of disorders. Music therapy has shown promising results in treating anxiety, chronic stress, pain, sleep disorders, autism, depression, psychosis, post-traumatic stress disorder, respiratory disease, and as an adjunct therapy for addiction. (Bauldoff 2009; Bradt and Dileo 2009; de Niet et al. 2009; Gold et al. 2009; Jung and Newton 2009; Mays et al. 2008; Nilsson 2008; Rossignol 2009). However, while musical appreciation is well documented in the human population, there is still significant disagreement in the literature about whether this is confined to the human species or extends to other members of the animal kingdom. Considering the enormous potential that music therapy offers, there is a growing need to develop preclinical models.
The goal of the present study was to address these issues by using a musical CS in a classical conditioning paradigm. To this end, we conditioned rats for one week with a contextual/musical CS (“Four” by Miles Davis) either in the presence or absence of methamphetamine (METH), our unconditioned stimulus (US). The effects of conditioning were then assessed in a non-drug state by measuring locomotor activity and extracellular levels of DA (using in vivo microdialysis); brain regions dialyzed included the BLA, NAcc, and mPFC. Based on previous findings, we postulated that, after conditioning, music alone would increase locomotor activity and increase extracellular DA in the brain regions examined.
2. Materials and Methods
2.1. Animals
Naïve female Sprague-Dawley rats (Taconic Germantown, NY), weighing between 225 and 275g at the start of the experiment, were housed individually in a temperature and humidity controlled colony room under a standard 12:12 light/dark cycle. Food and water were provided ad libitum. Protocols were designed and implemented in accordance with the Guide for the Care and Use of Laboratory Animals (1996) and were approved by the Institutional Animal Care and Use Committee of Albany Medical College. Rats were given one week of acclimation time prior to surgery or experimental procedures.
2.2. Drugs
Methamphetamine (Sigma-Aldrich Company, St. Louis, MO), was dissolved in 0.9% sodium chloride with a 1 mg/1 ml drug to saline ratio. METH (1 mg/kg) or saline was administered intraperitoneally (i.p.). Animals were anesthetized with pentobarbital (50 mg/kg, i.p.) for microdialysis cannulation surgery. All other reagents used in conjunction with microdialysis experiments were obtained from local suppliers and were of analytical grade.
2.3. Surgery
Stereotaxic surgery for implantation of microdialysis guide cannulae was conducted in accordance with a previously established protocol (Maisonneuve and Glick 1999). Each rat had two microdialysis guide cannulae (CMA/Microdialysis AB, Stockholm, Sweden) implanted into two of three target nuclei: the basolateral amygdala (BLA), nucleus accumbens (NAcc), and/or medial prefrontal cortex (mPFC). Coordinates were determined according to Paxinos and Watson (1986) such that, when inserted, the dialysis probe was located in the BLA (in mm, AP = −2.2; ML = ±4.6; DV = −5.0, 0° lateral angle insertion), NAcc (in mm, AP = +1.6; ML = ±3.0; DV = −4.0, 16° lateral angle insertion), or mPFC (in mm, AP = +2.7; ML = ±1.9; DV = −2.2, 14° lateral angle insertion). Probe locations were randomized and counterbalanced amongst animals, so that our final sample represented animals that had bilateral treatments balanced among the three brain regions. Rats were monitored for 3–4 days before the initiation of conditioning sessions.
2.4. Conditioning
On days one through seven, rats were transported from the colony room to a sound attenuated experimental room containing a stereo system and clear Plexiglas® 36cm cylindrical conditioning chambers with stainless steel bar floors. Rats were administered METH or vehicle (saline) and placed into individual conditioning chambers. Rats were immediately presented with the conditioning cue, a jazz trumpet selection (Miles Davis, Four) played continuously for 90 minutes. This musical selection was played at a volume that allowed the auditory range to remain between sixty five and seventy five decibels. After each 90 min. conditioning session, rats were returned to their home cages and transported back to the colony room. Refer to Figure 1 for a diagrammatic representation of the time course for classical conditioning and activity/microdialysis tests. Separate animals were used for the activity and microdialysis assays.
Figure 1.
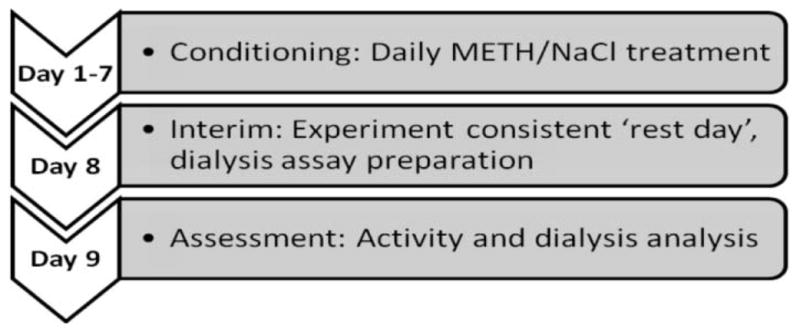
Diagrammatic representation of time course for classical conditioning and activity/microdialysis tests.
2.5. Activity Assay
On the day of assessment (day 9), rats were transported to a sound attenuated testing room. This was a different room than that used for conditioning, and housed activity chambers characteristically different from the conditioning chambers. Locomotion was studied in opaque, cylindrical (60 cm) photocell activity chambers with three intersecting light beams. The photocells were located equidistant from each other around the circumference of the apparatus, 3 cm above a grid floor. Each time a light beam was broken a single activity count was recorded by a PC computer interfaced with Med Associates Inc. software. Presentation of the musical conditioned cue was initiated and maintained for 90 minutes. After the ninety minute session, rats were removed from the activity chambers and were euthanized via carbon dioxide.
Several control groups were used to ensure that any conditioned response found on the activity test day was due to the musical CS, and not due to the residual presence of METH or other conditioning factors. These groups are outlined in Table 1, which summarizes the 8 groups that were tested for locomotor response. The Meth-Music and NaCl-Music groups were injected with METH or saline and conditioned in the presence of the music, then tested in a drug free state in the presence of music only. The Meth-NMCond and NaCl-NMCond groups were controls that were injected with METH or saline and received no music during conditioning, and tested in a drug free state in the presence of music only. The Meth-NonEx and NaCl-NonEx groups were controls that used explicitly non-paired presentations of the CS and US. Consequently, during conditioning, these groups were placed in the chambers with music, and then received an injection of METH or saline in their home cages approximately three hours after the music session. They were subsequently tested for activity in the presence of music only. The Meth-NMTest and NaCl-NMTest groups were controls that were injected with METH or saline and received music during conditioning, but no music during the test session.
Table 1.
Depiction of the eight groups that were tested for locomotor activity: combinations of drug/vehicle and conditioning assignments along with the respective test condition for each group.
| Group | Conditioning | Test |
|---|---|---|
| Meth-Music | Miles Davis + Meth | Miles Davis Only |
| NaCl-Music | Miles Davis + NaCl | Miles Davis Only |
| Meth-NMCond | Meth Only | Miles Davis Only |
| NaCl-NMCond | NaCI Only | Miles Davis Only |
| Meth-NonEx | Miles Davis Only/Meth 3Hr Later | Miles Davis Only |
| NaCl-Non-Ex | Miles Davis Only/NaCI 3Hr Later | Miles Davis Only |
| Meth-NMTest | Miles Davis + Meth | No Music |
| NaCl-NMTest | Miles Davis + NaCl | No Music |
2.6. In Vivo Microdialysis
On the afternoon prior to assessment (day 8), microdialysis probes were calibrated for DA, DOPAC, and HVA to ensure recovery higher than 15% (Glick et al. 1994). Probes were discarded if they did not meet the 15% criteria. The subjects were transiently anesthetized with 25 mg/kg of Pentothal (Hospira, INC., Lake Forest, IL), and then placed into dialysis chambers. These dialysis chambers were constructed of clear Plexiglas®, were rectangular in shape, and contained a grid floor. They were housed in a different room than the conditioning or activity chambers. A 2 mm dialysis probe (or 3mm probe for mPFC) was inserted through each guide cannula. The subjects were monitored until the effects of anesthesia had subsided.
On the day of microdialysis (day 9), samples were collected in tubes containing 2 μl of 1.1 N perchloric acid solution (containing 50 mg/l Na2EDTA and 50 mg/l sodium metabisulfite). The probe was continuously perfused at a flow rate of 1 μl/min with artificial cerebrospinal fluid (146 mM NaCl, 2.7 mM KCL, 1.2 mM CaCl2, 1.0 mM MgCl2). A test sample was collected for 20 min from each probe for each experimental subject. Six, 20 min baseline samples were obtained during the first two hours of sample collection. Immediately following baseline collection, the conditioned cue was presented by turning the music on, and then four 20 min samples were collected. The cue was removed by turning the music off, and an additional five 20 min samples were collected. The dialysate samples were transferred from collection to analysis vials for DA, DOPAC, and HVA analysis by high performance liquid chromatography with electrochemical detection (HPLC-EC). Immediately following the microdialysis experiment, subjects were sacrificed and their brains were removed and preserved for histological confirmation of guide cannulae placements.
2.7. Histology
Brains were frozen at −80°C until histology was performed utilizing a cryostat (Microm HM500M, Walldorf, Germany). Probe placements were mapped directly from the cryostat sections, and data were excluded from analysis if the probe was not located within region-specific boundaries for BLA, NAcc, and mPFC (refer to Figure 2).
Figure 2.
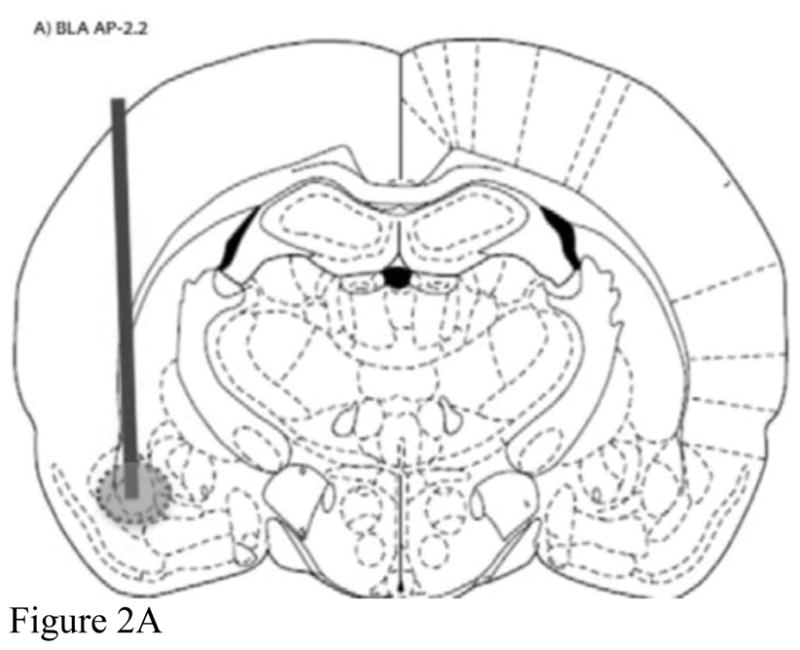
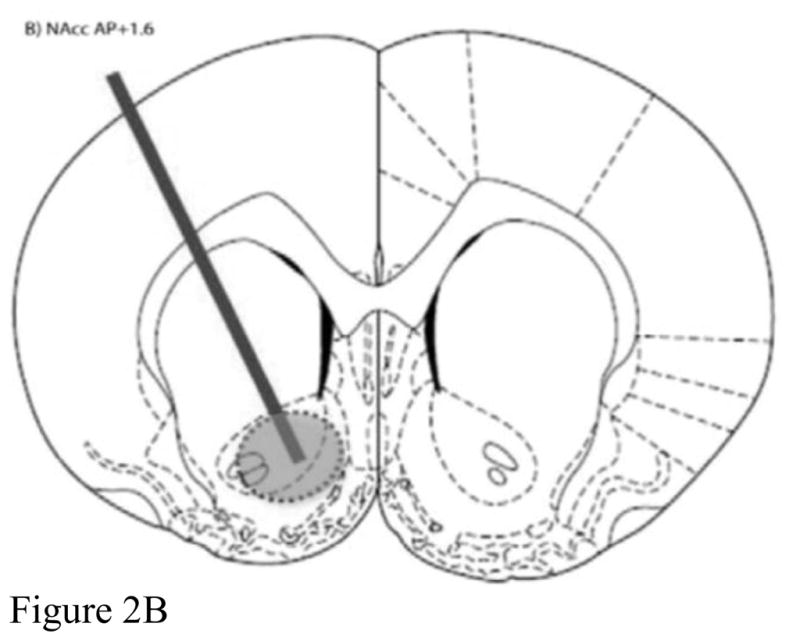
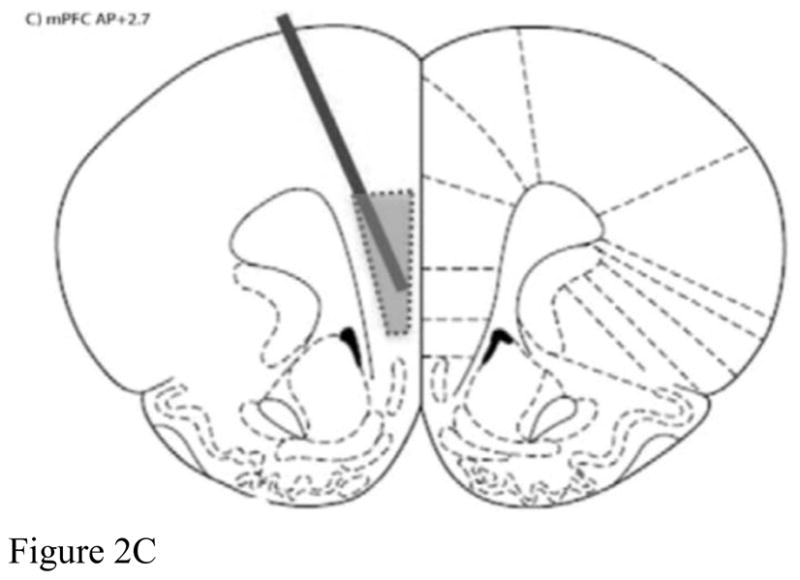
Representative probe placements for A) basolateral amygdala, B) nucleus accumbens, and C) medial prefrontal cortex.
2.8. HPLC
The dialysate samples were analyzed utilizing a high performance liquid chromatography system with electrochemical detection (HPLC-EC). The system consisted of an ESA 540 autosampler (ESA, North Chelmsford, MA), an ESA solvent delivery unit, an ESA column (MD-150/RP-C18; diameter =3.0 μm), and an ESA Coulochem II electrochemical detector with an ESA 5020 guard cell and an ESA 5014B analytical cell. The potential of the glass carbon working electrode was set at 300 mV with respect to the reference electrode. The MD-TM mobile phase (ESA, North Chelmsford, MA), composed of 75 mM sodium dihydrogen phosphate monohydrate, 1.7 mM 1-octanesulfonic acid sodium salt, 100 μl/l triethylamine, 25 μM EDTA in 10% acetonitrile (pH=3.0), was pumped at a flow rate of 0.530 ml/min. The electrochemical data were processed with Agilent Technologies Chem Station Plus software (Agilent Technologies, Wilmington, DE). The software produced chromatographs, visual depictions of DA, DOPAC, and HVA concentrations (in pmol) plotted on the y-axis against the temporal representation (in min) for ion affinity plotted along the x-axis.
2.9. Data Analysis
For the locomotor activity data, one-way independent samples analysis of variance (ANOVA) was used to make comparisons between test and control groups based on summed activity during the 90 min test. Specifically, statistical comparisons were made between our four METH groups; the original Meth-Music treatment group and three METH control groups. Additionally, one-way ANOVA was used to compare our Meth-Music treatment group to our four saline control groups. Significant differences were further examined using Newman-Keuls post-hoc tests. One-way ANOVA was also used to compare all the control groups to determine if there were any significant differences.
For analysis of the microdialysis data, basal levels of DA and metabolites were expressed as pm/10 μl and were analyzed using a repeated measures ANOVA with Treatment (METH vs. NaCl) as the independent factor and Time (20 min intervals, 15 total) as the repeated measures variable. If no significant differences were observed in the basal levels, DA and its metabolites were expressed as a percentage of the corresponding baseline means, and the percent baseline values were then used in subsequent analyses. A two-way ANOVA was used to evaluate two factors: Time and Treatment. Significant results were further examined by Newman-Keuls post-hoc tests.
3. Results
3.1. Music Conditioning: Activity Assay
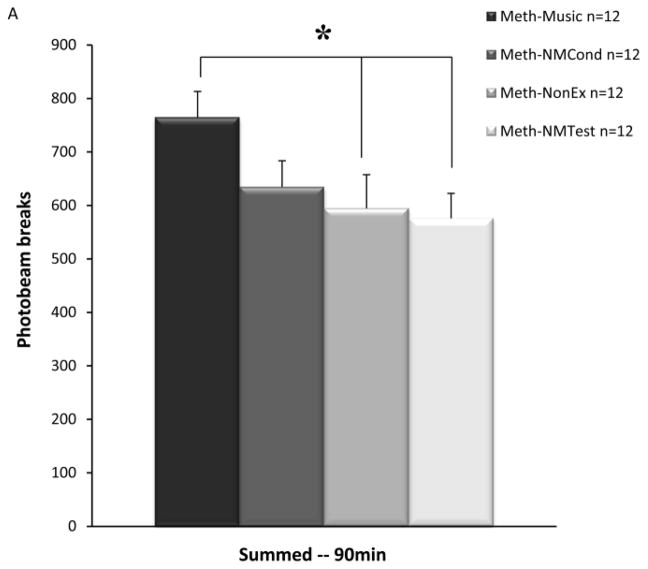
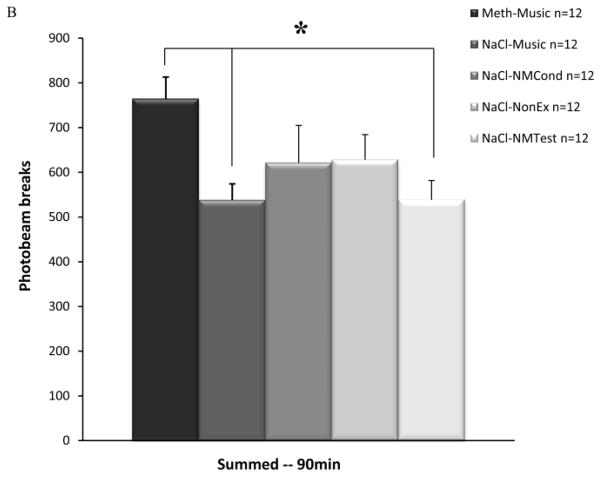
Figure 3a depicts the summed locomotor activity of the Meth-Music test group, and the three METH control groups as measured by automated photobeam breaks during 90 minute sessions. ANOVA tests revealed a significant difference between groups (F(3,44)=2.96, p<0.05). Newman-Keuls post-hoc tests showed significant differences between the Meth-Music test group when compared to both the Meth-NonEx and Meth-NMTest control groups (p<0.05). Figure 3b depicts the summed locomotor activity of the Meth-Music test group, and the four saline control groups. ANOVA testing indicated significant differences between groups (F(4,55)=2.79, p<0.05). Newman-Keuls post-hoc tests revealed significant differences between the Meth-Music test group when compared to both the NaCl-Music and NaCl-NMTest control groups (p<0.05). There were no significant differences revealed by ANOVA when the seven control conditions were compared.
Figure 3.
Effects of a conditioned musical cue on locomotor activity. Data points represent mean summed locomotor activity for the 90 min test session (± SEM). Refer to Table 1 for a detailed depiction of the conditioning and test conditions for each group. A) Meth-Music test group and the three METH control groups. * p<0.05 B) Meth-Music test group and the four saline control groups. * p<0.05
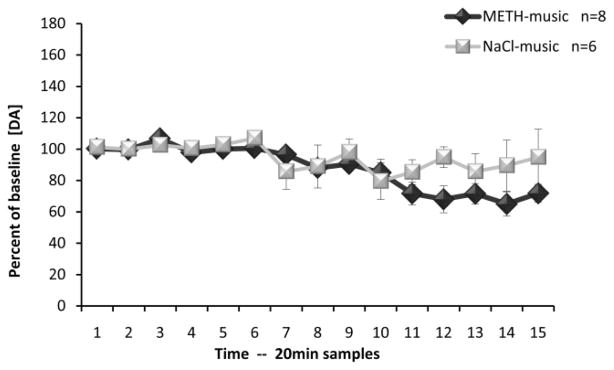
3.2. Musical Conditioning: Dialysis Assay
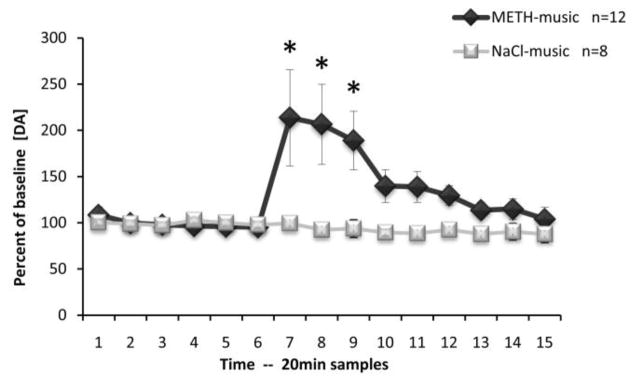
No group differences were observed among basal levels of DA or its metabolites in the BLA (DA: F(1,18)=0.044, p=0.837; DOPAC: F(1,18)=0.656, p=0.429; HVA F(1,18)=1.218, p=0.284; Table 2). Figure 4 shows the levels of extracellular DA (percent of baseline) in the BLA during the time-course of microdialysis testing. The auditory conditioned cue was presented to subjects immediately before the collection of sample 7. As can be observed from this graph, there was a robust increase in DA levels after presentation of the musical conditioned cue. These results were confirmed to be significantly different from both baseline samples (Time) and control samples (Group) utilizing ANOVA, (F(14,252)=2.87, p<0.001) and (F(1,18)=6.67, p<0.05), respectively. ANOVA also revealed a significant Time*Group interaction (F(14,252)=3.10, p<0.001). Newman-Keul’s post-hoc comparison tests revealed significant differences at samples 7, 8 and 9 (p<0.05). No difference was observed in the levels of either metabolite: DOPAC [Group effect: F(1,18)=0.00, p=0.99; Time effect: F(14,252)=0.47, p=0.95; Time*Group interaction: F(14,252)=0.78, p=0.69] or HVA [Group effect: F(1,18)=0.87, p=0.36; Time effect: F(14,252)=0.22, p=0.99; Time*Group interaction: F(14,252)=0.57, p=0.89].
Table 2.
Average basal levels of extracellular DA. DOPAC. and HVA in rats treated with methamphetamine or saline. Mean ± SEM expressed at pm/10 μl
| Region | Neurotransmitter | Treatment | N= | Mean ± SEM |
|---|---|---|---|---|
| BLA | Dopamine | NaCl | 8 | 0.016 ± 0.00007 |
| METH | 12 | 0.019 ± 0.00027 | ||
| DOPAC | NaCl | 8 | 3.203 ± 0.01116 | |
| METH | 12 | 4.976 ± 0.03966 | ||
| HVA | NaCl | 8 | 2.687 ± 0.01829 | |
| METH | 12 | 4.110 ± 0.00979 | ||
| NAcc | Dopamine | NaCl | 7 | 0.028 ± 0.00014 |
| METH | 10 | 0.026 ± 0.00034 | ||
| DOPAC | NaCl | 7 | 10.00 ± 0.06542 | |
| METH | 10 | 11.36 ± 0.09757 | ||
| HVA | NaCl | 7 | 4.794 ± 0.03798 | |
| METH | 10 | 5.921 ± 0.04659 | ||
| PEC | Dopamine | NaCl | 6 | 0.019 ± 0.00025 |
| METH | 8 | 0.016 ± 0.00037 | ||
| DOPAC | NaCl | 6 | 1.427 ± 0.01520 | |
| METH | 8 | 1.436 ± 0.00619 | ||
| HVA | NaCl | 6 | 2.191 ± 0.00818 | |
| METH | 8 | 1.955 ± 0.00434 |
Figure 4.
Effects of conditioned musical cue in the basolateral amygdala. Time course of extracellular dopamine (mean ± SEM) during microdialysis testing. Musical cue presentation was initiated immediately prior to collection of sample 7 and terminated immediately following collection of sample 11. * p<0.05
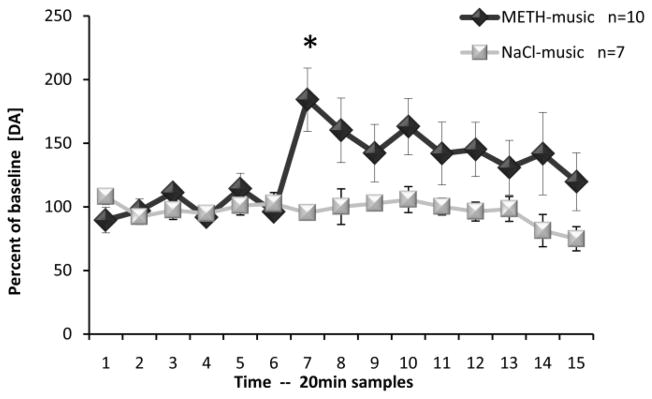
For the NAcc, analysis revealed no significant neurochemical differences between groups in average basal levels (DA: F(1,15)=0.042, p=0.840; DOPAC: F(1,15)=0.168, p=0.688; HVA F(1,15)=0.431, p=0.522; Table 2). Figure 5 shows the ability of the auditory cue to significantly increase the extracellular levels of DA in the NAcc when presented immediately before the collection of sample 7. A repeated measures ANOVA revealed significant differences from both baseline samples (Time) and control samples (Group) for DA (F(14,210)=2.281, p<0.01 and F(1,15)=5.355, p<0.05, respectively). A significant Time*Group interaction was also revealed by the ANOVA (F(14,210)=2.308, p<0.01). Post hoc Newman-Keul’s comparisons revealed a significant difference for sample 7 (p<0.05). No significant differences were observed for either metabolite (DOPAC [Group effect: F(1,15)=0.175, p=0.682; Time effect: F(14,210)=1.107, p=0.353; Time*Group interaction: F(14,210)=0.636, p=0.834] or HVA [Group effect: F(1,15)=0.021, p=0.887; Time effect: F(14,210)=0.956, p=0.50; Time*Group interaction: F(14,210)=0.609, p=0.856].
Figure 5.
Effects of conditioned musical cue in the nucleus accumbens. Time course of extracellular dopamine (mean ± SEM) during microdialysis testing. Musical cue presentation was initiated immediately prior to collection of sample 7 and terminated immediately following collection of sample 11. * p<0.05
Analysis of the basal levels of extracellular DA, DOPAC, and HVA showed no significant differences between groups in the PFC (DA: F(1,12)=2.86, p=0.117; DOPAC: F(1,12)=0.038, p=0.848; HVA F(1,12)=0.500, p=0.493; Table 2). There were also no significant effects of conditioning on DA levels in the mPFC (Figure 6). The ANOVA showed no effect of Group (F(1,12)=1.75, p=0.21), and there was no significant interaction between Group*Time (F(14,168)=1.47, p=0.12). Similar results were obtained for either metabolite: DOPAC [Group effect: F(1,12)=0.25, p=0.62; Group*Time interaction: F(14,168)=0.91, p=0.55] or HVA [Group effect: (F(1,12)=0.90, p=0.56); Group*Time interaction: (F(1,168)=0.50, p=0.49).
Figure 6.
Effects of conditioned musical cue in the medial prefrontal cortex. Time course of extracellular dopamine (mean ± SEM) during microdialysis testing. Musical cue presentation was initiated immediately prior to collection of sample 7 and terminated immediately following collection of sample 11.
4. Discussion
While the contribution of simple conditioned cues has been extensively investigated with regard to goal directed behavior, more complex contextual CS have not been comparably explored. The present study is the first to show that when a psychostimulant is paired with music in a Pavlovian conditioning paradigm, the music alone can later alter both neurochemical function and locomotor activity. These results indicate that music can serve as an effective contextual CS in rats, and that this species could potentially be used in other preclinical models utilizing musical interventions.
Using a classical conditioning paradigm, METH was an effective US using a continuous, repetitive musical cue as the CS. Presentation of the auditory cue alone significantly increased locomotor activity of rats in the Meth-Music cue condition group; this effect is consistent with previous research showing increases in locomotor activity after pairing music with MDMA (Feduccia and Duvauchelle 2008). This effect is unlikely a result of chronic METH injections alone: the locomotor activity of the Meth-NonEx and Meth-NMTest groups was each significantly lower than that of the Meth-Music treatment group. Furthermore, there were no significant differences among any of the seven METH and saline control groups. Thus, it is highly unlikely that residual effects from one week of chronic METH injections could account for the observed changes in locomotor activity of the music conditioned rats. Additionally, in either the locomotor or microdialysis paradigms, it is not possible for place conditioning to have played a role in producing the conditioned response due to the fact that the conditioning, activity, and microdialysis chambers were all characteristically distinct from one another, and each was housed in a different room. While CPP paradigms have yielded important contributions regarding contextual cues, we believe a stronger case can be made for the impact of a contextual cue when it is tested in an environment different than that used for conditioning. Use of different apparatus and environments allows the contextual cue to be the one variable that remains constant, and this eliminates any possible confounds related to conditioning and testing in the same apparatus.
The ANOVA results indicate that the increase in locomotor activity in the Meth-Music group was a function of condition, contingent upon which treatment the subjects received during conditioning. While music is a more complex CS, these results are consistent with locomotor increases seen in previous studies pairing rewarding drugs with simple CS (Bevins et al. 2001; Rodríguez-Borrero et al. 2006); and CPP results have also shown the effect that a contextual CS can have on a CR (Feduccia and Duvauchelle 2008; McCallum and Glick 2009).
The results of our in vivo microdialysis study show that a continuous, repetitive musical cue previously associated with drug administration can significantly increase extracellular DA in the BLA of rats. This suggests that elevated DA concentrations are in part involved in BLA modulation of drug-associations, conditioned responses, and cue recall/retrieval. This finding is supported by previous studies showing that inactivation of the BLA through lesion or drug blockade results in attenuation of drug seeking behaviors elicited by drug associated CS (Feltenstein and See 2007; Meil and See 1997). Dopaminergic neurons respond in an adaptive manner to events and cues that predict motivationally-significant stimuli; in the case of drug administration, re-exposure to a stimulus that has been paired with the rewarding effects of psychostimulants has been proven to reinstate drug-seeking behavior, promoting relapse by directly eliciting effects similar to the effects induced by the drugs themselves and resulting in anticipatory craving (Robinson and Berridge 1993).
The amygdala has been linked to many functional systems in the mammalian brain, including neuromodulatory, autonomic, endocrine, limbic, and neocortical circuits. Due to the anatomical position of this structure, as well as its extensive reciprocal and diverse neural projections, the amygdala serves as a confluence of sensory affective neurotransmission. Furthermore, the amygdala provides concatenate functions that facilitate communication between sensory encoding, interpretative systems, motor planning, and determination pathways (Balleine and Killcross 2006). Decades of research have implicated the amygdala as the neural substrate responsible for regulating and modifying behaviors in an adaptive manner that favors the most advantageous possible outcomes.
The amygdala’s role in emotion is related to its altering, modulating, and integrating the functions of other brain systems, i.e., implementing behavioral response patterns to accommodate internal affective states that are influenced by external, predictive stimuli (Mannella et al. 2007). Therefore, it is likely that the dopaminergic increases we observed in the BLA are a result of positive emotional experiences elicited by the pairing of METH administration with music. Emotion-related behavioral responding can be viewed as the coordinated processing of amygdaloid nuclei: the basolateral amygdala encodes and retrieves the affective value of environmental stimuli, and directs complex adaptive behavioral responses to an older central amygdala system that enables cortical structures to recruit incentive motivational processes and invigorate emotional responding (Everitt et al. 2003).
The memory consolidation function of the BLA is thought to be dependent on its cooperative effects with several forebrain structures, including the NAcc. Evidence has suggested that emotionally significant memories are modulated by the concurrent activation of the BLA and NAcc (LaLumiere et al. 2005). In the latter study, roles of the shell and core of the NAcc were investigated in order to differentiate the relative importance of each subdivision in the memory consolidation process. Their results strongly indicate that activation of DA receptors in the shell, but not in the core, are associated with the modulation of memory mediated by the BLA. Stimulation of the BLA increased DA release in the NAcc shell, suggesting that the shell serves as a downstream component critical in the consolidation process of associative learning involving reinforcing stimuli. In contrast, an experiment conducted by Di Ciano and Everitt (2004) neuropharmacologically disconnected the NAcc core and BLA from the limbic cortical-ventral striatopallidal circuitry to assess their role in cocaine-seeking behavior. The procedure reportedly interrupted conditioned stimulus-induced reinstatement of responding after extinction. The authors concluded that it is the combined influence of the BLA and NAcc core that selectively mediates conditioned-reinforcer-elicited goal-directed behavior. Moreover, several previous studies have also shown differential control over drug seeking behavior in the shell vs. core regions of the accumbens (Fuchs et al. 2008; Ito et al. 2000; Ito et al. 2004).
There are several factors that may explain differences in the literature regarding shell vs. core involvement in drug seeking behaviors. These are small anatomical regions, and therefore it is possible that microinjections into one region may diffuse into neighboring areas. Other differences in the literature reflect disparities in drug-delivery, e.g., contingent vs. non-contingent drug administration. In addition, DA, like other catecholamines, may exert its effect by means of volume transmission; this entails bulk release into the surrounding extracellular fluid where it can diffuse to local synaptic boutons. Consequently, isolation of neighboring but discrete DA-containing structures can prove extremely difficult and imprecise. Accordingly, in the present study, we chose not to distinguish between the two sub-regions of the NAcc when determining dialysis coordinates.
Much like our results in the BLA, in vivo microdialysis within the NAcc also demonstrated a dopaminergic response to our musical CS. We observed a significant increase in extracellular DA immediately following onset of the musical cue. This indicates that complex drug-associated cues can evoke an increase in extracellular DA within the NAcc, an observation supported by previous literature. Ikagami et al (2007) found that cues paired with cocaine were able to enhance typical NAcc DA responses to the drug itself, compared to non-reward cues. Additionally, it has been shown that animals in a drug-free state exhibit an increase in NAcc DA levels when placed in an environment previously paired with cocaine (Gerasimov et al 2001). In a self-administration paradigm, cocaine-associated stimuli elicited increases in extracellular DA within the NAcc while animals were in a drug-free state (Weiss et al 2000). It has also been previously reported that extracellular DA in the NAcc was increased when music was paired with MDMA during operant self-administration. (Feduccia and Duvauchelle 2008). Our results suggest that the NAcc, along with the BLA, may be involved in the association of drugs with complex environmental cues, such as music. Furthermore, it is changes in the levels of DA in response to cues that may mediate learning and responses to drug-cue associations.
The role of DA in learning has been well documented for midbrain DA neurons; however, the literature is limited with regard to dopaminergic responses to error prediction in cortical neurons. We found no significant changes in extracellular DA levels in the mPFC after presentation of the musical CS. Similarly, a recent report examining the effects of a cocaine-paired CS found PFC DA levels to be unaltered after presentation of the drug paired stimuli (Ikegami et al. 2007). In the present study, the mPFC was included as an area of interest because of its involvement in associative learning and its modulatory influence over key components of the incentive-salience pathway. Our initial expectation was that DA-evoking stimuli would have a pervasive effect on DA receiving terminals, and therefore dopaminergic alterations in the mPFC would follow suit with the other regions under investigation. However, this did not occur, perhaps due to the anatomical differences between regions of the mPFC.
Previous research indicates that the prelimbic portion of the mPFC is crucial for drug relapse, is involved in drug seeking behaviors, and can by activated by drug-associated cues (Rocha & Kalivas, 2010; McLaughlin and See 2003; Di Pietro et al., 2003; Kalivas and O’Brien, 2008; Miller and Marshal 2004; Zavala et al. 2003). In contrast, the infralimbic cortex appears to be extensively involved in extinction learning, is not necessary for methamphetamine-reinstatement, and is not activated by drug-paired cues (LaLumiere et al 2010; Peters et al 2008; Rocha & Kalivas, 2010; Miller and Marshal 2004). The current microdialysis study did not differentiate between these distinct regions of the mPFC, which may account for the lack of a significant response. Furthermore, previous research has also indicated that involvement of the prelimbic cortex in reinstatement models may be dependent upon the type of cue used (Di Pietro et al 2006). Therefore, further investigation would be required to determine if our lack of effect is due to regional discrimination or to the musical cue itself.
Replacement of a commonly utilized simple CS with a more complex contextual music cue allowed for investigation of associative learning that more closely resembles higher level functioning and mental processes that occur during subjective human drug experiences. While the literature is inconsistent with regard to whether animals appreciate music, this is certainly not necessary for its utilization in animal models of addiction. In fact, it may prove to be advantageous if subjects do not experience pleasure from the music alone, as this would further complicate analysis and interpretation. Results pertinent to this point indicate that rats have the ability to discriminate musical passages, and that when music is paired with drug reward in a conditioning paradigm, the music can influence drug seeking behavior (Feduccia and Duvauchelle 2008; Otsuka et al. 2009). However, it should be noted that both of these latter studies concluded that music alone did not have a primary reinforcing effect in rats. Phylogenetic considerations may determine the reinforcing properties of music; that is, the music used in the current experiments was obviously made and adjusted by humans. In order to truly address the reinforcing quality of music in other animal species, the music may have to match that particular animal’s audiogram. However, our goal was not to see if rats had an appreciation for Miles Davis, it was to determine whether a complex musical passage could effectively be used as a contextual CS in a Pavlovian learning paradigm.
Preoccupation with obtaining and consuming substances is the distinguishing characteristic of drug addiction. Learning-induced, plastic changes at dopaminergic synapses appear to be responsible for this compulsive drug-seeking behavior. Cue-elicited craving is a fundamental component of addiction, and is at least partially responsible for precipitating relapse (Volkow et al. 2006). The present study was the first classical conditioning paradigm to employ an abstract continuous musical stimulus as the conditioned cue in rats. The locomotor activity results established the effectiveness of the musical auditory cue. The BLA and NAcc DA data provided evidence that the musical cue has the ability to stimulate the dopaminergic circuitry associated with memory modulation and retrieval responsible for associative forms of learning. This work further solidifies the notion that lower order species have the ability to discriminate complex musical passages, and suggests that rats could be used in other preclinical models that utilize musical interventions.
Acknowledgments
This work was supported by National Institute on Drug Abuse training grant number 5T32DA007307-10 and National Institute on Drug Abuse grant number 5R01DA016283-05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends in Neurosciences. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bauldoff GS. Music: More than just a melody. Chron Respir Dis. 2009;6:195–7. doi: 10.1177/1479972309346752. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: Dopaminergic and GABAergic influences on conditioned expression. Pharmacology Biochemistry and Behavior. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98:11818–23. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. European Journal of Pharmacology. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bradt J, Dileo C. Music for stress and anxiety reduction in coronary heart disease patients. Cochrane Database Syst Rev. 2009:CD006577. doi: 10.1002/14651858.CD006577.pub2. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Niet G, Tiemens B, Lendemeijer B, Hutschemaekers G. Music-assisted relaxation to improve sleep quality: meta-analysis. J Adv Nurs. 2009;65:1356–64. doi: 10.1111/j.1365-2648.2009.04982.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behavioral Neuroscience. 2003;117:952–960. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–73. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Annals of the New York Academy of Sciences. 2003:233–250. [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Duvauchelle CL. Auditory stimuli enhance MDMA-conditioned reward and MDMA-induced nucleus accumbens dopamine, serotonin and locomotor responses. Brain Research Bulletin. 2008;77:189–196. doi: 10.1016/j.brainresbull.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiology of Learning and Memory. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Dong N, Keller RW, Jr, Carlson JN. Estimating extracellular concentrations of dopamine and 3,4- dihydroxyphenylacetic acid in nucleus accumbens and striatum using microdialysis: Relationships between in vitro and in vivo recoveries. Journal of Neurochemistry. 1994;62:2017–2021. doi: 10.1046/j.1471-4159.1994.62052017.x. [DOI] [PubMed] [Google Scholar]
- Gold C, Solli HP, Kruger V, Lie SA. Dose-response relationship in music therapy for people with serious mental disorders: systematic review and meta-analysis. Clin Psychol Rev. 2009;29:193–207. doi: 10.1016/j.cpr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Olsen CM, D’Souza MS, Duvauchelle CL. Experience-Dependent Effects of Cocaine Self-Administration/Conditioning on Prefrontal and Accumbens Dopamine Responses. Behavioral Neuroscience. 2007;121:389–400. doi: 10.1037/0735-7044.121.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. Journal of Neuroscience. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nature Neuroscience. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jung XT, Newton R. Cochrane Reviews of non-medication-based psychotherapeutic and other interventions for schizophrenia, psychosis, and bipolar disorder: A systematic literature review. Int J Ment Health Nurs. 2009;18:239–49. doi: 10.1111/j.1447-0349.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences. 2000:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Nawar EM, McGaugh JL. Modulation of memory consolidation by the basolateral amygdala or nucleus accumbens shell requires concurrent dopamine receptor activation in both brain regions. Learning and Memory. 2005;12:296–301. doi: 10.1101/lm.93205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Attenuation of the reinforcing efficacy of morphine by 18-methoxycoronaridine. European Journal of Pharmacology. 1999;383:15–21. doi: 10.1016/s0014-2999(99)00560-9. [DOI] [PubMed] [Google Scholar]
- Mannella F, Mirolli M, Baldassarre G. The role of amygdala in devaluation: A model tested with a simulated rat. Proceedings of the Seventh International Conference on Epigenetic Robotics: Modeling Cognitive Development in Robotic Systems; Lund University Cognitive Studies; 2007. [Google Scholar]
- Mays KL, Clark DL, Gordon AJ. Treating addiction with tunes: a systematic review of music therapy for the treatment of patients with addictions. Subst Abus. 2008;29:51–9. doi: 10.1080/08897070802418485. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Glick SD. 18-Methoxycoronaridine blocks acquisition but enhances reinstatement of a cocaine place preference. Neurosci Lett. 2009;458:57–9. doi: 10.1016/j.neulet.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Floresco SB. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience. 2007;146:1484–1494. doi: 10.1016/j.neuroscience.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self- administered cocaine. Behavioural Brain Research. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28:175–84. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Nilsson U. The anxiety- and pain-reducing effects of music interventions: a systematic review. Aorn J. 2008;87:780–807. doi: 10.1016/j.aorn.2007.09.013. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? Journal of Psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Otsuka Y, Yanagi J, Watanabe S. Discriminative and reinforcing stimulus properties of music for rats. Behavioural Processes. 2009;80:121–127. doi: 10.1016/j.beproc.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Borrero E, Bernardo Colón A, Burgos-Mártir MA, Álvarez Carillo JE, Estrada del Campo Y, Abella-Ramírez C, Maldonado-Vlaar CS. NMDA antagonist AP-5 increase environmentally induced cocaine-conditioned locomotion within the nucleus accumbens. Pharmacology Biochemistry and Behavior. 2006;85:178–184. doi: 10.1016/j.pbb.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a systematic review. Ann Clin Psychiatry. 2009;21:213–36. [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. European Journal of Pharmacology. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Sutoo D, Akiyama K. Music improves dopaminergic neurotransmission: demonstration based on the effect of music on blood pressure regulation. Brain Res. 2004;1016:255–62. doi: 10.1016/j.brainres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J Neurosci. 2007;27:3937–45. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: Effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]