Abstract
We previously identified metal-responsive transcription factor-1 (MTF-1) as a positive contributor to mouse fibrosarcoma growth through effects on cell survival, proliferation, tumor angiogenesis and extracellular matrix remodeling. In the present study, we investigated MTF-1 protein expression in human tissues by specific immunostaining of both normal and tumor tissue samples. Immunohistochemical (IHC) staining of a human tissue microarray (TMA), using a unique anti-human MTF-1 antibody, indicated constitutive MTF-1 expression in most normal tissues, with liver and testis displaying comparatively high levels of expression. Nevertheless, MTF-1 protein levels were found to be significantly elevated in diverse human tumor types, including breast, lung and cervical carcinomas. IHC analysis of a separate panel of full-size tissue sections of human breast cancers, including tumor and normal adjacent, surrounding tissue, confirmed and extended the results of the TMA analysis. Taken with our previous findings, this new study suggests a role for MTF-1 in human tumor development, growth or spread. Moreover, the study suggests that MTF-1 could be a novel therapeutic target that offers the opportunity to manipulate metal or redox homeostasis in tumor cells.
Keywords: MTF-1, human tumors, human tissue microarray, immunohistochemistry, human breast carcinomas
Introduction
Metal-responsive transcription factor-1 (MTF-1) is a unique Cys2His2 zinc finger protein that is evolutionarily conserved from Drosophila to humans. MTF-1 is activated by heavy metals, redox stresses, growth factors and cytokines, and its established targets are important for metal homeostasis, embryogenesis and hematopoiesis.1-3 MTF-1 may also possess RNA-binding properties that enable it to participate in the post-transcriptional control of certain stress-related cell survival pathways.4 Pathological consequences of MTF-1 activity in normal tissue were recently highlighted by reports that MTF-1 has a role in neurodegenerative diseases associated with expression of the prion protein receptor.5,6 Our own studies have revealed roles for MTF-1 in both extracellular matrix (ECM) remodeling and experimental tumorigenesis.7-11 In this connection, MTF-1 activity is modulated by metals (e.g., zinc, copper and cadmium), oxidants (e.g., reactive oxygen species, ROS; nitric oxide, NO), hypoxia, and the cytokine interleukin-6. All of these can contribute to tumorigenic phenotypes such as enhanced cell survival and proliferation, tumor angiogenesis, the establishment of tumor microenvironments involving tumor/host cell inflammatory signaling, and growth factor independence.2,9,10,12-16
The MTF-1 protein contains six highly conserved zinc finger domains (and an N-terminal cysteine cluster) that regulate its transcriptional activity through direct sensing and binding of intracellular free zinc (Zn2+) and subsequent DNA binding.2,17 Some activators of MTF-1 (e.g., ROS, NO, cadmium) exert their effects through release of Zn2+ from cellular storage proteins such as the metallothionein (MT) family, which is the prototypic MTF-1 target.2,8,12 The underlying mechanisms of action of other MTF-1 inducers, including hypoxia, are unknown but may involve more complex controls.18 Activated MTF-1 binds to metal response elements (MREs; TGCRCNC, in which R represents a purine and N is any nucleotide) within target promoters; the result is either induction or repression of expression, depending on the target gene, MRE flanking sequences and cellular context.2,18-22 Interactions with other transcription factors—e.g., nuclear factor-κB (NFκB), hypoxia-inducible transcription factor-1, (HIF-1), nuclear factor 1 and SP1—and posttranslational modifications (e.g., phosphorylation) of MTF-1 are also likely determinants of its targets and transactivational activity.2,11,18,23-26
MTF-1 directly induces the expression of several genes that are likely contributors to phenotypes found in solid tumor microenvironments, such as the genes for MTs (MT1, MT2A), Zn transporter-1 (ZNT1/SLC30A1) and placenta growth factor (PGF).1,9,18,27 Other MTF-1 targets or partners, such as HIF-1, transforming growth factor-β1 (TGFβ1), tissue transglutaminase 2 (TGM2), CCAAT/enhancer binding protein α, seleno-protein W, and N-myc downstream-regulated gene 1,7,10,20,21 are overexpressed (or activated) in some human tumors, including breast carcinomas; immunostaining patterns of these proteins are reported to correlate with tumor progression and disease recurrence.8,28-35 MTs, which are considered to protect cells against ROS and other electrophilic agents, contribute to proliferation, survival and energy-generating pathways in normal cell types;36-41 it is likely that they function similarly in tumor cells. Placenta growth factor (PGF) is a member of the vascular endothelial growth factor (VEGF) family of pro-angiogenic cytokines; PGF expression correlates with stage, vascularity, survival, metastasis and recurrence of cancers including breast carcinomas.29,30 HIF-1, which is a critical regulator of the cellular response to hypoxia, regulates the expression or activity of various proteins involved in tumorigenesis.42,43 High HIF-1α protein levels occur in several human tumor types and correlate with increased risk of mortality.34,43 Finally, increased expression of the ECM-modifying proteins TGFβ1 and TGM correlates with survival and metastasis in some aggressive tumor types.31-33
Our earlier studies demonstrated the importance of MTF-1 for tumor cell survival and proliferation, angiogenesis and ECM remodeling, and focused in vivo on mouse tumor xenografts.7-11 In the present study, for the first time we provide evidence demonstrating elevated MTF-1 protein expression in human carcinomas, including lung, cervical and breast carcinomas.
Results
Specific detection of MTF-1 protein on immunoblots
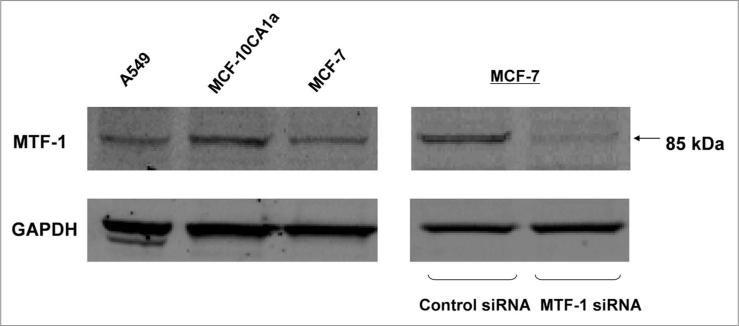
The MTF-1 antibody was tested for its ability to selectively detect MTF-1 in whole cell extracts by immunoblotting. Diverse human carcinoma cell lines—MCF-7, MCF-10CA1a, A549 cells—were selected for this purpose. Figure 1 (left) shows that these cell lines were found to express similar levels of MTF-1, which migrated at approximately 80–90 kDa. The specificity of antibody detection was verified by siRNA knockdown of MTF-1 in MCF-7 cultures (right).
Figure 1.
Detecion of MTF-1 protein in cultured human cells. human breast (MCF-10Ca1a, MCF-7) and lung (a549) carcinoma cells were grown to subconfluence, and whole cell extracts were used for immunoblotting. To confirm the specificity of the antibody for MTF-1 protein, MCF-7 cells were transfected with scrambled control or MTF-1 siRNa duplexes 6 d before harvesting for total protein.
MTF-1 visualization in fixed human carcinoma cells
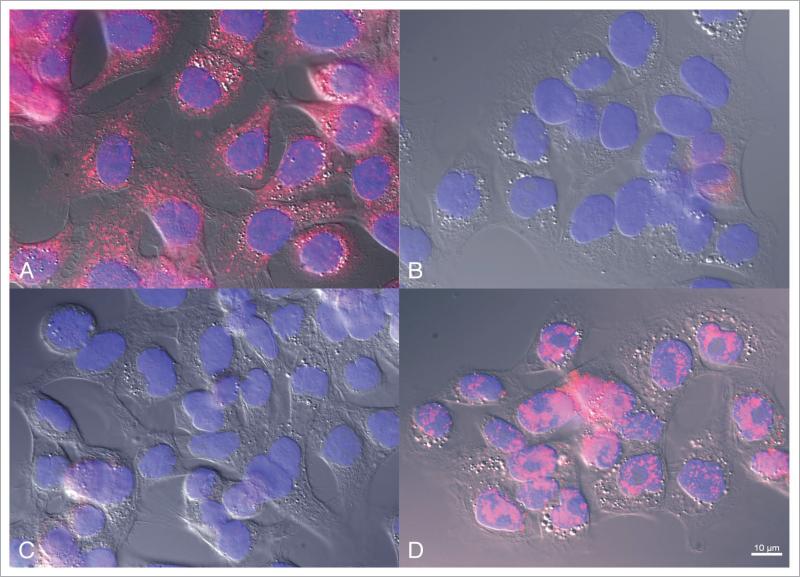
MCF-10CA1a human breast carcinoma cells were used to demonstrate the ability of the MTF-1 antibody to specifically detect MTF-1 protein in intact cells. MCF-10CA1a cells were cultured on glass cover slips, fixed and stained with the MTF-1 antibody as described in Materials and Methods. Negative controls included incubation and staining with pre-immune serum and peptide blocking of the primary MTF-1 antibody with its immunogen before the antibody was added to cells. Figure 2A shows that MTF-1 immunostaining was primarily cytoplasmic in cells, with some weak nuclear and peri-nuclear signals. When the primary antibody was replaced with pre-immune serum, MTF-1 signals were essentially undetectable (Fig. 2B). Similar results to those shown in Figure 2B were observed in fixed cells that were incubated with the immunizing peptide before treatment with the MTF-1 antibody (Fig. 2C). When a random, control peptide was pre-incubated with the primary MTF-1 antibody, no blocking effect was observed (Fig. 2D). Together, the results in Figures 1 and 2 demonstrate that the MTF-1 antibody is able to recognize human MTF-1 with high specificity, as indicated by immunoblotting of total cell protein and in fixed cells.
Figure 2.
MTF-1 immunofluorescence detection in a human breast cancer line. MCF-10Ca1a breast cancer cells were cultured and labeled with (A) affinity -purified MTF-1 antibody, (B) pre-immune serum, (C) immunogen-blocked MTF-1 antibody, and (D) random, control peptide-blocked MTF-1 antibody. Each panel is a merged composite of DAPI labeling (pseudo-colored blue), MTF-1 (red) and DIC (grey) in registration.
MTF-1 protein expression in human tumor and normal tissues
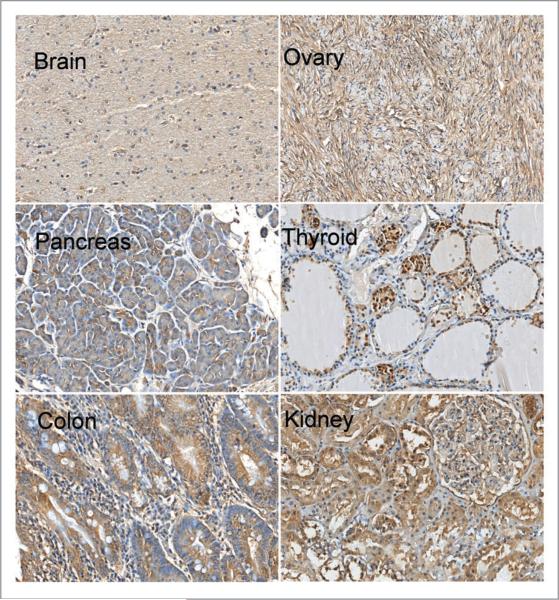
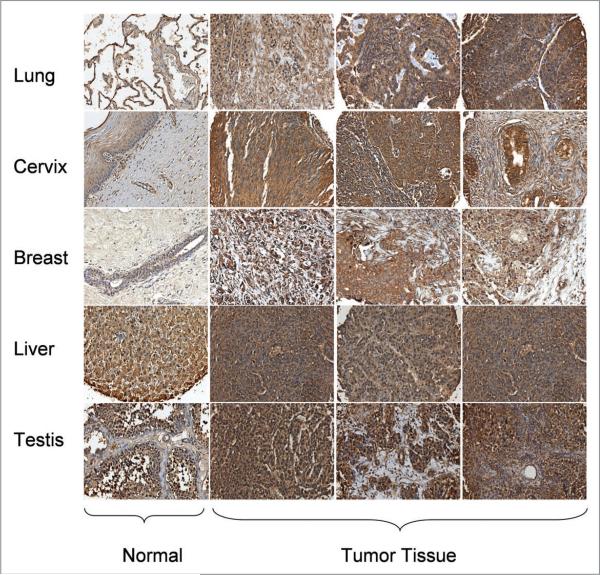
After validating the MTF-1 antibody, we used it to screen a National Disease Research Interchange (NDRI) Pan Cancer Tissue Microarray, which represents both tumor and normal human tissues (see Materials and Methods and Suppl. Table for details), for levels of MTF-1 protein expression. Figures 3 and 4 show representative immunostaining for MTF-1 protein in normal tissues. MTF-1 protein was detected with various levels of immunostaining intensity in all normal tissues in the array. This result is consistent with the ubiquitous expression of this transcription factor in rodent tissues.44 Overall, MTF-1 immunostaining was lowest in brain; moderately high in breast, ovary, pancreas and lung; and high in thyroid, colon, cervix and kidney. MTF-1 immunostaining was most intense in liver and testis. The elevated expression levels found in testis are consistent with the results of an earlier study reporting high mRNA levels of MTF-1 in mouse testis.45 MTF-1 immunostaining was generally cytoplasmic, but showed some nuclear localization, especially in testis; cytoplasmic MTF-1 immunostaining is consistent with that found in MCF-10CA1a cells (Fig. 2A). IHC analysis of the array showed relatively high levels of MTF-1 expression in certain human tumors (e.g., lung, breast and cervical carcinomas) compared with the corresponding normal tissues (Fig. 4). No significant differences in MTF-1 staining patterns were found in liver and testicular tumors as compared with normal tissues (Fig. 4). This observation may reflect the normally high MTF-1 expression patterns within these two tissue types. As a caveat, the tumor specimens in the array were derived from needle biopsy samples and thus do not contain normal adjacent, surrounding (called “surrounding” hereafter) tissue as an internal control; nevertheless, these findings are novel and indicate that MTF-1 protein expression is high in diverse human carcinomas.
Figure 3.
Representative IHC analysis of MTF-1 expression in normal tissue sections from a human. TMA (x200 magnification). IHC analysis of MTF-1 expression in six normal human tissues.
Figure 4.
Representative IHC analysis of MTF-1 expression in normal and cancer tissue sections from a human. TMA (x200 magnification). IHC analysis of MTF-1 expression in normal and tumor tissues of human lung, cervix, breast, liver and testis.
MTF-1 is highly expressed in human breast tumors
To address the potential caveat mentioned above, we investigated MTF-1 immunostaining in a panel of full-size, formalin-fixed, paraffin-embedded specimens of human breast carcinomas that were resected during routine surgical oncology procedures (see Materials and Methods for details). Figure 5 shows representative examples of MTF-1 immunostaining from this panel: MTF-1 immunostaining intensities varied widely among individual tumors, but tumor cells within each specimen generally had higher MTF-1 expression than the levels measured in normal tissue. To obtain a semiquantitative estimate of MTF-1 protein expression levels in the panel of breast carcinoma specimens, two pathologists (Shi Y and Amin K) independently evaluated MTF-1 immunostaining of the panel using a scoring system (see Materials and Methods for details). Table 1 shows that MTF-1 protein expression was significantly higher in tumor than in normal surrounding tissue for all 71 patient tumors (p value <0.0001).
Figure 5.
MTF-1 expression is elevated in human breast cancer tumor tissue compared with normal surrounding tissue. Clinical human breast tumor specimens (71) were immunostained using the MTF-1 antibody. (A) Representative image shows both tumor and normal surrounding tissues in one field taken at x100 magnification. (B and C) Increased magnification (x400) of tumor (B) and normal tissue (C).
Table 1.
Statistical analysis of protein levels of MTF-1 in human breast cancer patients
| Mean (±SEM; N = 71) | |
|---|---|
| Tumor tissue | 175.8 (±6.76) |
| Normal surrounding tissue | 123.5 (±5.54) |
| p-value | <0.0001 |
Immunostained sections from the 71 clinical human breast tumor specimens were evaluated. MTF-1 protein content was semi quantitatively measured in both the breast tumors and the normal surrounding tissue of each tumor, and a paired t-test was used to test for statistically significant differences. The data are presented as the mean ± standard error of the mean.
Discussion
The novel findings described here for MTF-1 protein expression in normal and transformed human tissues provide important evidence implying a role for MTF-1 in human tumor development. Our earlier studies showed that MTF-1 contributes to experimental mouse tumor biology through effects on cell survival, tumor angiogenesis and ECM remodeling.8-11,13 The present study extends this research to human tumor biology in two ways. First, we used specific immunostaining of a human tissue microarray with a new antibody to obtain evidence that MTF-1 expression is elevated in multiple human tumor types, including breast, lung and cervical carcinomas, compared with its expression in corresponding normal tissues, (Fig. 4). Second, we used immunostaining of a collection of resected human breast carcinomas to confirm that MTF-1 protein expression is significantly elevated in a common human carcinoma relative to expression in normal surrounding tissue (Fig. 5 and Table 1).
It is noteworthy that variable MTF-1 expression was detected by immunostaining in all the normal human tissues present in the tissue microarray. As mentioned in Results, the ubiquitous expression of MTF-1 in normal human tissues is consistent with its functional role in maintaining cellular metal and redox homeostasis.8,21,46 The relatively abundant levels of MTF-1 protein in human liver and testis tissues (Fig. 4) are consistent with results of previous studies that found a critical role for MTF-1 in liver development in the mouse,47 as well as high MTF-1 mRNA content in mouse testis.45 The functional importance of relatively high MTF-1 protein expression and, presumably, activity in these tissues remains unclear. MTF-1 immunostaining of liver and testicular tumors found no apparent differences between tumor tissues and the corresponding normal tissues (Fig. 4). This finding may simply reflect the already high MTF-1 protein expression levels within liver and testis; it is also consistent with a recent study that showed no significant differences in MTF-1 levels between human hepatocellular carcinoma samples and matched controls.48
The elevated expression of MTF-1 protein found in breast carcinoma cells in full-size clinical specimens is consistent with other reports of high expression of a prototypic MTF-1 target, MT2A, within human breast tumors.49,50 Furthermore, MT expression has been reported to correlate with tumor progression and to predict tamoxifen resistance in invasive ductal breast cancer.50,51 Other MTF-1 targets and transcriptional partners, such as PGF, HIF-1α and NFκB, are also overexpressed in breast tumors.29,35,43 In addition, high levels of Zn2+, an inducer of MTF-1 transactivation and some forms of drug resistance, accumulate in experimental breast tumors and human breast tumors.52,53 Additional studies are required to determine any possible correlation between MTF-1 protein levels and histological classification, proliferation index, tumor stage, prognosis and molecular markers such as estrogen and HER2/Neu receptors of human breast tumors. It will also be important to examine MTF-1 expression in full-size cervical, lung, liver and testicular tumors.
In summary, the findings of the present study, taken with our previous findings,8-11,13 highlight the importance of MTF-1 in human tumors. A recent report showing that a novel Zn2+ chelator, LOR-253, can inhibit lung and colon xenograft growth, proliferation and angiogenesis in association with changes in MTF-1 protein levels54 indicates that efforts to reduce MTF-1 protein expression or MTF-1 activity may represent a therapeutic opportunity for the treatment of some solid tumors. The underlying mechanisms that regulate MTF-1 expression and transcriptional activation in growing tumors remain unclear, but they may involve both genetic and microenvironmental components.
Materials and Methods
Production of a MTF-1 antibody for immunohistochemistry
An anti-MTF-1 rabbit polyclonal antibody (NEP-5717; called the “MTF-1 antibody” hereafter) was custom generated by New England Peptide LLC (Gardner, MA). A sequence at the N-terminus (but outside the Zn finger domain) of the human MTF-1 protein was chosen for its potential to generate a specific polyclonal antibody active in both IHC and immunoblotting analyses. A synthetic peptide, (H2N)-MGEHSPDNNIIYFEAEC-(amide) at the amino terminus (AA 1–16), was used to immunize rabbits and the resulting serum antibody was screened by ELISA and affinity purified. The cysteine at the carboxy terminus of the sequence was added for coupling purposes. Pre-immune serum was also collected and used as a control in the IHC studies described here. Negative controls included peptide titration of the antibody.
Cell lines
The MCF-7 human breast carcinoma cell line (maintained in MEM + 1 mM sodium pyruvate + 10% FBS) and the A549 human non-small cell lung cancer cell line (maintained in DMEM + 10% FBS) were purchased from the American Type Culture Collection (ATCC, Manassas, VA). The metastatic human MCF-10CA1a breast carcinoma cell line was purchased from the Michigan Cancer Foundation and grown in DMEM + 10% FBS.
RNA interference (RNAi) strategy
An siRNA sequence against human MTF-1 was designed from a published mouse sequence.55 The corresponding human sequence is 3'-CTG ATT CCC ATT GAA GCA CTA-5'. To determine the specificity of this siRNA against human MTF-1, we used RNAiMAX (Invitrogen, Carlsbad, CA) to transfect MCF-7 cells with the MTF-1 siRNA or a scrambled sequence negative control, and subsequently monitored MTF-1 protein and MT-IIA mRNA levels (by qRT-PCR, probes and primers were from Applied Biosystems, Foster City, CA, Cat #Hs02379661 g1). Briefly, first-strand cDNA was synthesized from total RNA using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). PCR condition: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Reactions were run in triplicates in a 7300 real-time PCR System (Applied Biosystems, Foster City, CA).
Immunoblotting
Cells were plated in 60 mm culture dishes at 0.7 × 106 cells/dish in DMEM + 10% FBS. Once the cultures reached about 70% confluency, whole cell extracts were prepared using an NP-40 based buffered solution (50 mM Tris pH 7.4, 250 mM NaCl, 50 mM NaF, 0.5% NP-40, 1 mM Na3VO4, 15.7 mM Na4P2O7) containing 2 μg/ml aprotinin, 2 μg/ml leupeptin and 120 μg/ml PMSF. Final lysate supernatants were prepared by centrifugation at 9,000 xg at 4°C. Total cell protein was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using NuPAGE Novex Bis-Tris 4–12% gels and a NuPAGE MOPS running buffer (Invitrogen). It was then transferred to Immobilon FL membranes (Millipore Corp., Bedford, MA). Blots were probed with specific antibodies, and proteins were detected using an Odyssey Infrared Imaging system according to the manufacturer's protocol (LI-COR, Lincoln, NE). The primary antibody against human MTF-1 is described above; an antibody for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cat #: sc-25778) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Ponceau S (Sigma Chemical Co., St. Louis, MO) staining was used to verify equal protein loading and transfer.
Cellular immunohistochemistry
MCF-10CA1a cells were grown on 18 mm2 glass cover slips to 50% density and processed for immunofluorescence. Briefly, cells were rinsed with PBS (37°); fixed (20 min) in freshly prepared 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer; rinsed three times (10 min each) in PBS; and permeabilized with PBS/0.1% Triton X-100 (30 min) prior to blocking (30 min) in 10% normal donkey serum in bovine serum albumin (BSA) buffer (2% BSA/0.1 M phosphate buffer, pH 7.4). This medium was aspirated, and the cells were incubated with the primary MTF-1 antibody (4 μg/ml in BSA buffer) for an additional 30–60 min at room temperature (RT). To control for primary MTF-1 antibody specificity, this antibody was preincubated with a x100 molar excess of the 17-mer immunogen (above) or an irrelevant peptide (NQEQVSP; mw 800.8) for 60 min at RT, centrifuged (50,000 rpm, 4°) to remove precipitates and incubated with cells as above. Cells were rinsed three times with PBS (10 min each), and then labeled with a Cy3-conjugated donkey anti-rabbit F(ab')2 fragment antibody [minimally cross-reactive IgG (H + L); Jackson ImmunoResearch, West Grove, PA] at 5 μg/ml in BSA buffer (30–60 min at room temperature). Cells were next rinsed two times with PBS (10 min each) followed by a final rinse in 0.1 M Tris, pH 8.5 (5 min). The cover slips were mounted on microscope slides under an anti-fade medium containing diamidinophenylindole dihydrochloride (DAPI; ProLong Gold, Molecular Probes/Invitrogen, Carlsbad, CA) and sealed. Dried salts were removed with de-ionized water, and the slides were dried and stored at -20°C. Images were obtained using a Leica DM 5500B microscope (Bannockburn, IL; e.g., at x63 magnification) equipped with a Retiga-SRV camera (Q-imaging, Surrey, BC) and Image Pro Plus acquisition software (Media Cybernetics, Bethesda, MD).
IHC of tissue microarray and clinical human breast tumor specimens
The Comprehensive Pan Cancer Tissue Microarray (TMA) was obtained from the NDRI. This array consists of five separate slides representing 522 tumors (20 tumor types; called TMA1-4 here) and 120 control biopsies (called TMA5 here) from human tissue. Clinical breast cancer samples (formalin-fixed, paraffin-embedded human breast cancer specimens) were obtained from Dr. Murat Ascaroy (Duke University), and handled by standard oncology procedures.
Tissue sections were deparaffinized with xylene and rehydrated in ethanol solution, and then endogenous peroxidase activity was quenched by treatment with 3% hydrogen peroxide for 15 min. MTF-1 antigen retrieval was achieved by immersing sections in CytoQ solution (Innovex Biosciences) followed by microwave irradiation (750 W, two exposures, 4 min/exposure). After the sections were blocked with 5% donkey serum (15 min), they were incubated with the primary MTF-1 antibody (1:100 dilution) overnight at 4°C. As a negative control, some sections were incubated with the non-immune rabbit serum instead of the primary antibody. All sections were thoroughly rinsed with PBS and incubated with a biotinylated donkey anti-rabbit secondary antibody (1:2,000 dilution) for about 30 min at 37°C. A streptavidin-peroxidase complex (ABC Kit, Vector Laboratories, Burlingame, CA) was added for 30 min at 37°C. Immunostaining was completed by incubation with the peroxidase substrate diaminobenzidine (DAB) for 5 min. Sections were rinsed and then counterstained with hematoxylin. Images of whole slides were acquired using an iScan slide scanner (BioImagene, Inc., Sunnyvale, CA). The resolution of the images was 0.46 μm/pixel at 20 magnification. Areas of each slide were prescreened, and an area of interest was manually selected for image processing.
Scoring of the clinical tumor specimens of human breast cancers
Immunostained sections from clinical human breast tumor specimens were evaluated by using an imaging system consisting of a charge couple device (CCD) camera coupled to a microscope (Axioskop 2 plus, Carl Zeiss, Berlin, Germany). To estimate the intensity of immunostaining for MTF-1 in the specimens, images were first inspected at low magnifications (x25 and x50) and the percentages of positively staining cells in both normal surrounding tissue and tumor tissue were evaluated (reviewed in ref. 56). Each sample was next inspected at medium and medium-high magnifications (x100, x200, respectively) to assign intensity values based on the semi-quantitative scale of 0 to 3, where 0 is no staining, 1 is weak, 2 is moderate and 3 is strong. Immunostaining intensity scores were calculated by using the following formula: weighted signal intensity = percentage of immunostained cells × average intensity score.
Statistical analysis
The paired t-test was used to test statistical differences between MTF-1 staining intensities within tumors and within normal surrounding tissues in 71 human breast cancer specimens.
Supplementary Material
Acknowledgements
We greatly appreciate the technical assistance of Theresamai Le and Guita Lalehzadeh. We also thank Dr. Gui-shuang Ying (University of Pennsylvania) for invaluable assistance with statistical analysis. This study was supported by NIH Grant CA57692, awarded to the late Brian J. Murphy.
Abbreviations
- MTF-1
metal-responsive transcription factor-1
- IHC
immunohistochemistry
- TMA
tissue microarray
- ECM
extracellular matrix
- Zn
zinc
- MT
metallothionein
- NFκB
nuclear factor-κB
- HIF-1
hypoxia-inducible transcription factor-1
- ZNT1
Zn transporter-1
- PGF
placenta growth factor
- TGFβ1
transforming growth factor-β1
- TGM2
tissue transglutaminase 2
- VEGF
vascular endothelial growth factor
- NDRI
national disease research interchange
Footnotes
Supplementary materials can be found at: www.landesbioscience.com/supplement/ShiCBT9-6-Sup.pdf
References
- 1.Lichtlen P, Schaffner W. Putting its fingers on stressful situations: the heavy metal-regulatory transcription factor MTF-1. Bioessays. 2001;23:1010–7. doi: 10.1002/bies.1146. [DOI] [PubMed] [Google Scholar]
- 2.Laity JH, Andrews GK. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1). Arch Biochem Biophys. 2007;463:201–10. doi: 10.1016/j.abb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wimmer U, Lichtlen P, Inderbitzin D, Stieger B, Meier PJ, et al. Metal-responsive transcription factor-1 (MTF-1) is essential for embryonic liver development and heavy metal detoxification in the adult liver. Faseb J. 2004;18:1071–9. doi: 10.1096/fj.03-1282com. [DOI] [PubMed] [Google Scholar]
- 4.Adilakshmi T, Laine RO. Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival or death. J Biol Chem. 2002;277:4147–51. doi: 10.1074/jbc.M109785200. [DOI] [PubMed] [Google Scholar]
- 5.Bellingham SA, Coleman LA, Masters CL, Camakaris J, Hill AF. Regulation of prion gene expression by transcription factors SP1 and metal transcription factor-1. J Biol Chem. 2009;284:1291–301. doi: 10.1074/jbc.M804755200. [DOI] [PubMed] [Google Scholar]
- 6.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–32. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haroon ZA, Amin K, Lichtlen P, Sato B, Huynh NT, Wang Z, et al. Loss of metal transcription factor-1 suppresses tumor growth through enhanced matrix deposition. Faseb J. 2004;18:1176–84. doi: 10.1096/fj.03-1205com. [DOI] [PubMed] [Google Scholar]
- 8.Murphy BJ. Regulation of malignant progression by the hypoxia-sensitive transcription factors HIF-1alpha and MTF-1. Comp Biochem Physiol B Biochem Mol Biol. 2004;139:495–507. doi: 10.1016/j.cbpc.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Green CJ, Lichtlen P, Huynh NT, Yanovsky M, Laderoute KR, Schaffner W, et al. Placenta growth factor gene expression is induced by hypoxia in fibroblasts: a central role for metal transcription factor-1. Cancer Res. 2001;61:2696–703. [PubMed] [Google Scholar]
- 10.Murphy BJ, Sato BG, Dalton TP, Laderoute KR. The metal-responsive transcription factor-1 contributes to HIF-1 activation during hypoxic stress. Biochem Biophys Res Commun. 2005;337:860–7. doi: 10.1016/j.bbrc.2005.09.124. [DOI] [PubMed] [Google Scholar]
- 11.Murphy BJ, Kimura T, Sato BG, Shi Y, Andrews GK. Metallothionein induction by hypoxia involves cooperative interactions between metal-responsive transcription factor-1 and hypoxia-inducible transcription factor-1{alpha}. Mol Cancer Res. 2008;6:483–90. doi: 10.1158/1541-7786.MCR-07-0341. [DOI] [PubMed] [Google Scholar]
- 12.Stitt MS, Wasserloos KJ, Tang X, Liu X, Pitt BR, St. Croix CM. Nitric oxide-induced nuclear translocation of the metal responsive transcription factor, MTF-1 is mediated by zinc release from metallothionein. Vascul Pharmacol. 2006;44:149–55. doi: 10.1016/j.vph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Murphy BJ, Andrews GK, Bittel D, Discher DJ, McCue J, Green CJ, et al. Activation of metallothionein gene expression by hypoxia involves metal response elements and metal transcription factor-1. Cancer Res. 1999;59:1315–22. [PubMed] [Google Scholar]
- 14.Kimura T, Itoh N, Takehara M, Oguro I, Ishizaki J, Nakanishi T, et al. MRE-binding transcription factor-1 is activated during endotoxemia: a central role for metallothionein. Toxicol Lett. 2002;129:77–84. doi: 10.1016/s0378-4274(01)00473-8. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 16.Naugler WE, Karin M. NFkappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He X, Ma Q. Induction of metallothionein I by arsenic via metal-activated transcription factor 1. Critical role of carboxyl-terminal cysteine residues in arsenic sensing. J Biol Chem. 2009 doi: 10.1074/jbc.M901204200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cramer M, Nagy I, Murphy BJ, Gassmann M, Hottiger MO, Georgiev O, et al. NFkappaB contributes to transcription of placenta growth factor and interacts with metal responsive transcription factor-1 in hypoxic human cells. Biol Chem. 2005;386:865–72. doi: 10.1515/BC.2005.101. [DOI] [PubMed] [Google Scholar]
- 19.Zheng D, Feeney GP, Kille P, Hogstrand C. Regulation of ZIP and ZnT zinc transporters in zebrafish gill: zinc repression of ZIP10 transcription by an intronic MRE cluster. Physiol Genomics. 2008;34:205–14. doi: 10.1152/physiolgenomics.90206.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtlen P, Wang Y, Belser T, Georgiev O, Certa U, Sack R, et al. Target gene search for the metal-responsive transcription factor MTF-1. Nucleic Acids Res. 2001;29:1514–23. doi: 10.1093/nar/29.7.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wimmer U, Wang Y, Georgiev O, Schaffner W. Two major branches of anti-cadmium defense in the mouse: MTF-1/metallothioneins and glutathione. Nucleic Acids Res. 2005;33:5715–27. doi: 10.1093/nar/gki881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Lorenzi I, Georgiev O, Schaffner W. Metal-responsive transcription factor-1 (MTF-1) selects different types of metal response elements at low vs. high zinc concentration. Biol Chem. 2004;385:623–32. doi: 10.1515/BC.2004.077. [DOI] [PubMed] [Google Scholar]
- 23.LaRochelle O, Gagne V, Charron J, Soh JW, Seguin C. Phosphorylation is involved in the activation of metal-regulatory transcription factor 1 in response to metal ions. J Biol Chem. 2001;276:41879–88. doi: 10.1074/jbc.M108313200. [DOI] [PubMed] [Google Scholar]
- 24.Saydam N, Adams TK, Steiner F, Schaffner W, Freedman JH. Regulation of metallothionein transcription by the metal-responsive transcription factor MTF-1: identification of signal transduction cascades that control metal-inducible transcription. J Biol Chem. 2002;277:20438–45. doi: 10.1074/jbc.M110631200. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Kimura T, Huyck RW, Laity JH, Andrews GK. Zinc-induced formation of a coactivator complex containing the zinc-sensing transcription factor MTF-1, p300/CBP and Sp1. Mol Cell Biol. 2008;28:4275–84. doi: 10.1128/MCB.00369-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Li Y, Okumura F, Itoh N, Nakanishi T, Sone T, et al. Chromium (VI) inhibits mouse metal-lothionein-I gene transcription by preventing the zinc-dependent formation of an MTF-1-p300 complex. Biochem J. 2008 doi: 10.1042/BJ20081025. [DOI] [PubMed] [Google Scholar]
- 27.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem. 2000;275:34803–9. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- 28.Cherian MG, Jayasurya A, Bay BH. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat Res. 2003;533:201–9. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Taylor AP, Goldenberg DM. Role of placenta growth factor in malignancy and evidence that an antagonistic PlGF/Flt-1 peptide inhibits the growth and metastasis of human breast cancer xenografts. Mol Cancer Ther. 2007;6:524–31. doi: 10.1158/1535-7163.MCT-06-0461. [DOI] [PubMed] [Google Scholar]
- 30.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–75. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 31.Verma A, Guha S, Diagaradjane P, Kunnumakkara AB, Sanguino AM, Lopez-Berestein G, et al. Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res. 2008;14:2476–83. doi: 10.1158/1078-0432.CCR-07-4529. [DOI] [PubMed] [Google Scholar]
- 32.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGFbeta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 33.Wakefield LM, Roberts AB. TGFbeta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–9. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxiainducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- 35.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 36.Kang YJ, Li Y, Sun X. Antiapoptotic effect and inhibition of ischemia/reperfusion-induced myocardial injury in metallothionein-overexpressing transgenic mice. Am J Pathol. 2003;163:1579–86. doi: 10.1016/S0002-9440(10)63514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye B, Maret W, Vallee BL. Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci. 2001;98:2317–22. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang GW, Zhou Z, Klein JB, Kang YJ. Inhibition of hypoxia/reoxygenation-induced apoptosis in metallothionein-overexpressing cardiomyocytes. Am J Physiol Heart Circ Physiol. 2001;280:2292–9. doi: 10.1152/ajpheart.2001.280.5.H2292. [DOI] [PubMed] [Google Scholar]
- 39.Cai L, Satoh M, Tohyama C, Cherian MG. Metallothionein in radiation exposure: its induction and protective role. Toxicology. 1999;132:85–98. doi: 10.1016/s0300-483x(98)00150-4. [DOI] [PubMed] [Google Scholar]
- 40.Vukovic V, Pheng SR, Stewart A, Vik CH, Hedley DW. Protection from radiation-induced DNA single-strand breaks by induction of nuclear metallothionein. Int J Radiat Biol. 2000;76:757–62. doi: 10.1080/09553000050028904. [DOI] [PubMed] [Google Scholar]
- 41.Cai L, Cherian MG. Zinc-metallothionein protects from DNA damage induced by radiation better than glutathione and copper- or cadmium-metallothioneins. Toxicol Lett. 2003;136:193–8. doi: 10.1016/s0378-4274(02)00359-4. [DOI] [PubMed] [Google Scholar]
- 42.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–50. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 43.Semenza GL. Development of novel therapeutic strategies that target HIF-1. Expert Opin Ther Targets. 2006;10:267–80. doi: 10.1517/14728222.10.2.267. [DOI] [PubMed] [Google Scholar]
- 44.Lichtlen P, Georgiev O, Schaffner W, Aguzzi A, Brandner S. The heavy metal-responsive transcription factor-1 (MTF-1) is not required for neural differentiation. Biol Chem. 1999;380:711–5. doi: 10.1515/BC.1999.089. [DOI] [PubMed] [Google Scholar]
- 45.Auf der Maur A, Belser T, Wang Y, Gunes C, Lichtlen P, Georgiev O, et al. Characterization of the mouse gene for the heavy metal-responsive transcription factor MTF-1. Cell Stress Chaperones. 2000;5:196–206. doi: 10.1379/1466-1268(2000)005<0196:cotmgf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 47.Gunes C, Heuchel R, Georgiev O, Muller KH, Lichtlen P, Bluthmann H, et al. Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J. 1998;17:2846–54. doi: 10.1093/emboj/17.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Datta J, Majumder S, Kutay H, Motiwala T, Frankel W, Costa R, et al. Metallothionein expression is suppressed in primary human hepatocellular carcinomas and is mediated through inactivation of CCAAT/enhancer binding protein {alpha} by phosphatidylinositol 3-Kinase signaling cascade. Cancer Res. 2007;67:2736–46. doi: 10.1158/0008-5472.CAN-06-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bay BH, Jin R, Huang J, Tan PH. Metallothionein as a prognostic biomarker in breast cancer. Exp Biol Med. 2006;231:1516–21. doi: 10.1177/153537020623100910. [DOI] [PubMed] [Google Scholar]
- 50.Jin R, Chow VT, Tan PH, Dheen ST, Duan W, Bay BH. Metallothionein 2A expression is associated with cell proliferation in breast cancer. Carcinogenesis. 2002;23:81–6. doi: 10.1093/carcin/23.1.81. [DOI] [PubMed] [Google Scholar]
- 51.Surowiak P, Matkowski R, Materna V, Gyorffy B, Wojnar A, Pudelko M, et al. Elevated metallothionein (MT) expression in invasive ductal breast cancers predicts tamoxifen resistance. Histol Histopathol. 2005;20:1037–44. doi: 10.14670/HH-20.1037. [DOI] [PubMed] [Google Scholar]
- 52.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology. 2008;149:4912–20. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 53.Lee R, Woo W, Wu B, Kummer A, Duminy H, Xu Z. Zinc accumulation in N-methyl-N-nitrosourea-induced rat mammary tumors is accompanied by an altered expression of ZnT-1 and metallothionein. Exp Biol Med. 2003;228:689–96. [PubMed] [Google Scholar]
- 54.Huesca M, Wang M, Cukier H, Nedunuri V, Jin H, Peralta R, et al. Mechanistic studies of a novel small-molecule anticancer drug, LOR-253, on cell cycle arrest and angiogenesis. P 100th Ann Meeting of the Am Assoc Canc Res. 2009:50. [Google Scholar]
- 55.Kimura T, Itoh N, Sone T, Kondoh M, Tanaka K, Isobe M. Role of metal-responsive transcription factor-1 (MTF-1) in EGF-dependent DNA synthesis in primary hepatocytes. J Cell Biochem. 2006;99:485–94. doi: 10.1002/jcb.20948. [DOI] [PubMed] [Google Scholar]
- 56.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, et al. 5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–47. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.