Abstract
Histone deacetylase (HDAC) 7 is a member of the HDAC family of deacetylases. Although some of the HDAC proteins have been shown to regulate neuronal survival and death, whether HDAC7 has a similar role is not known. In this study, we show that HDAC7 protects neurons from apoptosis. In cerebellar granule neurons (CGNs) primed to undergo apoptosis by low potassium treatment, expression of HDAC7 protein is reduced. Reduced expression is also observed in CGNs induced to die by pharmacological inhibition of the proteasome, in cortical neurons treated with homocysteic acid, and in the striatum of R6/2 transgenic mice, a commonly used genetic model of Huntington disease. Forced expression of HDAC7 in cultured CGNs blocks low potassium-induced death, and shRNA-mediated suppression of its expression induces death in otherwise healthy neurons. HDAC7-mediated neuroprotection does not require its catalytic domain and cannot be inhibited by chemical inhibitors of HDACs. Moreover, pharmacological inhibitors of the PI3K-Akt or Raf-MEK-ERK signaling pathways or that of PKA, PKC, and Ca2+/calmodulin-dependent protein kinase fail to reduce neuroprotection by HDAC7. We show that stimulation of c-jun expression, an essential feature of neuronal death, is prevented by HDAC7. shRNA-mediated suppression of HDAC7 expression leads to an increase in c-jun expression. Inhibition of c-jun expression by HDAC7 is mediated at the transcriptional level by its direct association with the c-jun gene promoter. Taken together, our results indicate that HDAC7 is a neuroprotective protein acting by a mechanism that is independent of its deacetylase activity but involving the inhibition of c-jun expression.
Keywords: Apoptosis, Cell Death, Histone Deacetylase, Neurodegeneration, Neuron
Introduction
Histone deacetylases (HDACs)2 are the catalytic subunits of multiprotein complexes that deacetylate specific lysines in the tail residues of histones, resulting in the compaction of chromatin into a transcriptionally repressed state (reviewed in Refs. 1, 2). Although best studied for their effects on histones and transcriptional activity, it is now known that HDACs regulate the acetylation status of a number of other non-histone proteins, suggesting complex functions of HDACs (1, 2). The 18 HDACs expressed in mammals have been grouped into four classes. Class I HDACs (HDAC1–3 and HDAC8) are composed primarily of a catalytic domain, expressed ubiquitously, and localize to the nucleus where they serve as transcriptional repressors. Class II HDACs (HDAC4–7, HDAC9, and HDAC10) are larger proteins with an N-terminal regulatory domain involved in protein-protein interactions. These HDACs are expressed tissue-specifically and can shuttle between the nucleus and the cytoplasm in a phosphorylation-dependent manner. HDAC11 is the sole member of the class IV HDAC subfamily. Members of the third class of deacetylases are called sirtuins (Sirt1–7). In contrast to proteins in the other three classes, which are zinc-dependent deacetylases, sirtuins are NAD-dependent enzymes.
There is growing consensus that HDACs regulate neuronal survival and that deregulated HDAC activity contributes to the loss of neurons in neurodegenerative disorders (reviewed in Refs. 3–5). Protection afforded by nonselective pharmacological inhibitors (inhibiting all HDAC proteins efficiently) in certain in vivo models of neurodegeneration has suggested that the activation of HDACs contributes to the promotion of neuronal death. However, because the HDAC inhibitors used in these studies block most HDACs effectively, the identity of the HDAC protein(s) responsible for promoting neurodegeneration remains unclear. Moreover, contrary to the conclusions drawn from the pharmacological studies, results obtained from the analysis of individual members of the HDAC family have revealed that several HDAC proteins, including HDAC1, HDAC4, HDAC6, HDRP (a truncated form of HDAC9), and Sirt1, protect neurons rather than promote degeneration (6–13).
We and others have previously reported that treatment of cultured cerebellar granule neurons (CGNs) with HDAC inhibitors induces apoptosis (7, 14, 15). Neurotoxicity by HDAC inhibitors has also been observed in cortical neurons, although in comparison with CGNs, where an exposure of 4–6 h was sufficient to induce toxicity, a more long term exposure is required for neurotoxicity in cortical neurons (16). Interestingly, the treatment of CGNs with either of two distinct HDAC inhibitors, TSA and MS-275, leads to reduced expression of HDAC7 and HDRP (7). Selective down-regulation of HDAC7 expression by another broad spectrum HDAC inhibitor, SAHA, has also been observed in a variety of normal, immortalized, genetically transformed, and human cancer-derived cell lines (17, 18). Furthermore, administration of SAHA to mice results in the down-regulation of HDAC7 in the brain (17). These observations raise the possibility that elevated levels of HDAC7 are required for neuronal survival and that the neurotoxic effect of HDAC inhibitors on CGNs results from the reduction in HDAC7 expression. We investigated this possibility in this study. We report that HDAC7 is indeed capable of preventing neuronal death and does so in a deacetylase-independent manner. We provide evidence indicating that HDAC7-mediated neuroprotection is obtained by inhibition of c-jun expression, a transcription factor that plays a pivotal role in promoting neuronal death.
EXPERIMENTAL PROCEDURES
Materials
Unless indicated otherwise, all cell culture media and reagents were purchased from Invitrogen, and all chemicals, including homocysteic acid (HCA) and cycloheximide (CHX), were from Sigma. LY294002, PD98059, U0126, Akt inhibitor-X, TSA, HDACi, KN62, Gö6983, and benzyloxycarbonyl-Val-Ala-Asp- fluoromethyl ketone (Z-VAD-fmk) were purchased from Calbiochem. t-Butoxycarbonyl-Asp-(O-methyl)-fluoromethyl ketone (boc-Asp-fmk) was from Enzyme Systems Products (Dublin, CA). Antibodies used in this study were as follows: HDAC7 (sc-11412) and c-Jun (sc-1694) were from Santa Cruz Biotechnology (Santa Cruz, CA); cleaved caspase3 (9661) was from Cell Signaling Technology (Beverly, MA); HDAC4 (H0163), HDRP (H9163), FLAG (F-1804), and tubulin (T-5168) were from Sigma. All antibodies were used at a 1:1,000 dilution for Western blots.
Plasmids
FLAG-tagged full-length HDAC7 and JC6-Luc, a luciferase vector containing −225/+150 fragment of the mouse c-jun promoter, were purchased from Addgene (Addgene, Cambridge, MA). FLAG-tagged C-terminally truncated HDAC7, HDAC7(1–519) (corresponding to amino acids 1–519), and FLAG-tagged N-terminally truncated HDAC7, HDAC7(514–953) (corresponding to amino acids 514–953) were obtained by PCR using the full-length HDAC7 plasmid as template and were subcloned into the p3F-CMV vector (Sigma). shHdac7-1 (TRCN0000039335) and shHdac7-2 (TRCN0000039337), two shRNA vectors targeting both rat and mouse HDAC7, were purchased from Sigma. pLKO.1, which has a no-hairpin 18-bp insert and serves as shRNA control, was obtained from Addgene (Addgene, Cambridge, MA).
Cerebellar Granule Neuron Cell Culture, Transfection, and Viability Assays
CGNs were cultured from dissociated cerebella of 7–8-day-old Wistar rats as described previously (6, 7, 9). In short, processed CGNs were plated in basal Eagle's medium with Earle's salts (BME) supplemented with 10% FBS, 25 mm KCl, 2 mm glutamine (Invitrogen), and 100 μg/ml gentamicin. Cells were plated on dishes coated with poly-l-lysine at a density of 106 cells/well (24-well dishes) and 107 cells/60-mm dishes. 10 μm cytosine arabinofuranoside was added to the culture medium 18–22 h after plating to prevent proliferation of non-neuronal cells. Cells were then maintained for 4–5 days in 24-well dishes prior to transient transfection experiments. Transfection of CGNs was done as described previously (6, 7, 9). Briefly, 1 μg/well of DNA was precipitated by the calcium-phosphate method for 30 min at room temperature. Cell culture medium of neurons was changed to DMEM without l-glutamine after one wash. Precipitated DNA was added to the cell culture medium for 30 min. Cells were then washed twice with DMEM without l-glutamine medium and placed in their original medium. A day after transfection, the cells were rinsed once and then maintained in serum-free BME medium containing either 5 mm KCl (low potassium, LK) or 25 mm KCl (high potassium, HK). Pharmacological inhibitors (dissolved in dimethyl sulfoxide) were added in HK or LK conditions at the time of treating. Time of treatments are described in the figure legends. Transfected neurons were detected by immuno-staining using FLAG antibody or GFP antibody as described previously (6, 7, 9). For immunocytochemistry, antibodies purchased from Sigma were used at a 1:200 dilution and antibodies from Santa Cruz Biotechnology at a 1:100 dilution. Neuronal viability was quantified by staining cell nuclei with 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI), which is widely used and well accepted to detect apoptosis in cerebellar granule neurons, as described previously (6, 7, 9). The morphology change of chromatin condensation or nuclear fragmentation is well known as a hallmark of apoptosis. Cells with condensed or fragmented nuclei were counted as dead, and cells with rounded and less stained nuclei were counted as living. In each individual experiment, more than 100 transfected cells were counted for each treatment. Cell viability data are presented as mean values plus standard deviations from at least three separate experiments. Statistical significance was determined by using the Student's t test. p values <0.05 were deemed significant.
Cortical Neuron Cultures, Transfection, and Treatments
Cortical neurons were cultured from the cerebral cortex of E17 Wistar rats as described previously (19). One day after plating, cells were transfected by calcium-phosphate precipitation as described above. Transfected cells were subjected to 1 mm HCA treatment for 16 h or 10 μm Aβ peptide for 48 h. Cell viability was quantified as described above.
Evaluation of Cellular c-jun Levels by Quantification of c-jun Fluorescence
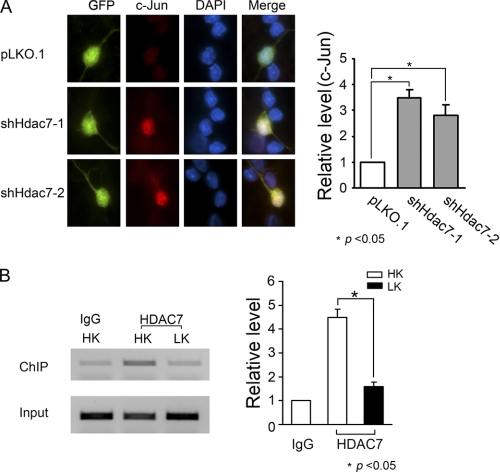
CGNs were transfected with shHdac7-1, shHdac7-2, or control vector pLKO.1. GFP was cotransfected to label transfected cells. Two days after transfection, cells were fixed and subjected to immunocytochemistry with a mouse anti-GFP and a rabbit anti-c-Jun as primary antibodies followed by goat anti-mouse conjugated with FITC (green) and goat anti-rabbit conjugated with Texas red (red) as secondary antibodies. c-Jun fluorescence images were taken by a Nikon eclipse 80i fluorescence microscope with a CCD camera using the same magnification and exposure time. Transfected cells were identified by GFP staining. The c-Jun fluorescent signals in transfected cells were quantified by calculating the mean pixels of the transfected cells on the unprocessed fluorescent images using the histogram function in Adobe Photoshop CS2 (20). To correct for background fluorescence, the pixel intensity of the same size area was measured immediately adjacent to the transfected cell, and this value was subtracted. For each individual experiment, about 100 transfected cells from each group were subjected to c-Jun fluorescence quantification. Results are from three independent experiments.
Treatment of HT-22 Cells
The mouse HT-22 neuroblastoma cell line was from ATCC (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (DMEM) with 4.5 g/liter glucose (without sodium pyruvate) supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. HT-22 cells were transfected with GFP or HDAC7 plasmid by Lipofectamine 2000 (Invitrogen) for 24 h. To induce apoptosis, HCA was added at a final concentration of 1 mm as described previously (6, 9). Time of treatments are described in the figure legends.
RNA Preparation and Semiquantitative RT-PCR
RNA was extracted from cultured neurons or HT-22 cells by using TRIzol (Invitrogen) according to the manufacturer's instructions (6, 7, 9). Three micrograms of total RNA from each sample was reverse-transcribed by ThermoScript reverse transcriptase PCR (RT-PCR) system (Invitrogen) according to the manufacturer's instructions. PCR was performed with PCR master mix (Promega). The primers used for PCR amplification were as follows: c-jun forward, 5′-TGGGCACATCACCACTACAC-3′, and c-jun reverse, 5′-AGTTGCTGAGGTTGGCGTA-3′; hdac7 forward, 5′-AGCCAGACACACCAGGCTCT-3′, and hdac7 reverse, 5′-GCTGCTACTACTGGGGGAGGA-3′; β-actin forward, 5′-AGGACTCCTATGTGGGTGACGA-3′, and β-actin reverse, 5′-CGTTGCCAATAGTGATGACCTG-3′.
Western Blot Analysis
The culture medium was removed, and the cells were washed once with ice-cold phosphate-buffered saline (PBS) and lysed in lysis buffer (1% Triton, 20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin, and 1× protease inhibitor mixture). Protein concentrations were measured and normalized using Bradford protein assay reagent (Bio-Rad). After normalization, 60 μg of protein was subjected to Western blotting. Immunoreactivity was examined by enhanced chemiluminescence (Amersham Biosciences).
Luciferase Assay
c-jun promoter activity was determined by luciferase assay. HT-22 cells were transfected with JC6-Luc, the c-jun promoter luciferase vector, with or without HDAC7-FLAG by using Lipofectamine 2000 (Invitrogen). One day after transfection, cells were treated with 1 mm HCA for 6 h. Cells were harvested, and c-jun promoter activity was determined by using Monolight enhanced luciferase assay kit (BD Biosciences) following the manufacturer's instructions.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were carried out using a kit from Upstate and the protocol provided by the manufacturer as described previously (7). Briefly, cell cultures were fixed by the addition of formaldehyde to a final concentration of 1% and incubated for 10 min at 37 °C. The medium was aspirated, and cells were washed twice with ice-cold PBS. Cells were then harvested and lysed in 200 μl of SDS lysis buffer per 106 cells (1% SDS, 10 mm EDTA, 50 mm Tris, pH 8.1). Each 200-μl aliquot of lysate was sonicated with three pulses of 10 s at 30% power. Samples were diluted 1:10 in ChIP dilution buffer (Upstate Cell Signaling Solutions) and followed by immunoprecipitation with FLAG or HDAC7 antibody. Immunocomplex was washed and then eluted by the addition of 200 μl of elution buffer (1% SDS, 0.1 m NaHCO3). DNA-protein cross-links were reversed by adding 8 μl of 5 m NaCl and incubating at 65 °C for 4 h. Protein was degraded by the addition of 4 μl of 0.5 m EDTA, 8 μl of 1 m Tris-HCl, pH 6.5, and 10 μg of proteinase K followed by incubation for 1 h at 45 °C. Samples were then phenol/chloroform-extracted, and nucleic acids were precipitated with ethanol and subjected to PCR. Sequences of primer designed to detect a 174-bp fragment of c-jun promoter are as follows: forward, 5′-CTAGACAGCCAAACCAAGAC-3′; and reverse, 5′-GCTCACGGGATGAGGTAAT-3′.
Huntington Disease Mouse Model
Breeding pairs of R6/2 mice made up of an ovarian transplant hemizygote female and a B6CBAF1/J male, both on a C57BL/6J background strain, were purchased from The Jackson Laboratory (Bar Harbor, ME). The breeding pairs were maintained and crossed at the institutional animal care facility. All animals had unlimited access to water and food and were maintained in a 12-h light/12-h dark cycle. Experimental procedures were performed in accordance with National Institutes of Health guidelines. Genotyping of litters was performed 10–14 days after birth by PCR of tail tip DNA. At 6 weeks after birth, mice homozygous for the transgene (R6/2tg/tg) and wild-type littermates were sacrificed by carbon dioxide inhalation. The brains were dissected, and the striatum was separated. The rest of the brain tissue was termed as extra-striatal tissue. RNA or protein lysates were prepared from striatal and nonstriatal tissue and used for RT-PCR or Western blotting as described above.
RESULTS
HDAC7 Expression Is Reduced in Neurons Induced to Die
The treatment of cultured CGNs with pharmacological inhibitors of HDACs induces apoptosis (7, 14, 15, 21). We previously reported (7) that the expression of HDAC7 is sharply down-regulated in neurons treated with HDAC inhibitors, TSA, and sodium butyrate. To examine whether HDAC7 expression was also reduced by apoptotic stimuli other than HDAC inhibitors, we treated CGNs with LK, a commonly used mode of inducing apoptosis in these neurons. LK treatment did not have any effect on the hdac7 mRNA expression (Fig. 1A). The level of HDAC7 protein, however, was dramatically reduced within 6 h of LK treatment (Fig. 1B). Multiple HDAC7 bands are observed in CGN lysates, all of which are reduced, albeit to differing extents. A similar pattern of HDAC7 immunoreactivity (multiple bands) was observed with another antibody generated against a different epitope (data not shown). It is unclear whether the multiple bands represent different isoforms of HDAC7 or whether they are proteolytic products or post-translational modifications.
FIGURE 1.
HDAC7 protein but not mRNA level is reduced in CGNs that undergo apoptosis. A, CGNs were treated with HK or LK medium for 4 and 6 h. RNA was prepared, and RT-PCR analysis was performed with HDAC7-specific primers. β-Actin served as a loading control. Right panel shows quantification of hdac7 mRNA expression from three independent experiments. Densitometric analysis of hdac7 mRNA was first normalized with respect to β-actin. Relative levels were normalized to HK 4 h, which was set as 1. B, neurons were treated as above, and cell lysates were prepared and subjected to Western blotting using a HDAC7 antibody. Membranes were probed with tubulin antibody to ensure equal loading. Densitometric analysis of HDAC7 protein expression was normalized with respect to tubulin. Relative levels were normalized to HK 4 h, which was set as 1. * indicates p < 0.05 using the Student's t test (HK 4 h versus LK 4 h and HK 6 h versus LK 6 h); n = 3. C, CGNs were treated with 1 μm CHX for 1, 3, and 6 h. Cells treated with vehicle served as control (CTR). Western blotting was performed to determine HDAC7 protein levels. Densitometric analysis was performed to quantify HDAC7 protein expression. Relative levels were normalized to CTR. Statistical analysis was performed by using one-way ANOVA followed by Bonferroni's post test; * indicates p < 0.05 compared with control, n = 3.
The dramatic reduction of HDAC7 protein in LK-treated neurons suggested that HDAC7 protein, but not hdac7 mRNA, is relatively unstable. To examine this issue further, we measured the half-life of HDAC7 protein in CGNs using CHX to block protein synthesis. HDAC7 protein level decreased ∼35% within 1 h of CHX treatment and over 50% by 3 h (Fig. 1C) confirming that HDAC7 is a relatively unstable protein, at least in neurons.
Protein degradation is most commonly mediated through the ubiquitin-proteasome system. To examine if neuronal death-associated reduction of HDAC7 was due to proteasomal degradation, we treated neurons with MG132, a potent and widely used ubiquitin-proteasome system inhibitor. Interestingly, and as described by others (22), treatment with MG132 by itself induced death of otherwise healthy CGNs (Fig. 2A). As shown in Fig. 2B, the level of HDAC7 protein was also reduced by MG132 treatment. This result indicates that the reduction of HDAC7 is not due to proteasomal degradation. In cells such as T lymphocytes, activation of the extrinsic cell death machinery with tumor necrosis factor-related apoptosis-inducing ligand or FasL leads to the proteolysis of HDAC7 by caspase-8 (23). Treatment with pan-caspase inhibitors such as Z-VAD-fmk inhibits tumor necrosis factor-related apoptosis-inducing ligand or Fas-induced proteolysis of HDAC7 (23). Although death of CGNs by LK treatment is caspase-independent (24, 25), we investigated whether the reduction of HDAC7 was due to proteolysis by caspases such as caspase-8. However, as shown in Fig. 2C, treatment with either of two separate pan-caspase inhibitors, Z-VAD-fmk and boc-Asp-fmk, had no effect on the LK-induced reduction of HDAC7 protein, although both inhibitors blocked caspase activation. This suggests that the reduction of HDAC7 protein levels is not due to caspase-mediated proteolytic degradation.
FIGURE 2.
Proteasome system and caspases do not mediate HDAC7 protein down-regulation. A, CGNs were treated for 24 h with HK or LK either in the absence or presence of 10 μm MG132 (MG). Phase contrast micrographs showing appearance of cultures at 24 h are shown in the left panel. Right panel shows viability of the cultures. The survival rates were normalized to HK. Data represent the mean ± S.D. from three independent experiments. * indicates p < 0.05 using the Student's t test compared with HK. B, CGNs were treated with HK or LK in the absence or presence of 10 μm MG132 for 6 h. Western blotting was performed to determine HDAC7 protein levels. Right panel shows quantification of HDAC7 protein expression. Relative levels were normalized to HK. Data represent the mean ± S.D. from three independent experiments. C, CGNs were treated with HK, LK, or LK medium containing 50 μm Z-VAD-fmk (Z-VAD) or 100 μm boc-Asp-fmk (BOC). HDAC7 protein levels were measured by Western blotting. Membranes were reprobed with cleaved caspase-3 antibody to ensure the effectiveness of Z-VAD-fmk and boc-Asp-fmk. HDAC7 protein levels were quantified by densitometric analysis with respect to tubulin. Relative levels were normalized to HK. Data represents the mean ± S.D. from three independent experiments.
HDAC7 Protects Neurons from Apoptosis
Because reduced expression of HDAC7 was associated with neuronal death, we examined whether forced expression of HDAC7 could be neuroprotective. As shown in Fig. 3A, LK treatment of CGNs led to a loss of ∼50% of the neurons within 24 h. Forced expression of HDAC7 in LK-treated neurons offered almost complete protection (Fig. 3A). Although also present in the nucleus,3 ectopically expressed HDAC7 is predominantly cytoplasmic in healthy CGNs. Upon switching from HK to LK medium, however, cytoplasmic HDAC7 translocated to the nucleus (Fig. 3B) suggesting that neuroprotection is mediated within the nucleus. To examine the versatility of HDAC7-mediated neuroprotection, we treated cortical neurons with Aβ peptide. Strong protection by HDAC7 was also observed in this commonly used cell culture model of Alzheimer disease (Fig. 3C). Furthermore, forced expression of HDAC7 protected primary cortical neurons from HCA-induced toxicity (Fig. 3D). Taken together, our results demonstrate that an elevated level of HDAC7 is protective against diverse neurotoxic stimuli.
FIGURE 3.
HDAC7 protects neurons from apoptosis. A, CGNs were transfected with GFP or FLAG-tagged HDAC7 and then switched to HK or LK medium for 24 h. Transfected cells were visualized by immunocytochemistry with GFP or FLAG antibody. Viability of transfected neurons was quantified by DAPI staining. The survival rates were normalized to GFP-transfected cultures treated with HK. Data represent the mean ± S.D. from five independent experiments. * indicates the value of p < 0.05 using the Student's t test (GFP LK versus HDAC7 LK). B, CGNs transfected with FLAG-HDAC7 were treated with HK or LK for 24 h after which immunocytochemical analysis was performed using FLAG antibody. Left panel shows typical localization pattern. Percentages of HDAC7 signal in cytoplasm (Cyt) or nuclei (Nuc) were counted. Right panel shows quantification from three independent experiments. * indicates the value of p < 0.05 using the Student's t test (HK cytoplasm versus LK Cyt, HK nuclei versus LK nuclei). C, cortical neurons were transfected with FLAG-HDAC7 or GFP for 8 h. The cells were then treated with 10 μm Aβ or vehicle (CTR). Two days later, cells were fixed and subjected to immunocytochemistry with GFP or FLAG antibody. Cell viability was quantified as described above. Result represents of mean ± S.D. from three independent experiments. * indicates the value of p < 0.05 using the Student's t test (GFP Aβ versus HDAC7 Aβ). D, cortical neurons were transfected with FLAG-HDAC7 or GFP. One day after transfection, cells were treated with 1 mm HCA or vehicle (CTR) for 16 h. Immunocytochemistry was performed to visualize transfected cells, and cell viability was quantified. Result represents of mean ± S.D. from three independent experiments. * indicates the value of p < 0.05 using the Student's t test (GFP HCA versus HDAC7 HCA).
Although our results demonstrate that forced expression of HDAC7 protects CGNs, they do not show that the down-regulation of endogenous HDAC7 following LK treatment is causally involved in promoting neuronal death. To examine this issue, we suppressed HDAC7 expression using two shRNA vectors, shHdac7-1 and shHdac7-2. Because CGNs are not efficiently transfected, the ability of these two shRNA constructs to suppress HDAC7 expression was evaluated in HT-22 cells, a mouse hippocampal cell line. As shown in Fig. 4A, both shRNAs knocked down HDAC7 protein levels but had no effect on HDAC4 or HDRP, two HDAC family members that protect CGNs and other neuronal types from death (6, 7). This result indicates that the two shRNAs suppress HDAC7 expression specifically. As shown in Fig. 4B, when transfected in CGNs, both shHdac7-1 and shHdac7-2 were able to induce cell death even in HK medium.
FIGURE 4.
Knockdown of HDAC7 induces cell death in CGNs. A, HT-22 cells were transfected with shHdac7-1, shHdac7-2, two vectors encoding small hairpin RNA targeting HDAC7, or control vector pLKO.1. Two days after transfection, cells were harvested and Western blotting was performed with HDAC7 antibody. Membranes were reprobed with HDAC4, HDRP, and tubulin antibody, respectively. HDAC7 protein levels were quantified by densitometric analysis. Relative levels were normalized to pLKO.1. Statistical analysis was performed by using one-way ANOVA followed by Bonferroni's post test; * indicates p < 0.05 compared with pLKO.1; n = 3. B, CGNs were transfected with shHdac7-1, shHdac7-2, or control vector pLKO.1. GFP was cotransfected to visualize transfected cells. Two days after transfection, cells were switched to HK or LK medium for 24 h. Viability was quantified as described above. Statistical analysis was performed by using one-way ANOVA followed by Bonferroni's post test; * indicates p < 0.05 compared with pLKO.1 HK; n = 3.
HDAC7-mediated Neuroprotection Does Not Require Its Deacetylase Activity
Although HDACs are generally believed to exert their effect through their catalytic activity, emerging evidence suggests that actions of these proteins can also be deacetylase-independent. For example, HDAC7 inhibits the transcriptional activity of Runx2 in maturing osteoblasts in a deacetylase-independent manner (26). To examine whether the catalytic activity of HDAC7 was necessary for its neuroprotective effect, we generated a C- terminal truncated construct HDAC7(1–519) that completely lacks the HDAC catalytic domain. As shown in Fig. 5A, HDAC7(1–519) was as effective as wild-type HDAC7 in protecting neurons. In contrast to HDAC7(1–519), the expression of HDAC7(514–953), an N-terminal deletion construct, blocked survival in HK (Fig. 5A). It remains to be determined whether this is due to a dominant-negative effect of the truncated protein or whether elevated deacetylase activity is detrimental to neuronal survival. Control experiments confirmed that the truncated HDAC7 constructs were expressed as efficiently as wild-type HDAC7 (Fig. 5B), ruling out the possibility that the different effect on neuronal survival was due to differences in expression levels. Because class II HDACs can interact with class I HDAC proteins, it was possible that HDAC7 acquired deacetylase activity through recruitment of other catalytically active members. To rule out this possibility, we inhibited HDAC activity using two structurally independent but potent pan-HDAC inhibitors, TSA and HDACi (7). Neither of these inhibitors reduced neuroprotection by HDAC7 (Fig. 5C).
FIGURE 5.
Neuroprotection of HDAC7 is not mediated by its histone deacetylase activity. A, CGNs were transfected with FLAG-tagged HDAC7(1–519), a C-terminal truncated form of HDAC7 lacking the catalytic domain (N-HDAC7), or FLAG-tagged HDAC7(514–953), an N-terminal truncated form of HDAC7 containing the intact histone deacetylase domain (C-HDAC7). GFP served as control. Transfected cultures were switched to HK or LK medium for 24 h, and viability was quantified following immunocytochemistry and DAPI staining. Data represents the mean ± S.D. from three independent experiments. * indicates the value of p < 0.05 using the Student's t test (GFP LK versus N-HDAC7 LK, GFP HK versus C-HDAC7 HK). B, 293T cells were transfected with equal amount of N-HDAC7, C-HDAC7, or GFP. Western blotting was performed to measure the expression of HDAC7 constructs with a FLAG antibody. Membranes were reprobed with tubulin antibody to ensure equal loading. The expression levels of HDAC7 constructs were quantified by densitometric analysis. Relative levels were normalized to N-HDAC7. Data represent the mean ± S.D. from three independent experiments. C, CGNs were transfected with GFP or FLAG-HDAC7. FLAG-HDAC7-transfected cultures were treated with HK, LK, or LK containing 2 μm TSA or 50 μm HDACi for 24 h. Viability was determined as described above and compared with GFP-transfected cultures treated with HK. Data represent the mean ± S.D. from three independent experiments.
Inhibitors of Several Other Signaling Molecules and Pathways Do Not Inhibit the Neuroprotective Activity of HDAC7
To obtain insight into the mechanism by which HDAC7 exerts its neuroprotective effect, we used pharmacological inhibitors against pathways and molecules known to promote survival of neurons. One of the best studied survival-promoting pathways in neurons and other cell types is the PI3K-Akt signaling pathway (27). Treatment of CGNs with LY294002 or Akt inhibitor-X, inhibitors of PI3K and Akt, respectively, did not reduce neuroprotection by HDAC7 (Fig. 6) suggesting that the PI3K-Akt signaling pathway was not necessary for neuroprotection by HDAC7. Another potent survival-promoting mechanism is the Raf-MEK-ERK pathway (27). Treatment with two separate inhibitors of Raf-MEK-ERK signaling, U0126 and PD98059, had no inhibitory effect indicating that this signaling pathway was also not necessary for neuroprotection by HDAC7 (Fig. 6). Additionally, the PKC inhibitor, Gö6983, and the Ca2+/calmodulin-dependent protein kinase inhibitor, KN62, were also without effect on HDAC7-mediated neuroprotection (Fig. 6). Control experiments confirmed that all the inhibitors used in these analyses were effective in inhibiting their targets at the doses used in this analysis (data not shown).
FIGURE 6.
Effects of pharmacological inhibitors on HDAC7 neuroprotection. CGNs transfected with FLAG-HDAC7 were treated with different inhibitors 10 μm LY294002 (LY), 20 μm U0126, 50 μm PD98059 (PD), 10 μm Gö6983 (Gö), 50 μm KN62 (KN), or 5 μm AKT inhibitor X (AKTX) in either HK or LK for 24 h. HK or LK medium containing DMSO was used as control (CTR). Viability was determined by immunocytochemistry and DAPI staining and compared with GFP-transfected cultures treated with HK. Data represent the mean ± S.D. from three independent experiments.
HDAC7 Inhibits c-jun Gene Transcription
It is well established that the expression of c-Jun is increased during neuronal death in a variety of experimental models and that suppression of c-Jun expression or activity is sufficient to protect against neuronal death (28–30). We investigated the possibility that HDAC7 protects neurons by inhibiting c-jun gene expression and that reduced HDAC7 expression would therefore result in an increase in c-jun expression leading to neuronal death. Consistent with this possibility, immunocytochemical analysis of neurons transfected with HDAC7 shRNA constructs displayed a higher level of c-Jun expression (Fig. 7A).
FIGURE 7.
HDAC7 inhibits c-jun expression and binds to c-jun promoter in CGNs. A, CGNs were transfected with shHdac7-1, shHdac7-2, or control vector pLKO.1. GFP was cotransfected to visualize transfected cells. Two days after transfection, cells were fixed and subjected to immunocytochemistry with GFP antibody to label transfected cells (green) and c-Jun antibody to stain the endogenous c-Jun protein (red). Fluorescent signals of c-Jun were quantified as described under “Experimental Procedures.” Relative levels were normalized to pLKO.1. Statistical analysis was performed by using one-way ANOVA followed by Bonferroni's post test; * indicates p < 0.05 compared with pLKO.1; n = 3. B, CGNs were treated with HK or LK for 3 h. Cells were subjected to ChIP assays with HDAC7 antibody or normal IgG as control. Right panel shows quantitative data from three independent experiments. Relative levels were normalized to HK IgG. * indicates the value of p < 0.05 using the Student's t test (HK versus LK).
The expression of c-jun is regulated at the transcriptional level (31). This, along with findings of other laboratories that HDAC7 is a transcriptional repressor (26, 32–34), raised the possibility that the reduction of c-Jun expression by HDAC7 was transcriptionally mediated. Indeed, results from ChIP assays revealed that HDAC7 does associate with the c-jun promoter and that the level of association is reduced in neurons primed to die by LK treatment (Fig. 7B).
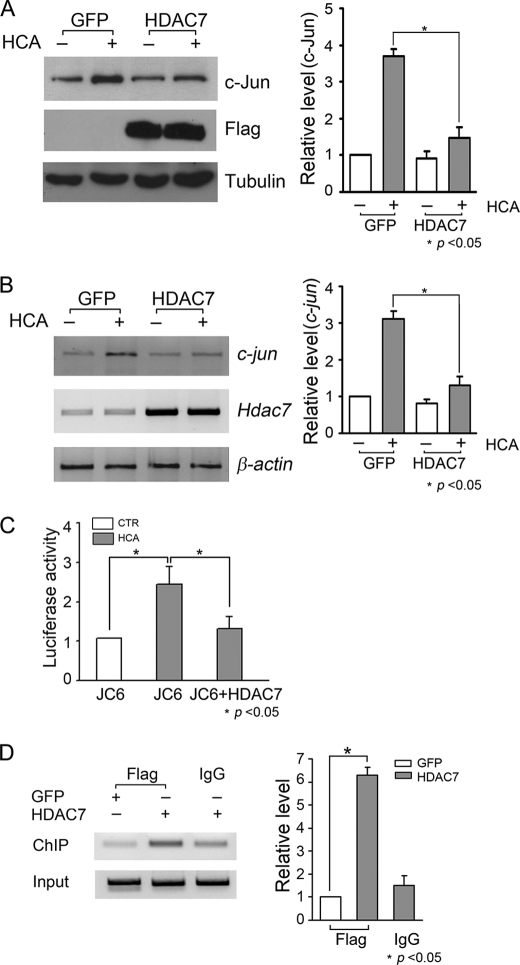
To garner stronger evidence that neuroprotection by HDAC7 involves the inhibition of c-jun transcription, we used the easily transfectable HT-22 cell line. As observed with LK-treated CGNs, HDAC7 protein levels were reduced in HT-22 cells by HCA treatment, and mRNA expression was not altered (Fig. 8, A and B). Also, as observed in LK-treated CGNs, HCA treatment led to an increase in c-jun mRNA expression (Fig. 8A). Forced expression of HDAC7 protected against HCA-induced neuronal death (Fig. 8C). We examined the effect of HDAC7 levels on c-jun expression. As shown in Fig. 9, A and B, the HCA-mediated increase in c-jun mRNA and protein expression was abrogated by HDAC7 overexpression. The incomplete inhibition of c-jun expression is likely due to the fact that not all HT-22 cells were successfully transfected.
FIGURE 8.
HDAC7 protein but not mRNA level is down-regulated, and forced expression of HDAC7 protects HT-22 cells against HCA treatment. A, HT-22 cells were treated with 1 mm HCA for 3, 6, and 9 h. Cells treated with vehicle served as control (CTR). Total RNA was extracted, and RT-PCR was performed to measure the mRNA levels of hdac7 or c-jun, respectively. Densitometric analysis was performed to quantify hdac7 mRNA expression levels. Relative levels were normalized to HCA for 3 h. Data represent the mean ± S.D. from three independent experiments. B, cell lysates from HT-22 cells treated with HCA or vehicle were prepared and subjected to Western blotting analysis with HDAC7 antibody. Quantification of HDAC7 protein was performed. * indicates p < 0.05 using the Student's t test; n = 3. C, HT-22 cells were transfected with GFP or FLAG-tagged HDAC7 for 24 h. The cells were then treated with 1 mm HCA for 18 h, and cells treated with vehicle served as control (CTR). Transfected cells were visualized by immunocytochemistry with GFP or FLAG antibody. Cell viability was quantified by DAPI staining as described under “Experimental Procedures.” Result represents of mean ± S.D. from three independent experiments. * indicates the value of p < 0.05 using the Student's t test (GFP HCA versus HDAC7 HCA).
FIGURE 9.
HDAC7 inhibits c-jun transcription by directly binding to c-jun promoter in HT-22 cells. A, HT-22 cells were transfected with GFP or FLAG-tagged HDAC7 plasmid. Twenty four hours after transfection, cells were treated with 1 mm HCA or vehicle for 6 h. Cells were harvested and subjected to Western blotting analysis with c-Jun antibody. To confirm the expression of HDAC7 plasmid, the membranes were reprobed with FLAG antibody. Tubulin antibody was used to ensure equal loading. c-Jun protein levels were quantified by densitometric analysis. Relative levels were normalized to GFP control. * indicates p < 0.05 using the Student's t test (GFP HCA versus HDAC7 HCA); n = 3. B, HT-22 cells were transfected with GFP or FLAG HDAC7 plasmid and treated as described in A. c-jun mRNA levels were determined by RT-PCR. HDAC7 primer was used to confirm the expression of HDAC7 plasmid, and β-actin was used as loading control. Densitometric analysis was performed to quantify c-jun mRNA levels. Relative levels were normalized to GFP control. * indicates p < 0.05 using the Student's t test (GFP HCA versus HDAC7 HCA); n = 3. C, HT-22 cells were transfected with JC6-luc plasmid with or without HDAC7 plasmid. Twenty four hours after transfection, cells were treated with 1 mm HCA or vehicle (CTR) for 6 h. c-jun promoter activity was determined by luciferase assay as described under “Experimental Procedures.” The changes of c-jun promoter activity were normalized to the promoter activity of control. Data represent the mean ± S.D. from three independent experiments. * indicates the value of p < 0.05 (compared with JC6 HCA) using one-way ANOVA followed by Bonferroni's post test. D, HT-22 cells were transfected with GFP or FLAG-tagged HDAC7 for 24 h. Cells were cross-linked by adding formaldehyde at final concentration of 1%, and DNA was sheared by sonication. FLAG antibody was used to immunoprecipitate chromatin bound by FLAG-HDAC7, and normal IgG served as control. PCR was performed by using primers amplifying a 174-bp fragment of c-jun promoter. Aliquots of cell lysates were taken before immunoprecipitation, and DNA was purified and c-jun promoter was amplified by PCR and served as DNA input. c-jun promoter amplification was quantified by densitometric analysis. Relative levels were normalized to GFP FLAG. * indicates the value of p < 0.05 using the Student's t test (GFP versus HDAC7), n = 3.
To look at c-jun transcription more directly, we co-transfected JC6-Luc, a luciferase vector driven by a c-jun promoter, in the presence or absence of HDAC7. c-jun promoter activity increased after HCA treatment mimicking the profile of the endogenous c-jun gene (Fig. 9C). When cotransfected with HDAC7, the transcriptional activity of the c-jun promoter was robustly suppressed (Fig. 9C).
To examine whether the repression of c-jun transcription was a direct effect involving association of HDAC7 with the c-jun promoter (as opposed to an indirect effect involving modulation of another molecule), we performed chromatin immunoprecipitation analysis of HDAC7 expressed in HT-22 cells. As shown in Fig. 9D, HDAC7 immunoprecipitation pulled down the c-jun promoter efficiently. In contrast, the c-jun promoter was barely detectable when lysates from GFP-transfected cultures were used.
HDAC7 Expression Is Reduced in a Mouse Model of HD
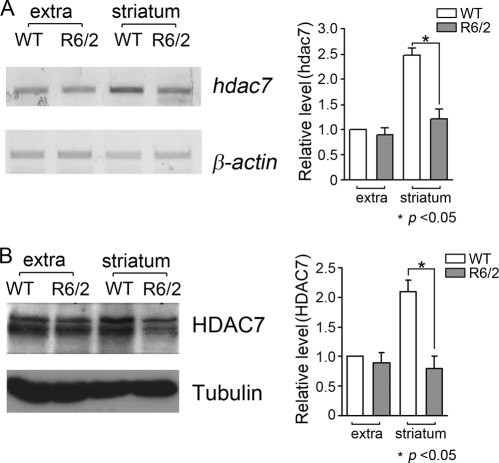
One of the most widely utilized models of neurodegenerative disease is the R6/2 transgenic mouse model of HD (17), generated by the expression of an expanded form of exon 1 of the huntingtin gene. Mice homozygous for the transgene display selective striatal atrophy and neurological deficits within 6–8 weeks after birth. We examined the expression pattern of HDAC7 in striatal and nonstriatal brain tissue from R6/2 transgenic mice (R6/2tg/tg) and wild-type littermates. As shown in Fig. 10, A and B, HDAC7 expression can be detected in both striatal and nonstriatal brain tissue. The level of HDAC7 protein was substantially reduced in the R6/2 striatum compared with wild-type control (Fig. 10B). In contrast to the lack of any effect at the mRNA level in cultured neurons undergoing apoptosis, hdac7 mRNA was also decreased in R6/2 striatum (Fig. 10A). This suggests that HDAC7 expression can be regulated at both the mRNA and protein level during the process of neuronal degeneration.
FIGURE 10.
HDAC7 expression decreases in striatum of huntington R6/2 mouse. Whole brains from 6-week-old R6/2 transgenic mice (R6/2 tg/tg) or wide type (WT) littermates were removed. Striatum and extra striatal brain tissues (whole brain without striatum) were dissected. A, RNA was extracted from the dissected tissue, and RT-PCR was performed to measure hdac7 mRNA levels. β-Actin served as control. Densitometric analysis was performed to quantify hdac7 mRNA levels. Relative levels were normalized to WT extra. * indicates the value of p < 0.05 using the Student's t test (WT extra versus R6/2 striatum); n = 3. B, protein lysates were prepared and subjected to Western blotting using HDAC7 antibody. HDAC7 protein levels were quantified by densitometric analysis. Relative levels were normalized to WT extra. * indicates the value of p < 0.05 using the Student's t test (WT extra versus R6/2 striatum); n = 3.
DISCUSSION
HDAC inhibitors prevent neurodegeneration and improve behavioral performance in a variety of in vivo models of neurodegenerative disease (3–5). But because the inhibitors used so far are not selective, the specific HDAC protein(s) that they inhibit in affording neuroprotection remain to be identified. A recent study examined the possibility that one target of these inhibitors is HDAC7 (17). Indeed, administration of HDAC inhibitors results in the down-regulation of HDAC7 expression in the brains of wild-type and R6/2 mice, a commonly used mouse model of Huntington disease (17). It was found, however, that genetic knockdown of HDAC7 in R6/2 mice does not improve disease phenotype arguing against HDAC7 being the target of the pharmacological inhibitors (17). In this study, we show that not only is HDAC7 not responsible for promoting neuronal death but that elevated levels of this protein have a protective effect in neurons. It has already been described that treatment of CGNs with HDAC inhibitors induces death, which is preceded by a sharp down-regulation of HDAC7 expression (7). We report that down-regulation of HDAC7 expression is also observed during LK-induced death of these neurons. Re-establishing high levels of HDAC7 protects neurons against LK-induced death, whereas shRNA-mediated suppression of its expression promotes death of otherwise healthy neurons that are maintained in HK medium. Suppression of HDAC7 expression does not alter the levels of HDAC4 or HDRP, two other neuroprotective HDACs, ruling out the possibility that compensatory mechanisms involving up-regulation of other HDACs are involved. Our results suggest that reduced expression of HDAC7 plays an important role in the neurotoxic effect of HDAC inhibitors in CGNs. In addition to LK-treated CGNs, down-regulation of HDAC7 protein also occurs in cortical neurons exposed to HCA,3 as well as HT-22 cells induced to die by HCA treatment. Furthermore, we find that HDAC7 expression is reduced in the striatum of R6/2 mice at a time when behavioral deficits can be observed. In contrast to LK or HCA-induced reduction in cultured neurons, in R6/2 mice the down-regulation of HDAC7 expression is seen at the mRNA level also. This finding, along with the down-regulation of HDAC7 mRNA by treatment with HDAC inhibitors, demonstrates that HDAC7 expression can be regulated at both the mRNA and protein level.
We find that LK treatment down-regulates HDAC7 protein, but it has no effect at the mRNA level. Although pointing to post-translational mechanisms, pharmacological inhibition of the proteasome with MG132 did not reduce the down-regulation of HDAC7 protein caused by LK treatment. On the contrary, treatment with MG132 promoted neuronal death by itself, which was accompanied by reduced HDAC7 expression. HDAC7 has been reported to be a substrate of caspase-8 in vitro and in thymocytes undergoing extrinsically triggered apoptosis (23). However, several results and observations suggest that caspase-8-induced HDAC7 proteolysis is not relevant to neuronal death. For example, LK-induced death of CGNs or HCA-induced death of HT-22 cells is caspase-independent (24, 25). Furthermore, we have not been able to detect the 50- and 31-kDa caspase-8-generated fragments of HDAC7 described by Scott et al. (23) in neuronal lysates. The reduction of endogenous HDAC7 in LK-treated CGNs is also not inhibited by treatment with Z-VAD-fmk, a pan-caspase inhibitor that inhibits HDAC7 proteolysis in thymocytes. In thymocytes, cleavage of HDAC7 by caspase-8 results in the accumulation of the C-terminal domain in the cytosol. In LK-treated neurons, the C-terminal half of the HDAC7 protein localizes to the nucleus when overexpressed. Further work is necessary to determine whether other mechanisms such as autophagic degradation or proteolysis by non-caspase proteases mediate the reduction of HDAC7 protein in apoptotic neurons.
Neuroprotection by HDAC7 does not require its catalytic activity. Indeed, deletion of the C-terminal half of the protein containing the HDAC domain has no effect on neuroprotection. This is reminiscent of HDRP, a deacetylase-deficient truncated form of HDAC9 generated by alternative splicing, which is also neuroprotective (7). In the case of HDRP, however, deacetylase activity is acquired through association with HDAC1. Treatment with HDAC inhibitors was found to inhibit neuroprotection by HDRP indicating the need for deacetylase activity (7). In contrast, neuroprotection by HDAC7 is not inhibited by the same inhibitors that block HDRP-mediated survival. Studies by other laboratories have also described deacetylase-independent actions of HDAC7. For example, repression by HDAC7 of the transcriptional activities of the androgen receptor, the estrogen receptor, MEF2, and Runx2 has been suggested to be independent of its deacetylase activity (26, 32–34).
We find that the neuroprotective effect of HDAC7 is not affected by inhibition of the PI3K-Akt or the Raf-MEK-ERK signaling pathways. Likewise, inhibition of other molecules known to promote neuronal survival, such as Ca2+/calmodulin-dependent protein kinase or PKC, has no inhibitory effect on HDAC7-mediated neuroprotection. We find that neuroprotection by HDAC7 involves the inhibition of c-jun expression. Forced expression of HDAC7 results in reduced c-jun expression, whereas suppression of HDAC7 expression leads to elevated c-jun levels. Furthermore, our results indicate that the inhibition of c-jun expression is transcriptionally mediated by direct association of HDAC7 with the c-jun promoter.
Ectopically expressed HDAC7 is predominantly cytoplasmic, although a portion of it is detectable in the nucleus. In neurons primed to undergo apoptosis, HDAC7 translocates to the nucleus where it acts by repressing c-jun expression. Increased c-jun expression is a necessary event in neuronal death resulting from a variety of apoptotic stimuli (31, 35). As observed with ectopically expressed HDAC7, we find that endogenous HDAC7 is mainly in the cytoplasm with lower levels in the nucleus.3 We propose that the low level of nuclear HDAC7 is sufficient to maintain neuronal survival under normal circumstances (for example, in CGNs maintained in HK medium), Because of the degradation of HDAC7 in neurons induced to die, however, c-jun transcription is derepressed.
In summary, we show that elevated HDAC7 expression promotes neuronal survival. Reduced expression of HDAC7 is seen in neurons induced to die by a number of different stimuli, including treatment with LK, HCA, and proteasomal inhibition. In addition, we and others have found that HDAC7 expression is decreased in the striatum of R6/2 mice. Our results suggest that reduced HDAC7 expression could cause or contribute to neuronal death in neurodegenerative conditions. Our analysis reveals that reduction of HDAC7 in dying neurons can occur either at the mRNA level or post-translationally by degradation of the protein. Although the regulation of HDAC7 expression itself is complex and may depend on the cell type, our results suggest that its neuroprotective effect in different neuronal types is mediated by a common mechanism involving the inhibition of c-jun expression. The finding that elevated HDAC7 protects cultured neurons from death raising the possibility that increasing HDAC7 levels in the brain by pharmacologically stimulating endogenous expression, or by delivering it to vulnerable neuronal populations via viral or other delivery systems, might represent a therapeutic approach to reduce cell death in neurodegenerative disorders.
This work was supported, in whole or in part, by National Institutes of Health Grants NS047201 and NS040408.
C. Ma and S. R D'Mello, unpublished data.
- HDAC
- histone deacetylase
- CGN
- cerebellar granule neurons
- HCA
- homocysteic acid
- HK
- high potassium
- LK
- low potassium
- boc
- t-butoxycarbonyl
- fmk
- fluoromethyl ketone
- Z
- benzyloxycarbonyl
- CHX
- cycloheximide
- TSA
- trichostatin acid A
- ANOVA
- analysis of variance
- HDRP
- histone deacetylase-related protein.
REFERENCES
- 1. Yang X. J., Seto E. (2008) Nat. Rev. Mol. Cell Biol. 9, 206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haberland M., Montgomery R. L., Olson E. N. (2009) Nat. Rev. Genet. 10, 32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D'Mello S. R. (2009) Drug News Perspect. 22, 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kazantsev A. G., Thompson L. M. (2008) Nat. Rev. Drug Discov. 7, 854–868 [DOI] [PubMed] [Google Scholar]
- 5. Sleiman S. F., Basso M., Mahishi L., Kozikowski A. P., Donohoe M. E., Langley B., Ratan R. R. (2009) Expert Opin. Investig. Drugs 18, 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Majdzadeh N., Wang L., Morrison B. E., Bassel-Duby R., Olson E. N., D'Mello S. R. (2008) Dev. Neurobiol. 68, 1076–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morrison B. E., Majdzadeh N., Zhang X., Lyles A., Bassel-Duby R., Olson E. N., D'Mello S. R. (2006) Mol. Cell. Biol. 26, 3550–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pandey U. B., Nie Z., Batlevi Y., McCray B. A., Ritson G. P., Nedelsky N. B., Schwartz S. L., DiProspero N. A., Knight M. A., Schuldiner O., Padmanabhan R., Hild M., Berry D. L., Garza D., Hubbert C. C., Yao T. P., Baehrecke E. H., Taylor J. P. (2007) Nature 447, 859–863 [DOI] [PubMed] [Google Scholar]
- 9. Pfister J. A., Ma C., Morrison B. E., D'Mello S. R. (2008) PLoS ONE 3, e4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim D., Frank C. L., Dobbin M. M., Tsunemoto R. K., Tu W., Peng P. L., Guan J. S., Lee B. H., Moy L. Y., Giusti P., Broodie N., Mazitschek R., Delalle I., Haggarty S. J., Neve R. L., Lu Y., Tsai L. H. (2008) Neuron 60, 803–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim D., Nguyen M. D., Dobbin M. M., Fischer A., Sananbenesi F., Rodgers J. T., Delalle I., Baur J. A., Sui G., Armour S. M., Puigserver P., Sinclair D. A., Tsai L. H. (2007) EMBO J. 26, 3169–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qin W., Yang T., Ho L., Zhao Z., Wang J., Chen L., Zhao W., Thiyagarajan M., MacGrogan D., Rodgers J. T., Puigserver P., Sadoshima J., Deng H., Pedrini S., Gandy S., Sauve A. A., Pasinetti G. M. (2006) J. Biol. Chem. 281, 21745–21754 [DOI] [PubMed] [Google Scholar]
- 13. Chen J., Zhou Y., Mueller-Steiner S., Chen L. F., Kwon H., Yi S., Mucke L., Gan L. (2005) J. Biol. Chem. 280, 40364–40374 [DOI] [PubMed] [Google Scholar]
- 14. Salminen A., Tapiola T., Korhonen P., Suuronen T. (1998) Brain Res. Mol. Brain Res. 61, 203–206 [DOI] [PubMed] [Google Scholar]
- 15. Boutillier A. L., Trinh E., Loeffler J. P. (2002) Ann. N.Y. Acad. Sci. 973, 438–442 [DOI] [PubMed] [Google Scholar]
- 16. Langley B., D'Annibale M. A., Suh K., Ayoub I., Tolhurst A., Bastan B., Yang L., Ko B., Fisher M., Cho S., Beal M. F., Ratan R. R. (2008) J. Neurosci. 28, 163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benn C. L., Butler R., Mariner L., Nixon J., Moffitt H., Mielcarek M., Woodman B., Bates G. P. (2009) PLoS One 4, e5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dokmanovic M., Perez G., Xu W., Ngo L., Clarke C., Parmigiani R. B., Marks P. A. (2007) Mol. Cancer Ther. 6, 2525–2534 [DOI] [PubMed] [Google Scholar]
- 19. Chen H. M., Wang L., D'Mello S. R. (2008) Eur. J. Neurosci. 28, 2003–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kundel M., Jones K. J., Shin C. Y., Wells D. G. (2009) J. Neurosci. 29, 13630–13639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boutillier A. L., Trinh E., Loeffler J. P. (2003) J. Neurochem. 84, 814–828 [DOI] [PubMed] [Google Scholar]
- 22. Butts B. D., Hudson H. R., Linseman D. A., Le S. S., Ryan K. R., Bouchard R. J., Heidenreich K. A. (2005) Mol. Cell. Neurosci. 30, 279–289 [DOI] [PubMed] [Google Scholar]
- 23. Scott F. L., Fuchs G. J., Boyd S. E., Denault J. B., Hawkins C. J., Dequiedt F., Salvesen G. S. (2008) J. Biol. Chem. 283, 19499–19510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller T. M., Moulder K. L., Knudson C. M., Creedon D. J., Deshmukh M., Korsmeyer S. J., Johnson E. M., Jr. (1997) J. Cell Biol. 139, 205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D'Mello S. R., Kuan C. Y., Flavell R. A., Rakic P. (2000) J. Neurosci. Res. 59, 24–31 [PubMed] [Google Scholar]
- 26. Jensen E. D., Gopalakrishnan R., Westendorf J. J. (2009) J. Biol. Chem. 284, 2225–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D'Mello S. R., Chin P. C. (2005) Curr. Drug Targets CNS Neurol. Disord. 4, 3–23 [DOI] [PubMed] [Google Scholar]
- 28. Watson A., Eilers A., Lallemand D., Kyriakis J., Rubin L. L., Ham J. (1998) J. Neurosci. 18, 751–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Estus S., Zaks W. J., Freeman R. S., Gruda M., Bravo R., Johnson E. M., Jr. (1994) J. Cell Biol. 127, 1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ham J., Babij C., Whitfield J., Pfarr C. M., Lallemand D., Yaniv M., Rubin L. L. (1995) Neuron 14, 927–939 [DOI] [PubMed] [Google Scholar]
- 31. Ham J., Eilers A., Whitfield J., Neame S. J., Shah B. (2000) Biochem. Pharmacol. 60, 1015–1021 [DOI] [PubMed] [Google Scholar]
- 32. Kao H. Y., Downes M., Ordentlich P., Evans R. M. (2000) Genes Dev. 14, 55–66 [PMC free article] [PubMed] [Google Scholar]
- 33. Dressel U., Bailey P. J., Wang S. C., Downes M., Evans R. M., Muscat G. E. (2001) J. Biol. Chem. 276, 17007–17013 [DOI] [PubMed] [Google Scholar]
- 34. Chang S., Young B. D., Li S., Qi X., Richardson J. A., Olson E. N. (2006) Cell 126, 321–334 [DOI] [PubMed] [Google Scholar]
- 35. Herdegen T., Waetzig V. (2001) Oncogene 20, 2424–2437 [DOI] [PubMed] [Google Scholar]