Abstract
Voltage-gated potassium (KV) channels, such as KCNQ1 (KV7.1), are modulated by accessory subunits and regulated by intracellular second messengers. Accessory subunits belonging to the KCNE family exert diverse functional effects on KCNQ1, have been implicated in the pathogenesis of various genetic disorders of heart rhythm, and contribute to transducing intracellular signaling events into changes in KV channel activity. We investigated the interactions between calmodulin (CaM), the ubiquitous Ca2+-transducing protein that binds and confers Ca2+ sensitivity to the biophysical properties of KCNQ1, and KCNE4. These studies were motivated by the observed similarities between the suppression of KCNQ1 function by pharmacological disruption of KCNQ1-CaM interactions and the effects of KCNE4 co-expression on the channel. We determined that KCNE4, but not KCNE1, can biochemically interact with CaM and that this interaction is Ca2+-dependent and requires a tetraleucine motif in the juxtamembrane region of the KCNE4 C terminus. Furthermore, disruption of the KCNE4-CaM interaction either by mutagenesis of the tetraleucine motif or by acute Ca2+ chelation impairs the ability of KCNE4 to inhibit KCNQ1. Our findings have potential relevance to KCNQ1 regulation both by KCNE accessory subunits and by an important intracellular signaling molecule.
Keywords: Calmodulin, Ion Channels, Potassium Channels, Protein Domains, Signal Transduction
Introduction
Regulation of many voltage-gated potassium (KV)2 channels occurs through a variety of intracellular second messengers and by interactions with accessory subunits. Several KV channels, including KCNQ1 (KV7.1), are modulated by accessory subunits belonging to the KCNE family of single-transmembrane domain proteins (1–3). KCNE1 associates with KCNQ1 to form the channel complex responsible for generating the slow component of the cardiac delayed rectifier current (IKs). IKs is a repolarizing K+ current critical during later phases of the cardiac action potential (4, 5) that is impaired in various genetic or acquired cardiac arrhythmia syndromes (6–11) arising from mutations in the genes encoding either KCNQ1 or KCNE1.
In some circumstances, accessory subunits are required for transducing intracellular signaling events into physiologically relevant changes in KV channel activity. For example, in the heart KCNQ1 is modulated by β-adrenergic signaling through cAMP-mediated channel phosphorylation. However, this effect results in physiological changes in potassium current only when certain KCNE subunits are also expressed (12, 13). Elucidating other functional and biochemical events that accompany KCNE modulation of KV channels is important for understanding the physiological importance of this accessory subunit family.
A comprehensive understanding of KCNQ1 regulation by KCNE proteins may require that we consider properties of these interactions in the context of the relevant intracellular milieu such as the cycling of internal Ca2+ concentration that occurs in cardiac myocytes. Each ionic component of the cardiac action potential, including IKs, is a potential target for feedback regulation by intracellular Ca2+. Indeed, calmodulin (CaM), the ubiquitous Ca2+-transducing protein (14, 15), is recognized to bind and confer Ca2+ sensitivity to the biophysical properties of several cardiac ion channels, including KCNQ1 (15–20). Of note, KCNQ1 interacts biochemically with CaM through a domain in the channel C terminus, and pharmacological disruption of the KCNQ1-CaM interaction causes complete suppression of KCNQ1 current, suggesting that CaM is required for KCNQ1 activity (15, 20). In this study, we asked whether certain KCNE accessory subunits might interact biochemically or functionally with CaM. Specifically, because the dramatic inhibitory modulation of KCNQ1 by KCNE4 resembles the effect of disrupting the binding of CaM to KCNQ1, we hypothesized that an interaction between KCNE4 and CaM could be involved in the modulation of KCNQ1 by KCNE4.
Our results demonstrate that KCNE4, but not KCNE1, can biochemically interact with CaM and that this interaction requires a tetraleucine motif in the juxtamembrane region of the KCNE4 C terminus. Furthermore, mutation of this tetraleucine motif abolishes CaM association with KCNE4 and impairs functional interactions of KCNE4 with KCNQ1. Our findings have potential relevance to KCNQ1 regulation by an important and ubiquitous intracellular signaling molecule.
EXPERIMENTAL PROCEDURES
cDNA Constructs
Full-length KCNQ1 was expressed from the pIRES2-DsRed vector, whereas KCNE1 and KCNE4 were subcloned into pIRES2-EGFP (Clontech), as described previously (3). A triple HA epitope (YPYDVPDYAGYPYDVPDYAGSYPYDVPDYA) was introduced into the KCNE4 vector and a triple FLAG epitope (DYKDHDGDYKDHDIDYKDDDDK) into the KCNE1 cDNA, immediately upstream of the stop codon. HA sequence was PCR-amplified from a plasmid provided by Sabina Kupershmidt (Vanderbilt University, Nashville, TN); FLAG sequence was PCR-amplified from p3×FLAG-CMVTM-13 (Sigma). Addition of the epitope tags did not affect the modulatory properties of KCNE4 or KCNE1, as reported previously (21). Point mutations were engineered using QuikChange mutagenesis (Stratagene). All constructs were verified by complete sequencing of the open reading frames.
Cell Culture and Transfection
Chinese hamster ovary cells (CHO-K1, American Type Culture Collection) were grown at 37 °C with 5% CO2 in F-12 nutrient mixture medium supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals), penicillin (50 units ml−1)/streptomycin (50 μg ml−1), and l-glutamine (2 mm). CHO cells stably expressing KCNQ1 were generated using transposon-mediated gene transfer (22). Stable clones were identified by G418 resistance, tested by patch clamp recording, and maintained at 37 °C with 5% CO2 in CHO cell medium plus 600 μg/ml G418. Unless otherwise stated, all tissue culture media were obtained from Invitrogen. CHO cells were transiently transfected using FuGENE 6 (Roche Applied Science). For electrophysiology studies shown in Fig. 5, cells were transfected with a 2:1 ratio of KCNQ1 to E4-ha, L(69–72)A-ha, or empty vector DNA.
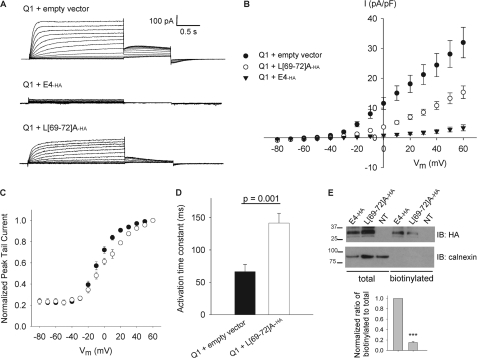
FIGURE 5.
L(69–72)A-ha modulation of KCNQ1. A, representative whole-cell currents from CHO cells transiently transfected with Q1 plus empty vector, Q1 plus E4-ha, or Q1 plus L(69–72)A-ha. B, mean (± S.E.) current density versus voltage plot. At all test potentials more positive than −40 mV, p < 0.05 for comparison of Q1 + L(69–72)A-ha with each Q1 + empty vector and Q1 + E4-ha by t test. C, voltage dependence of activation curves for Q1 plus empty vector versus Q1 plus L(69–72)A-ha. D, means ± S.E. for time constant of activation determined by fitting single-exponential function to +60-mV current recorded between 0.075 and 0.475 s after voltage step. E, representative immunoblot (IB) following cell-surface biotinylation using CHO cells transiently transfected with E4-ha, L(69–72)A-ha, or nontransfected cells (NT). Separate parts of the same blot probed with monoclonal anti-HA to detect KCNE4 in the total protein and biotinylated fractions, and with polyclonal anti-calnexin to assess stringency of the biotinylation. Bars below each lane indicate average biotinylated-to-total protein signal ratio, normalized to wild-type E4-ha from same experimental replicate. Means ± S.E. for normalized ratios are as follows: L(69–72)A-ha, 0.15 ± 0.03, n = 3; ***, p < 0.001 versus wild-type by t test.
Protein Isolation
Forty eight hours post-transfection, CHO cells were lysed for recovery of protein as described previously (21). Briefly, one 100-mm dish of cells for each transfected construct was washed twice with ice-cold phosphate-buffered saline (PBS) (137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 2 mm KH2PO4, pH 7.4), then lysed with Nonidet P-40 lysis buffer (1% Nonidet P-40, 150 mm NaCl, 50 mm Tris, pH 8.0), and supplemented with Complete mini protease inhibitor tablet (Roche Applied Science). Lysates were rocked for 30 min at 4 °C and then centrifuged twice at 14,000 × g for 10 min to remove insoluble debris. Total protein concentration was quantified using a Bradford reagent (Bio-Rad).
Peptides
CaM inhibitory peptides CaMKII-P, LKKFNARRKLKGAILTTMLA (Enzo Life Sciences) and MLCK-P, RRKWQKTGHAVRAIGRL, and control peptide CTRL-P, RRKEQKTGHAVRAIGRE (Calbiochem), were used in biochemical and functional experiments in Figs. 1 and 3. In a previous characterization of MLCK-P (“Trp peptide”), the Kd value of calmodulin and MLCK-P was calculated to be 6 pm from assay of MLCK inhibition, yielding the conclusion that the peptide inhibits MLCK by trapping calmodulin and leaving free calmodulin concentrations very low (23). Similarly, prior characterization of CaMKII-P (“peptide 290–309”) determined that CaMKII-P inhibits CaMKII and CaM-dependent phosphodiesterase activity by peptide interaction with CaM and not by a direct effect on the enzyme (24). Our experiments in Figs. 1 and 3 make use of the ability of these peptides to bind CaM and induce its dissociation from KCNQ1 or KCNE4. CTRL-P is an analog of MLCK-P with two amino acid substitutions that disrupt its interaction with CaM (23).
FIGURE 1.
Biochemical and functional interaction between KCNQ1 and CaM. A, representative immunoblot (IB) following CaM-agarose pulldown using lysates from CHO cells transiently transfected with full-length KCNQ1 (Q1) or nontransfected cells (NT) performed in duplicate in the presence of 2 mm CaCl2 or 2 mm EDTA + 2 mm EGTA. Bound (beads) and unbound (supernatant, s/n) fractions were each probed for KCNQ1 using anti-KCNQ1. Bars below each lane indicate average bound-to-unbound signal ratio, normalized to CaCl2 condition from same experimental replicate. Means ± S.E. for normalized ratios as follows: EDTA + EGTA, 0.03 ± 0.02, n = 3; ***, p < 0.001 versus CaCl2 by t test. B, representative immunoblot following CaM-agarose pulldown of KCNQ1 in the presence of 2 mm CaCl2 and CaMKII-P, MLCK-P, or CTRL-P at indicated concentrations in micromolars. Bars below each lane indicate average bound-to-unbound signal ratio, normalized to no peptide condition from same experimental replicate. Means ± S.E. for normalized ratios as follows: CaMKII-P, 0.07 ± 0.06, n = 2; MLCK-P, 0.06 ± 0.04, n = 3; CTRL-P, 0.65 ± 0.16, n = 3; ***, p < 0.001 by Tukey post-test pairwise comparisons to no peptide following one-way ANOVA (p < 0.001). n.s., not significant. C, decay of steady-state whole-cell current over time recorded in CHO cells expressing KCNQ1 in the presence of 50 μm CTRL-P, 50 μm MLCK-P, or no peptide in the pipette solution. Left, average (± S.E.) steady-state current for each sweep during 0.1-Hz train of 2-s depolarizations to +60 mV from holding potential −80 mV, each normalized to current recorded in same cell 60 s after obtaining whole-cell configuration; n = 4; p ≤ 0.05 for one-way ANOVA and Tukey post-test pairwise comparison of MLCK-P versus CTRL-P and MLCK-P versus no peptide. Right, representative current traces recorded at 100 and 370 s, normalized to steady-state current recorded at 60 s.
FIGURE 3.
CaM-inhibitory peptides disrupt KCNE4-CaM interaction. Representative immunoblot following CaM-agarose pulldown of E4-ha in the presence of CaMKII-P, MLCK-P, or CTRL-P at indicated concentrations in micromolars. Pulldown performed in the presence of 2 mm CaCl2. Bound (beads) and unbound (supernatant, s/n) fractions were each probed for KCNE4 using monoclonal anti-HA. Bars below each lane indicate average bound-to-unbound signal ratio, normalized to no peptide condition. Means ± S.E. for normalized ratios are as follows: 50 μm CaMKII-P, 0.25 ± 0.05, n = 4; 5 μm MLCK-P, 0.33 ± 0.11, n = 3; 16 μm MLCK-P, 0.41 ± 0.09, n = 3; 50 μm MLCK-P, 0.24 ± 0.02, n = 4; 50 μm CTRL-P, 0.76 ± 0.09, n = 4; ***, p < 0.01 for Tukey pairwise comparison to no peptide following one-way ANOVA (p = 0.001). IB, immunoblot; NT, nontransfected cells; n.s., not significant.
CaM-Agarose Pulldown
Cellular lysate (100 μg) was added to 50 μl of CaM-agarose bead slurry (Sigma) in 1.7-ml microcentrifuge tubes, and volume was brought to 0.5 ml with wash buffer consisting of Nonidet P-40 lysis buffer with protease inhibitors and either 2 mm CaCl2 or 2 mm EGTA + 2 mm EDTA. Protein was incubated with beads for 2 h at 4 °C with rocking, in the presence or absence of CaM inhibitory peptides CaMKII-P, MLCK-P, or CTRL-P. Beads were pelleted, and supernatant was reserved as an unbound fraction. Beads were washed six times in 1 ml of CaCl2 or EGTA + EDTA wash buffer and then resuspended in 25 μl of 2× SDS-PAGE sample buffer (100 mm Tris, pH 7.5, 20% glycerol, 4% SDS, 0.008% bromphenol blue, 5% β-mercaptoethanol, 5 m urea). Proteins were eluted from beads by heating for 5 min at 95 °C.
Preparation of Cross-linked Antibody
As described previously (21), 10 μg of antibody were combined with 750 μl of borate buffer (200 mm sodium tetraborate decahydrate, pH 9.0), and 50 μl of protein-G-SepharoseTM 4 Fast Flow (GE Healthcare) and rocked at room temperature for 1 h. The beads were washed with borate buffer supplemented with 20 mm dimethyl pimelimidate dihydrochloride and rocked for 30 min at room temperature. The cross-linking reaction was quenched by 1 h of incubation with 200 mm ethanolamine, pH 8.0. Beads were washed in PBS and stored at 4 °C until use.
Co-immunoprecipitation
Cellular lysates were pre-cleared with protein-G-SepharoseTM 4 Fast Flow and then incubated for 1 h at 4 °C with 50 μl of cross-linked antibody. Beads were washed three times in ice-cold lysis buffer plus protease inhibitors and then proteins were eluted with 2× SDS-PAGE sample buffer for 5 min at 55 °C.
Cell-surface Biotinylation
Forty eight hours after transfection, CHO cells were bathed in 1.5 mg·ml−1 sulfo-NHS-biotin (Pierce) in PBS for 1 h on ice. The biotinylation reaction was quenched with 100 mm glycine in PBS and then cellular lysates were prepared, as described above, in ice-cold RIPA buffer (150 mm NaCl, 50 mm Tris base, pH 7.5, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, supplemented with a Complete mini-protease inhibitor tablet). Lysates were incubated with ImmunoPure immobilized streptavidin beads (Pierce) overnight at 4 °C. Beads were pelleted, and the supernatant was reserved as the nonbiotinylated fraction. Beads were washed three times and then biotinylated proteins were eluted in 2× SDS-PAGE sample buffer for 30 min at room temperature.
SDS-PAGE and Western Blotting
Protein samples were subjected to electrophoresis on pre-cast 4–20% Tris-HCl gels and then transferred to Immun-BlotTM PVDF membranes (Bio-Rad). Membranes were blocked for 1 h with 10% nonfat dry milk in TBS-T before applying the appropriate primary antibody in 4% nonfat dry milk in TBS-T as follows: 1:5000 mouse monoclonal anti-HA (Covance), 1:200 goat polyclonal anti-KCNQ1 (Santa Cruz Biotechnology), 1:2000 mouse monoclonal anti-calmodulin (Millipore Corp.), or 1:1000 rabbit polyclonal anti-calnexin (Sigma). Membranes were washed in TBS-T and then probed with the appropriate secondary antibody in 4% nonfat dry milk in TBS-T as follows: 1:5000 goat anti-mouse IgG-HRP, rabbit anti-goat IgG-HRP, or goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology). Membranes were washed in TBS-T, and HRP signal was detected using ECL Plus (GE Healthcare) and Kodak BioMax MS film (Eastman Kodak Co.). Protein band densitometry was performed on scanned films using ImageJ software (National Institutes of Health). Differences among groups were determined by one-way ANOVA, with Tukey post-test for pairwise comparison.
Electrophysiology
Currents were measured in CHO cells using the broken patch, whole-cell configuration of the patch clamp technique (25). For experiments using transiently transfected cells (Fig. 5), only yellow fluorescent cells (positive for both dsRed-MST and EGFP fluorescence) were recorded. Whole-cell currents were recorded at room temperature (20–23 °C) using Axopatch 200 and 200B amplifiers (MDS Analytical Technologies). Pulses were generated using Clampex 8.1 (MDS Analytical Technologies), and whole-cell currents were filtered at 1 kHz and acquired at 5 kHz. The access resistance and apparent membrane capacitance were estimated as described previously (26). Whole-cell currents were not leak subtracted. Unless otherwise indicated, whole-cell currents were measured during a series of 2-s voltage steps from a holding potential of −80 mV to test potentials ranging from −80 to +60 mV (in 10-mV increments) followed by a 1-s step to −30 mV to record tail currents. The extracellular solution contained (in mm) the following: 132 NaCl, 4.8 KCl, 1.2 MgCl2, 1 CaCl2, 5 glucose, and 10 HEPES, pH 7.4. The standard intracellular solution contained (in mm) the following: 110 K+ aspartate, 1 CaCl2, 10 HEPES, 11 EGTA, 1 MgCl2, and 5 K2ATP, pH 7.35. Pipette solution was diluted 5–10% to prevent activation of swelling-activated currents. For experiments using CaM-inhibitory peptides, 50 μm peptide was added to the diluted pipette solution. For experiments using varying concentrations of intracellular free Ca2+ (Fig. 6 and supplemental Fig. S1), see supplemental Table S1 for composition of intracellular solution. We also tested the effects of including EDTA along with EGTA to the 30 nm [Ca+2]i pipette solution (supplemental Fig. S1) to control for nonspecific effects of EDTA. Patch pipettes were pulled from thick wall borosilicate glass (World Precision Instruments, Inc.) with a multistage P-97 Flaming-Brown micropipette puller (Sutter Instrument Co.) and heat-polished with a Micro Forge MF 830 (Narashige). After heat polishing, the resistance of the patch pipettes was 2–5 megohms in the standard solutions. As a reference electrode, a 2% agar bridge with composition similar to the control bath solution was used. Junction potentials were zeroed with the filled pipette in the bath solution. Unless otherwise stated, all chemicals were obtained from Sigma. Data were collected for each experimental condition from at least three distinct transient transfections and analyzed using a combination of Clampfit (MDS Analytical Technologies), OriginPro 7 (OriginLab Corp.), and SigmaPlot 2000 (Systat Software, Inc.). Steady-state current at each test potential was measured 1.9 s following the voltage step, and current density was obtained by normalizing steady-state current to cell capacitance. Voltage dependence of activation was determined by normalizing peak tail current for each test potential to the maximal value for each cell, and plotting normalized peak tail current versus voltage. Activation curves were fitted with a Boltzmann function to determine the voltage for half-maximal activation (V½) and slope (k). To analyze kinetics of activation, the current recorded between 0.075 and 0.475 s after the voltage step to +60 mV was fitted with a single exponential function to determine the time constant, τ. Statistical analyses were performed using SigmaStat 2.03 (Systat Software, Inc.).
FIGURE 6.
KCNE4 inhibition of KCNQ1 is impaired upon reducing [Ca2+]i. A, representative whole-cell currents from CHO cells stably expressing Q1 and transiently transfected with empty vector or E4, recorded in the presence of 10 or 3 nm free [Ca2+]. B, mean (± S.E.) current density versus voltage plot (n = 8). At all test potentials more positive than −20 mV, p < 0.05 for Q1 + GFP, 3 nm (Δ) versus Q1 + E4, 3 nm (○) and p < 0.01 for Q1 + E4, 10 nm (●) versus Q1 + E4, 3 nm (○) by t test.
RESULTS
KCNQ1 Interacts Biochemically and Functionally with CaM
To demonstrate that KCNQ1 interacts biochemically with CaM in our experimental system (i.e. heterologous expression in CHO cells), we used CaM-agarose beads to pull down KCNQ1 in the presence of either CaCl2 or the Ca2+ chelators EGTA and EDTA. KCNQ1 was immunodetected robustly in eluate from beads incubated in the presence of CaCl2, but less was detectable when the incubation was performed in the presence of Ca2+ chelators (Fig. 1A). To examine the specificity of this finding, we performed pulldown assays in the presence of CaCl2 plus either of two CaM-inhibitory peptides, CaMKII-P and MLCK-P (named according to the binding regions of calmodulin-dependent kinase II and myosin light chain kinase, respectively), or a control peptide, CTRL-P. Compared with the peptide-free condition, both CaM-inhibitory peptides impeded pulldown of KCNQ1 by CaM-agarose, whereas CTRL peptide did not diminish the interaction (Fig. 1B).
We next examined the functional significance of the interaction between KCNQ1 and CaM in CHO cells. We recorded whole-cell currents in CHO cells transiently transfected with KCNQ1, using the MLCK inhibitory peptide in the pipette solution to disrupt the biochemical interaction between KCNQ1 and CaM. In the presence of 50 μm MLCK peptide, steady-state current amplitude decayed rapidly over time, whereas cells dialyzed with 50 μm CTRL or with no peptide showed minimal decay (Fig. 1C). We also observed that KCNQ1 function is Ca2+-sensitive. Specifically, we observed no effect of varying [Ca2+]i on current density, but a shift toward more depolarized voltage dependence of activation was observed at lower intracellular Ca2+ concentrations (supplemental Fig. S1). Overall, our results demonstrate a Ca2+-dependent interaction of KCNQ1 with CaM that is necessary for channel activity.
Ca2+-dependent Interaction of KCNE4 with CaM
The suppression of KCNQ1 current resulting from CaM inhibition closely resembles the effects of KCNE4 co-expression with the channel. We hypothesized that the mechanism of KCNQ1 inhibition by KCNE4 may involve the ability of KCNE4 to mimic a CaM-inhibitory peptide. To test this hypothesis, we first sought to demonstrate interaction between KCNE4 and CaM.
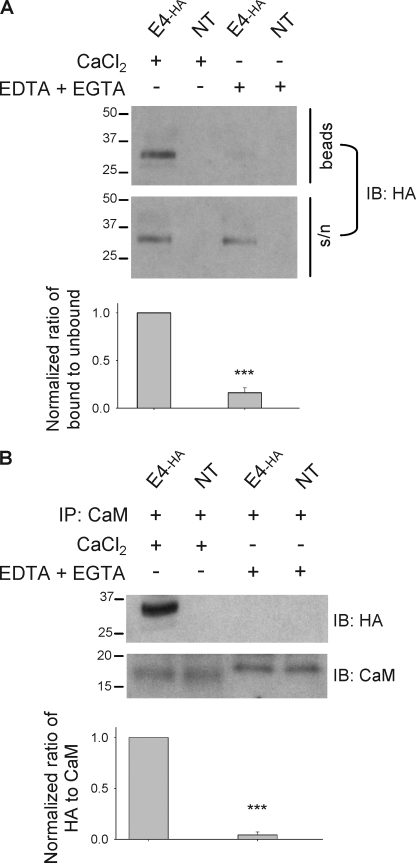
We used multiple biochemical approaches to assess whether KCNE4 can interact with CaM. First, CaM-agarose pulldown assays using lysates from CHO cells transiently transfected with epitope-tagged KCNE4 (KCNE4-ha) revealed that KCNE4-ha interacts with CaM in a Ca2+-dependent manner (Fig. 2A). Next, we validated the biochemical interaction between KCNE4-ha and CaM by co-immunoprecipitation using an anti-CaM primary antibody. KCNE4-ha was detected following immunoprecipitation performed in the presence of CaCl2 but not Ca2+ chelators, whereas CaM was detectable in the immunoprecipitate regardless of Ca2+ availability (Fig. 2B). Finally, we demonstrated that the interaction of KCNE4-ha with CaM could be significantly disrupted by CaM-inhibitory peptides but not by the control peptide (Fig. 3). These data indicate that KCNE4-ha and CaM biochemically interact in CHO cells.
FIGURE 2.
Biochemical interaction between KCNE4 and CaM. A, representative immunoblot following CaM-agarose pulldown using lysates from CHO cells transiently transfected with E4-ha or nontransfected cells (NT), performed in duplicate in the presence of 2 mm CaCl2 or 2 mm EDTA + 2 mm EGTA. Bound (beads) and unbound (supernatant, s/n) fractions were each probed for KCNE4 using monoclonal HA antibody. Bars below each lane indicate average bound-to-unbound signal ratio, normalized to CaCl2 condition from the same experimental replicate. Means ± S.E. for normalized ratios are as follows: EDTA + EGTA, 0.16 ± 0.09, n = 3; ***, p < 0.001 versus CaCl2 by t test. B, representative immunoblot (IB) following co-immunoprecipitation (IP) with anti-CaM in the presence of 2 mm CaCl2 or 2 mm EDTA + 2 mm EGTA. The apparent higher molecular weight of apo-CaM versus holo-CaM following SDS-PAGE is consistent with previous findings (20) and is attributable to conformational changes that allow CaM to adopt a more compact form upon binding Ca2+ (38). Bars below each lane indicate average HA-to-CaM signal ratio, normalized to CaCl2 condition from same experimental replicate. Means ± S.E. for normalized ratios are as follows: EDTA + EGTA, 0.04 ± 0.06, n = 4; ***, p < 0.001 versus CaCl2 by t test.
We also tested whether another KCNE subunit can interact biochemically with CaM by CaM-agarose pulldown using CHO cells transiently transfected with FLAG epitope-tagged KCNE1. No signal was detected in the bound fraction, regardless of whether the pulldown was performed in the presence of CaCl2 or Ca2+ chelators (supplemental Fig. S2). These results suggest that CaM interaction is not a universal property of all KCNE subunits.
Juxtamembrane Tetraleucine Motif Is Critical for KCNE4-CaM Interaction
To survey where CaM might interact with KCNE4, we examined the full-length KCNE4 amino acid sequence using an on-line CaM-binding site prediction algorithm (Calmodulin Target Database at the Ontario Cancer Institute). Based upon various peptide properties, including hydropathy, α-helical propensity, residue weight, side chain charge, and helical class, we identified one potential CaM interaction site located between KCNE4 residues 44 and 73. This candidate interaction site spans part of the transmembrane domain plus 15 residues of the adjacent intracellular juxtamembrane region of the KCNE4 C terminus (Fig. 4A). Because critical binding energy in protein-protein interactions involving CaM is often provided by bulky hydrophobic amino acids (27), we targeted a tetraleucine motif (residues 69–72) within the candidate-binding site for disruption of the interaction between KCNE4 and CaM. Specifically, we mutated the four leucine residues to alanines (designated L(69–72)A-ha) and then performed CaM pulldown assays to determine effects on the protein-protein interaction. CaM-agarose assays comparing pulldown of wild-type KCNE4-ha to L(69–72)A-ha demonstrated that this juxtamembrane tetraleucine motif is required for the biochemical interaction of KCNE4 with CaM (Fig. 4B) most likely because this sequence is part of a CaM-binding site. Importantly, this tetraleucine motif is conserved in KCNE4 orthologues across mammals, birds, and amphibians. Similar motifs are absent in the other KCNE subunits, and this is consistent with the observed lack of KCNE1 biochemical interaction with CaM (supplemental Fig. S2).
FIGURE 4.
KCNE4 juxtamembrane tetraleucine motif is critical for biochemical interaction with CaM. A, amino acid sequence of human KCNE4. Box encloses predicted transmembrane domain; underline denotes region identified by on-line prediction tool (Calmodulin Target Database at the Ontario Cancer Institute) as most likely to contain putative CaM-binding site. B, representative immunoblot (IB) following CaM-agarose pulldown comparing lysates from CHO cells transiently transfected with wild-type E4-ha, L(69–72)A-ha, or nontransfected cells (NT). Pulldown was performed in the presence of 2 mm CaCl2. Bound (beads) and unbound (supernatant, s/n) fractions each probed for KCNE4 using monoclonal anti-HA. Bars below each lane indicate average bound-to-unbound signal ratio, normalized to wild-type E4-ha from same experimental replicate. Means ± S.E. for normalized ratios are as follows: L(69–72)A-ha, 0.12 ± 0.08, n = 3; ***, p < 0.001 versus wild-type by t test.
Altered KCNQ1 Modulation by L(69–72)A-ha
To test whether disruption of the biochemical interaction between KCNE4 and CaM is accompanied by any functional consequences, we compared whole-cell currents recorded from CHO cells expressing KCNQ1 + empty vector, KCNQ1 co-expressed with wild-type KCNE4-ha, and KCNQ1 co-expressed with L(69–72)A-ha. Representative current traces and a current density-voltage plot illustrate that the dramatic KCNQ1 suppression normally exerted by wild-type KCNE4 is not evident with L(69–72)A-ha (Fig. 5, A and B). The degree of KCNQ1 suppression was significantly less for L(69–72)A-ha than for wild-type KCNE4. Interestingly, L(69–72)A-ha exerts other modulatory effects on KCNQ1, including a significant shift in the voltage dependence of activation curve to more depolarized potentials (Fig. 5C; Table 1) and a significant slowing of the kinetics of activation (Fig. 5D).
TABLE 1.
Electrophysiological parameters characterizing E4-haversus L(69–72)A-ha modulation of KCNQ1
Electrophysiological parameters characterizing the modulation of Q1 by L(69–72)A-ha in comparison with Q1 + empty vector and Q1 modulation by E4-ha. p values reported for t test were in comparison with Q1 + L(69–72)A-ha. NA, not applicable.
| n | Current density, +60 mV |
V½ |
k |
τ |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.E. | p | Mean ± S.E. | p | Mean ± S.E. | p | Mean ± S.E. | p | ||
| pA/picofarad | mV | mV | ms | ||||||
| Q1 + empty vector | 8 | 32.0 ± 5.1 | 0.02 | −7.0 ± 1.9 | 0.005 | 10.4 ± 0.5 | 0.004 | 66.4 ± 11.2 | 0.001 |
| Q1 + L(69–72)A-ha | 6 | 15.3 ± 2.1 | NA | 2.4 ± 2.0 | NA | 15.6 ± 1.5 | NA | 141.6 ± 15.1 | NA |
| Q1 + E4-ha | 7 | 3.3 ± 1.0 | 0.001 | ||||||
It is important to note that cell-surface biotinylation assays revealed that L(69–72)A-ha is detectable at the cell surface (Fig. 5E), albeit at a reduced level. However, the modulatory effects of L(69–72)A-ha on KCNQ1 described in Fig. 5, C and D, imply that mutant KCNE4 subunits still associate with KCNQ1 at the plasma membrane to a significant extent. Overall, these findings demonstrate that disrupting the KCNE4-CaM interaction hinders the inhibition of KCNQ1 by KCNE4 and suggests possible mechanisms for the regulation of KCNQ1 function.
KCNE4 Inhibition of KCNQ1 Is Ca2+-sensitive
To assess whether KCNE4 inhibition of KCNQ1 is sensitive to [Ca2+]i, we compared whole-cell currents recorded from CHO cells stably expressing KCNQ1 and transiently transfected with KCNE4 or empty vector, measured using a pipette solution that contained either 10 or 3 nm free [Ca2+]. Representative current traces and a current density versus voltage plot (Fig. 6) illustrate that KCNE4 fails to completely inhibit KCNQ1 current when [Ca2+]i is chelated acutely. CHO cells expressing KCNE4 in the absence of KCNQ1 generated no current when recorded using 3 nm [Ca2+] pipette solution, suggesting that the recorded currents illustrated in Fig. 6 are not from endogenous channels activated by low [Ca2+]i (data not shown). These findings are consistent with the Ca2+ sensitivity of the biochemical interaction between KCNE4 and CaM (Fig. 1A) and the impaired KCNQ1 inhibition by KCNE4 upon disrupting interactions between KCNE4 and CaM by mutagenesis. Importantly, the impairment of KCNE4 inhibition of KCNQ1 upon acutely chelating [Ca2+]i is unlikely explained by an acute decrease in KCNE4 cell-surface expression, favoring the interpretation that the KCNE4 function is directly affected by disrupting its interaction with CaM.
DISCUSSION
Regulation of KCNQ1 is physiologically important especially in heart where this protein constitutes the pore-forming subunit required to generate IKs, an essential repolarizing current. KCNQ1 is part of a macromolecular complex containing accessory subunits and regulatory proteins such as yotiao (28), phosphodiesterase PDE4D3 (29), and CaM (15, 20). In addition to regulation by multiple intracellular second messengers, including cAMP, phosphatidylinositol 4,5-bisphosphate (30, 31), and Ca2+ (15, 16, 18–20), KCNQ1 is modulated by KCNE proteins, some of which can exert dramatic effects on the channel (1, 3).
Interactions between accessory subunits (e.g. KCNE) and other regulatory proteins associated with the macromolecular IKs complex are emerging as another layer of complexity in regulating KCNQ1 function (12). In this study, we uncovered a new and intriguing example of an interaction between a KCNE subunit and another important KCNQ1 regulatory protein. Specifically, we identified an interaction between KCNE4 and CaM that is linked to the robust inhibitory effect of this accessory protein on KCNQ1. The observation that pharmacological inhibition of CaM dramatically blunts KCNQ1 activity (15, 20), which resembles the modulatory effect of KCNE4 on KCNQ1, prompted our hypothesis that KCNQ1 inhibition by KCNE4 could involve CaM.
Our demonstration of a Ca2+-dependent biochemical interaction between KCNE4 and CaM, and that KCNQ1 functional modulation by KCNE4 is impaired both upon mutation of a candidate CaM interaction site in the juxtamembrane region of KCNE4 and by acutely chelating [Ca2+]i to displace CaM from KCNE4, suggests a connection between the mechanism of KCNQ1 inhibition by KCNE4 and the effect of CaM on the channel. We previously assumed that the inhibition of KCNQ1 by KCNE4 is caused by a direct effect of KCNE4 on the channel (32, 33). In demonstrating that the interaction between CaM and KCNE4 is critical for the inhibitory effect of KCNE4, the data presented here introduce the new possibility that KCNE4 inhibits KCNQ1 by disrupting CaM-mediated KCNQ1 activation.
The KCNE4 region we identified to be critical for its biochemical interaction with CaM lacks typical motifs shared by many (but not all) CaM-interacting proteins, such as an IQ domain or a 1–5-10 motif (27). However, there are other examples of membrane proteins known to interact with CaM at an intracellular juxtamembrane region (as we demonstrated for KCNE4), including the epidermal growth factor receptor (34–36). We previously demonstrated that the cytoplasmic domain of KCNE4 is necessary for its inhibition of KCNQ1 and is sufficient to confer inhibitory function to a chimera consisting of the N terminus and transmembrane domain of KCNE1 but the C terminus of KCNE4 (32). This overlap between the CaM-binding site and the KCNE4 domain necessary for inhibition of KCNQ1 activity supports our hypothesis that KCNQ1 inhibition by KCNE4 may involve its ability to interact with CaM.
The primary structure of KCNE4 is distinct from the other four KCNE proteins at the juxtamembrane site identified here as a CaM-binding region. Specifically, the region containing four sequential hydrophobic leucine residues in KCNE4 is occupied by polar residues in the other four KCNE proteins, which would likely not support interactions with CaM. As we demonstrated in this study, KCNE1 does not interact with CaM when expressed alone (supplemental Fig. S2).
The identification of critical residues for CaM binding also allowed us to study the functional consequences of disrupting the KCNE4-CaM interaction. We demonstrated that KCNE4 L(69–72)A-ha subunits fail to robustly inhibit KCNQ1. However, expression of this KCNE4 mutant does modulate the kinetics and voltage dependence of KCNQ1 activation and changes kinetic properties of tail currents consistent with removal of inactivation (Fig. 5). Overall these findings indicate that the mutant KCNE4 with impaired biochemical interaction with CaM also exhibits impaired ability to inhibit KCNQ1, although its ability to function as a modulatory subunit to KCNQ1 is not lost. We speculate that these residual functional effects of KCNE4 L(69–72)A-ha on KCNQ1 could be mediated through interactions between the pore-forming subunit and the KCNE4 transmembrane domain. In support of this notion, there are similarities in KCNQ1 modulation evoked by KCNE4 L(69–72)A-ha and a chimera consisting of the N and C termini of KCNE1 coupled to the transmembrane domain of KCNE4 (32).
The physiological implications of the observations presented here may be significant, but they are difficult to assign because of the primary lack of understanding of the physiological contributions of KCNE4. In heart, where KCNE4 could modulate repolarizing currents, such as IKs, and where local free Ca2+ concentrations cycle dramatically, the Ca2+-CaM sensitivity described here for KCNE4 may enable Ca2+-dependent regulation of repolarization time. Because repolarization time is critical for action potential duration as well as Ca2+ release and contractility (37), CaM-KCNE4 interactions might constitute part of a feedback loop for regulating intracellular Ca2+. Further characterization of KCNE4 L(69–72)A in native cardiac myocytes will be valuable toward establishing the physiological consequences of disrupting the interaction between KCNE4 and CaM. The novel protein-protein interaction we demonstrated in this study reinforces the notion that KV channels function as dynamic protein complexes subject to regulation by both accessory subunits and intracellular signaling molecules, which may themselves interact to impact channel function.
Supplementary Material
Acknowledgments
We thank Dr. Roger Colbran and Dr. Walter Chazin for helpful advice.
This work was supported, in whole or in part, by National Institutes of Health Grant HL077188 (to A. L. G.) and USPHS Award T32 GM07347 from the NIGMS (to Vanderbilt Medical-Scientist Training Program). This work was also supported by American Heart Association Predoctoral Research Fellowship 09PRE2220323 (to E. J. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Table S1.
- KV channel
- voltage-gated potassium channel
- CaM
- calmodulin
- CaMKII
- CaM-inhibitory peptide based on the CaM-binding site of calmodulin kinase II
- MLCK
- CaM-inhibitory peptide based on the CaM-binding site of myosin light chain kinase
- CTRL
- control peptide
- Q1
- KCNQ1
- ANOVA
- analysis of variance
- IB
- immunoblot
- NT
- non-transfected
- n.s.
- not significant
- IP
- immunoprecipitation
- s/n
- supernatant.
REFERENCES
- 1. McCrossan Z. A., Abbott G. W. (2004) Neuropharmacology 47, 787–821 [DOI] [PubMed] [Google Scholar]
- 2. Li Y., Um S. Y., McDonald T. V. (2006) Neuroscientist 12, 199–210 [DOI] [PubMed] [Google Scholar]
- 3. Lundquist A. L., Manderfield L. J., Vanoye C. G., Rogers C. S., Donahue B. S., Chang P. A., Drinkwater D. C., Murray K. T., George A. L., Jr. (2005) J. Mol. Cell. Cardiol. 38, 277–287 [DOI] [PubMed] [Google Scholar]
- 4. Sanguinetti M. C., Curran M. E., Zou A., Shen J., Spector P. S., Atkinson D. L., Keating M. T. (1996) Nature 384, 80–83 [DOI] [PubMed] [Google Scholar]
- 5. Barhanin J., Lesage F., Guillemare E., Fink M., Lazdunski M., Romey G. (1996) Nature 384, 78–80 [DOI] [PubMed] [Google Scholar]
- 6. Abbott G. W., Goldstein S. A. (2002) FASEB J. 16, 390–400 [DOI] [PubMed] [Google Scholar]
- 7. Melman Y. F., Krumerman A., McDonald T. V. (2002) J. Biol. Chem. 277, 25187–25194 [DOI] [PubMed] [Google Scholar]
- 8. Yang Y., Xia M., Jin Q., Bendahhou S., Shi J., Chen Y., Liang B., Lin J., Liu Y., Liu B., Zhou Q., Zhang D., Wang R., Ma N., Su X., Niu K., Pei Y., Xu W., Chen Z., Wan H., Cui J., Barhanin J., Chen Y. (2004) Am. J. Hum. Genet. 75, 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma K. J., Li N., Teng S. Y., Zhang Y. H., Sun Q., Gu D. F., Pu J. L. (2007) Chin. Med. J. 120, 150–154 [PubMed] [Google Scholar]
- 10. Lundby A., Ravn L. S., Svendsen J. H., Hauns S., Olesen S. P., Schmitt N. (2008) Cell. Physiol. Biochem. 21, 47–54 [DOI] [PubMed] [Google Scholar]
- 11. Ravn L. S., Aizawa Y., Pollevick G. D., Hofman-Bang J., Cordeiro J. M., Dixen U., Jensen G., Wu Y., Burashnikov E., Haunso S., Guerchicoff A., Hu D., Svendsen J. H., Christiansen M., Antzelevitch C. (2008) Heart Rhythm. 5, 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurokawa J., Chen L., Kass R. S. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurokawa J., Bankston J. R., Kaihara A., Chen L., Furukawa T., Kass R. S. (2009) Channels 3, 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haitin Y., Yisharel I., Malka E., Shamgar L., Schottelndreier H., Peretz A., Paas Y., Attali B. (2008) PLoS ONE 3, e1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh S., Nunziato D. A., Pitt G. S. (2006) Circ. Res. 98, 1048–1054 [DOI] [PubMed] [Google Scholar]
- 16. Shen Z., Marcus D. C. (1998) Hear. Res. 123, 157–167 [DOI] [PubMed] [Google Scholar]
- 17. Kerst G., Beschorner U., Unsöld B., von Hahn T., Schreiber R., Greger R., Gerlach U., Lang H. J., Kunzelmann K., Bleich M. (2001) Pflugers Arch. 443, 146–154 [DOI] [PubMed] [Google Scholar]
- 18. Boucherot A., Schreiber R., Kunzelmann K. (2001) J. Membr. Biol. 182, 39–47 [DOI] [PubMed] [Google Scholar]
- 19. Gamper N., Li Y., Shapiro M. S. (2005) Mol. Biol. Cell 16, 3538–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shamgar L., Ma L., Schmitt N., Haitin Y., Peretz A., Wiener R., Hirsch J., Pongs O., Attali B. (2006) Circ. Res. 98, 1055–1063 [DOI] [PubMed] [Google Scholar]
- 21. Manderfield L. J., George A. L., Jr. (2008) FEBS J. 275, 1336–1349 [DOI] [PubMed] [Google Scholar]
- 22. Kahlig K. M., Saridey S. K., Kaja A., Daniels M. A., George A. L., Jr., Wilson M. H. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Török K., Cowley D. J., Brandmeier B. D., Howell S., Aitken A., Trentham D. R. (1998) Biochemistry 37, 6188–6198 [DOI] [PubMed] [Google Scholar]
- 24. Payne M. E., Fong Y. L., Ono T., Colbran R. J., Kemp B. E., Soderling T. R., Means A. R. (1988) J. Biol. Chem. 263, 7190–7195 [PubMed] [Google Scholar]
- 25. Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. (1981) Pflügers Arch. 391, 85–100 [DOI] [PubMed] [Google Scholar]
- 26. Lindau M., Neher E. (1988) Pflügers Arch. 411, 137–146 [DOI] [PubMed] [Google Scholar]
- 27. Rhoads A. R., Friedberg F. (1997) FASEB J. 11, 331–340 [DOI] [PubMed] [Google Scholar]
- 28. Marx S. O., Kurokawa J., Reiken S., Motoike H., D'Armiento J., Marks A. R., Kass R. S. (2002) Science 295, 496–499 [DOI] [PubMed] [Google Scholar]
- 29. Terrenoire C., Houslay M. D., Baillie G. S., Kass R. S. (2009) J. Biol. Chem. 284, 9140–9146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park K. H., Piron J., Dahimene S., Mérot J., Baró I., Escande D., Loussouarn G. (2005) Circ. Res. 96, 730–739 [DOI] [PubMed] [Google Scholar]
- 31. Loussouarn G., Park K. H., Bellocq C., Baró I., Charpentier F., Escande D. (2003) EMBO J. 22, 5412–5421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manderfield L. J., Daniels M. A., Vanoye C. G., George A. L., Jr. (2009) J. Physiol. 587, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vanoye C. G., Welch R. C., Daniels M. A., Manderfield L. J., Tapper A. R., Sanders C. R., George A. L., Jr. (2009) J. Gen. Physiol. 134, 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martín-Nieto J., Villalobo A. (1998) Biochemistry 37, 227–236 [DOI] [PubMed] [Google Scholar]
- 35. Li H., Villalobo A. (2002) Biochem. J. 362, 499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li H., Ruano M. J., Villalobo A. (2004) FEBS Lett. 559, 175–180 [DOI] [PubMed] [Google Scholar]
- 37. Sah R., Ramirez R. J., Kaprielian R., Backx P. H. (2001) J. Physiol. 533, 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu M. M., Rempel D. L., Zhao J., Giblin D. E., Gross M. L. (2003) Biochemistry 42, 15388–15397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.