Abstract
Phosphoinositide 3-kinases (PI3Ks) are critical regulators of pancreatic β cell mass and survival, whereas their involvement in insulin secretion is more controversial. Furthermore, of the different PI3Ks, the class II isoforms were detected in β cells, although their role is still not well understood. Here we show that down-regulation of the class II PI3K isoform PI3K-C2α specifically impairs insulin granule exocytosis in rat insulinoma cells without affecting insulin content, the number of insulin granules at the plasma membrane, or the expression levels of key proteins involved in insulin secretion. Proteolysis of synaptosomal-associated protein of 25 kDa, a process involved in insulin granule exocytosis, is impaired in cells lacking PI3K-C2α. Finally, our data suggest that the mRNA for PI3K-C2α may be down-regulated in islets of Langerhans from type 2 diabetic compared with non-diabetic individuals. Our results reveal a critical role for PI3K-C2α in β cells and suggest that down-regulation of PI3K-C2α may be a feature of type 2 diabetes.
Keywords: Diabetes, Exocytosis, Insulin Secretion, Phosphatidylinositol 3-Kinase, Signal Transduction
Introduction
Intracellular signaling pathways regulated by phosphoinositide 3-kinase (PI3K) are critical for the regulation of pancreatic β cell mass and survival. Eight mammalian PI3K isoforms exist, grouped into three classes, which generate lipid second messengers by phosphorylating position 3 within the inositol ring of distinct phosphoinositides (1). The most studied pathway involves activation of class I isoforms, generation of phosphatidylinositol 3,4,5-trisphosphate, and activation of the downstream effector protein kinase B/Akt, which plays a pivotal role in β cell regulation (2–4). Although the importance of class I PI3Ks in the control of β cell mass and survival is well established, conflicting evidence exists in the literature about their role in insulin secretion. It has been reported that the classical PI3Ks inhibitors wortmannin and LY294002 enhance insulin secretion in rat (5) and mouse islets (6) and pancreatic β cell lines (7) and that wortmannin does not inhibit insulin secretion or synthesis (8). However, knock-out mice for the class I isoform p110γ lack the first phase of insulin secretion (9, 10), and specific blockade of p110γ impairs insulin secretion (11). Recent evidence has revealed that PI3K isoforms other than the class I members are also present in pancreatic β cell. Specifically, the mRNAs for class II PI3Ks were detected in human pancreatic β cells (12), and the percentage of β cells expressing the class II isoform PI3K-C2α mRNA was higher than any other PI3K analyzed (13). More recently, protein expression of PI3K-C2α has been shown in human and mouse islets and in insulin-producing cell lines (14).
Although less investigated than class I, the class II subfamily of PI3Ks is currently emerging as critical for several intracellular functions (15). Interest has been fuelled by our data demonstrating that class II PI3Ks generate a different lipid product in vivo, namely phosphatidylinositol 3-phosphate (PtdIns3P, 16, 17), which would interact with distinct molecules compared with the class I product phosphatidylinositol 3,4,5-trisphosphate and, therefore, activate distinct signaling cascades. In particular, we demonstrated that PI3K-C2α generates a pool of PtdIns3P in muscle cells upon insulin stimulation (17) and that this enzyme is required for full insulin-induced glucose transport and translocation of the glucose transporter GLUT46 to the plasma membrane (17). These data indicate that PI3K-C2α regulates the exocytosis of GLUT4-containing vesicles and are consistent with the reported role of this enzyme in the regulated exocytosis of neurosecretory granules (18). Furthermore, the PI3K-C2α-dependent synthesis of PtdIns3P is critical for both GLUT4 translocation (17, 19) and neurosecretory granule release (20). Taken together these data suggest that PI3K-C2α and its lipid product PtdIns3P may have a general role in exocytosis in different cellular contexts.
The exocytosis of insulin is regulated by complex mechanisms (21, 22). The main events in this process involve conversion of uptake glucose to glucose 6-phosphate by the enzyme β glucokinase, its metabolism, and a subsequent increase in the ATP/ADP ratio, which leads to the closure of the K+ATP channel, depolarization of the plasma membrane, and opening of the L-type voltage-dependent Ca2+ channel. Although other regulatory processes are also likely to be involved (23), the resulting Ca2+ influx and rise in intracellular Ca2+ concentration are key events that trigger insulin secretion. Indeed, any treatment able to induce closure of the K+ATP channel can increase intracellular Ca2+ levels and can trigger insulin secretion, even in the absence of glucose metabolism. In particular and relevant to this study, treatment of pancreatic β cells in vitro with high concentrations of KCl can induce insulin secretion through this mechanism, and therefore, allows investigation of the critical steps regulating insulin granule exocytosis irrespective of the glucose-mediated effect (metabolic component). For the hormone to be released, the insulin-containing granules must dock to the plasma membrane and be “primed” (i.e. they must become competent for release through chemical modifications) before the actual fusion of the granules can occur (24). Fusion of the granules involves a complex interaction between the molecules synaptosomal-associated protein of 25 kDa (SNAP25), syntaxin 1 at the plasma membrane, and the vesicle-associated membrane protein VAMP2, probably modulated by synaptotagmin V (25) and VII (26). It has been suggested that a complex of these three proteins anchors the granule to the membrane under resting conditions. Upon stimulation, SNAP25 proteolysis allows fusion of the granules and subsequent insulin release (27). In particular, proteolysis of SNAP25 seems to be critical for insulin secretion (27).
Recent data have indicated a role for PI3K-C2α in glucose-induced insulin secretion in a mechanism involving an insulin-dependent feedback loop (14), implying that this enzyme is involved in the metabolic regulation of insulin secretion. The aim of this work was to determine whether PI3K-C2α had also a direct role in the late steps of insulin granule exocytosis (i.e. docking, priming, fusion). Here we report that selective down-regulation of this PI3K isoform in INS1 rat insulinoma cells specifically impairs insulin secretion irrespective of the metabolic effect, without affecting insulin content or the number of insulin granules associated with the plasma membrane in resting cells. Down-regulation of PI3K-C2α does not affect the expression levels of key proteins involved in insulin secretion, whereas it reduces the proteolysis of SNAP25, suggesting a role for this enzyme in fusion of the insulin granules to the membrane. Data further suggest that the mRNA for PI3K-C2α may be selectively down-regulated in islets of Langerhans from type 2 diabetic compared with non diabetic individuals, suggesting that down-regulation of PI3K-C2α may be a feature of type 2 diabetes.
EXPERIMENTAL PROCEDURES
Materials
Anti PI3K-C2α (dilution for Western blotting. 1:500) was from BD Transduction Laboratories, anti-tubulin (1:1000) was from Sigma, anti-p110α (1:500), anti-p110β (1:500), anti-phospho-Thr-308Akt (1:500), anti-Akt (1:1000), anti-ERK2 (1:1000), anti-IRS2 (1:500), anti-syntaxin 1 (1:1000), anti-syntaxin 4 (1:500), and anti-SNAP25 (1:1000) were from Santa Cruz Biotechnology (Santa Cruz, CA), anti-phosphotyrosine (1:1000) and anti-phospho-Ser-473 Akt (1:500) were from Cell Signaling Technology (Danvers, MA), anti-p110γ (1:500) was from Alexis Biochemicals (Enzo Life Sciences UK Ltd, Exeter, UK), anti-hVps34 (1:500) was from Zymed Laboratories Inc. (Invitrogen). The anti-VAMP1/2/3 used in confocal microscopy analysis was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-VAMP2 (69.1) used in Western blotting analysis (1:1000) was a kind gift from Dr. Giampietro Schiavo (Cancer Research UK London Research Institute). The insulin receptor inhibitor AG1024 was from Santa Cruz Biotechnology (Santa Cruz, CA). “Scrambled” siRNA (Silencer® negative control siRNA) was from Ambion (Applied Biosystems, Foster City, CA). Specific siRNAs targeting PI3K-C2α were designed based on the same sequences used to generate the stable cell lines (sequence 1, GTCCAGTCACAGTGCAAAG; sequence 2, GTACAGAATGAGGAGGTGG) and obtained from Eurofins MWG Operon (Ebersberg, Germany).
Cell Culture
INS1 cells were cultured in RPMI 1640 containing 10% FBS, 10 mm Hepes, 2 mm l-glutamine, 1 mm sodium pyruvate, and 50 μm β-mercaptoethanol. Stable cell lines were generated by retroviral infection using recombinant vectors as described (17) and selected in media containing 0.25 μg/ml puromycin. Alternatively, cells were transfected with the specific siRNAs (or scrambled siRNAs as control) using OligofectAMINE (Invitrogen) and stimulated for insulin secretion 72h after transfection. Cell proliferation was determined by manual counting. For flow cytometry analysis of cell cycle profile, cells were washed once in PBS and incubated for 30 min in 20 mm Tris-HCl, pH 7.5, 100 mm NaCl, 50 mg/ml propidium iodide before analysis on a BD Biosciences LSR II analyzer.
Insulin Secretion
Cells were treated with Krebs-Ringer buffer (KRB in the absence of Ca2+) or stimulated with KRB containing a secretagogue mixture (13.5 mm glucose, 10 mm leucine, 10 mm glutamine, 1 mm phorbol 12-myristate 13-acetate, 1 mm isobutylmethylxanthine, 1 mm tolbutamide, 1 mm Ca2+) for 3 h. Alternatively, cells were stimulated with 60 mm KCl or 20 mm glucose (in KRB supplemented with 1 mm Ca2+) for 30 min. Supernatants were collected, and total released insulin was determined using the Mercodia high range rat insulin ELISA kit (Mercodia, Uppsala, Sweden). Cells were then washed twice with PBS and lysed as described below. Protein concentration was determined in each sample using Bradford reagent. Alternatively, cells were detached and counted. Total released insulin was normalized by the total protein content or the total number of cells. In the experiments using the insulin receptor inhibitor, cells were treated for 30 min with KRB containing the indicated concentration of AG1024 (or vehicle) and then treated with 60 mm KCl in the absence or presence of AG1024 for further 30 min.
Insulin Content
Cells were resuspended in PBS and incubated with 500 μl of extraction solution (15% HCl, 80% ethanol) at 4 °C. After 24 h, 500 μl of 1 m NaOH was added to neutralize the samples, and insulin content was assayed as above. Total protein content was used to normalize insulin content. Alternatively, insulin content was measured by radioimmunoassay and normalized by the total number of cells.
Calcium Measurements
Cells were seeded on Lab-TekTM chambered #1.0 borosilicate coverglass (Nunc GmbH & Co. KG, Langenselbold, Germany) for confocal live microscopy and incubated with 250 μl of Hanks' balanced salt solution containing 0.5% BSA, 2 mm CaCl2,4 μm Fluo-4 AM (Invitrogen) for 1 h at 37 °C. Cells were then washed twice with Hanks' balanced salt solution containing 0.5% BSA, 2 mm CaCl2, and incubated at 37 °C in KRB containing 1 mm Ca2+ for 30 min to allow de-esterification of Fluo-4. Samples were analyzed using the Zeiss confocal microscope LSM 510 equipped with a chamber for live imaging at 37 °C supplied with 5% CO2 using a 10× objective. After recording basal fluorescence, cells were stimulated with 60 mm KCl (in KRB containing 1 mm Ca2+). ZEN Carl Zeiss software was used for acquisition and analysis of raw data. Fluorescence values of 40 cells per chamber were analyzed and expressed as the mean of fluorescence.
Immunoprecipitation and Western Blotting Analyses
For immunoprecipitation studies, cells were treated with KRB for 30 min before a further 30-min stimulation with 60 mm KCl or 100 nm insulin (in KRB supplemented with 1 mm Ca2+). Alternatively, cells were treated for 3 h with the secretagogue mixture. After washing in ice-cold PBS, cells were scraped in lysis buffer (containing 1% Triton X-100) supplemented with protease and phosphatase inhibitors cocktails (Sigma). Five hundred micrograms of proteins were immunoprecipitated using the Catch and Release® kit (Millipore UK Ltd, Walford, UK) and 2 μg of anti-IRS2 (1:100 of the stock, Santa Cruz Biotechnology, Santa Cruz, CA). For analysis of Akt activation, cells were treated with KRB for 3 h before a 30-min stimulation with 60 mm KCl (in KRB supplemented with 1 mm Ca2+) or with serum-free RPMI containing either 0.5 mm glucose, 15 mm glucose and 10 ng/ml insulin-like growth factor 1, or 15 mm glucose and 100 nm insulin. Alternatively, cells were treated for 3 h with the secretagogue mixture. Cells were then lysed as above, and Akt activation was monitored by analyzing phosphorylation at its residues Ser-473 and Thr-308 (phospho-Ser-473 Akt from Cell Signaling 1:500; phospho-Thr-308 Akt from Santa Cruz Biotechnology 1:1000). Equal loading was assessed using an anti-ERK2 antibody (Santa Cruz Biotechnology, 1:1000).
Confocal Microscopy Analysis
Cells plated on glass coverslips were fixed with 4% paraformaldehyde for 15 min and incubated in PBS containing 1% BSA for further 30 min. Coverslips were then incubated with the specific primary antibodies for 2 h at room temperature (dilution 1:50 for all of them), washed in PBS, and further incubated with the corresponding secondary antibodies for 1 h at room temperature. Coverslips were analyzed using a Zeiss LSM510 microscope with a Meta detector.
Total Internal Reflection Fluorescence (TIRF) Microscopy
TIRF microscopy (28) was performed as described (29–32). Briefly, cells were infected with NPY-Venus-expressing adenovirus 48 h before imaging at 32 °C in modified KRB (140 mm NaCl, 3.6 mm KCl, 0.5 mm NaH2PO4, 0.5 mm MgSO4, 1.5 mm CaCl2, 10 mm Hepes, 2 mm NaHCO3 saturated with O2/CO2 and pH 7.4) with 0.1% BSA and 3 mm glucose using a custom-built lens-type TIRF microscope based on an inverted stand (Axiovert 200M, Carl Zeiss) and equipped with a 100×/1.45 Apo lens (Carl Zeiss). Cells were excited with a DPSS laser (473 nm, 80 milliwatt; Crystalasers). Images were captured on a charge-coupled device (CCD) camera (iXonEM + DU-897, and IQ software Andor Technology, Belfast, UK) as an average of four 50-ms exposures. Cells were selected based on yellow fluorescence and morphology. Granules were detected automatically using ImageJ software with the particle detector plugin. A threshold was set by the operator once for the entire data set. Data are expressed as the number of granules/μm2 of the visible cell “footprint.”
Real-time Quantitative PCR (qPCR) Analysis of INS1 Cell Lines
Primers for real-time PCR analysis were designed using Primer Express 3.0 (Applied Biosystems) using mRNA sequences for rat on the Ensembl data base. Sequence specificity for all primers was verified using BLAST (www.ncbi.nlm.nih.gov). Real time-PCR was performed on an ABI-Prism Fast 7500 device (Applied Biosystems) using powerSYBR reagent (Applied Biosystems).
Microarray Analysis of Human Islets
Pancreata were obtained with the approval of the local Ethics Committee and consent by donors' relatives and handled as described (33). Specifically, human islets were isolated from 7 non-diabetic (age 58 ± 17 year; gender, 4 males/3 females; body mass index, 24.8 ± 2.5 kg/m2) and 6 type 2 diabetic (age 71 ± 9 year; gender, 3 males/3 females; body mass index, 26.0 ± 2.2 Kg/m2) organ donors by collagenase digestion followed by density gradient purification as previously reported (33). For microarray studies, total RNA was extracted and treated as reported elsewhere (34). Briefly, after two-cycle amplification, RNA was biotinylated, fragmented, and hybridized onto HG-U133A Affymetrix chips. These were then scanned and normalized, and differential gene expression was assessed. Genes with a p value lower than 0.01 were considered as differentially expressed. The row data have been deposited in a MIAME compliant data base (GEO, accession number GSE25724).
Real-time Quantitative qPCR of Human Samples
For qPCR, 1 μg of total RNA was extracted from human islets from 13 non-diabetic and 6 type 2 diabetic individuals (as above) and reverse-transcribed with iScript cDNA Synthesis Kit (Bio-Rad) before analysis using the Applied Biosystems 7500 Real-time PCR system. The cDNA was amplified using Maxima SYBR (Fermentas, Sheriff Hutton, UK) according to the manufacturer's protocol using primers (Invitrogen) for PI3K-C2α (forward, 5′-tggaaaatccctttctgtgg; reverse, 5′-aaaaagctgccatctcctca) and actin (forward, 5′-agaaaatctggcaccacacc; reverse, 5′- ggggtgttgaaggtctcaaa), which was used as reference gene. All primer pairs were tested for specificity and efficiency.
RESULTS
Down-regulation of PI3K-C2α Does Not Affect Cellular Growth
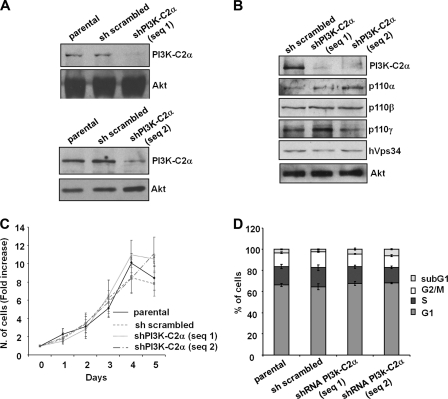
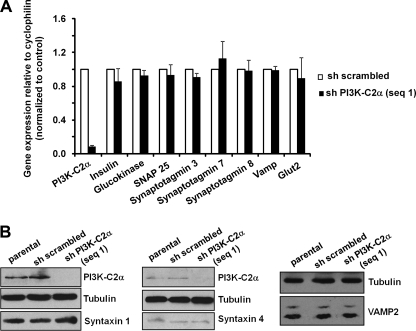
Recent data have revealed a role for PI3K-C2α in glucose-induced insulin secretion from pancreatic β cells and suggested that this enzyme is important for the regulation of the metabolic component of insulin secretion (14). However previous data, including data from our own laboratory, have suggested that PI3K-C2α (and its lipid product PtdIns3P) can regulate late steps in the process of exocytosis (17, 18, 20). We, therefore, decided to investigate whether PI3K-C2α may also play a role in insulin secretion by regulating the final steps of insulin granule exocytosis (i.e. docking, priming, fusion of the granules). Because no specific PI3K-C2α inhibitors are currently available, we generated two stable rat insulinoma INS1 cell lines expressing recombinant vectors containing distinct shRNAs targeting the enzyme. A vector containing a scrambled shRNA was used to generate control (“sh scrambled”) cells. Both shRNAs were able to down-regulate PI3K-C2α expression levels (Fig. 1, A and B) with no effect on the levels of other PI3Ks (Fig. 1B). No difference in proliferation was observed when cells were cultured in complete medium (Fig. 1C), and only a slight increase in the sub-G1 population was observed in cells lacking PI3K-C2α (Fig. 1D). These data indicate that PI3K-C2α does not regulate cell proliferation and survival in pancreatic β cells under the experimental conditions used here.
FIGURE 1.
Generation and characterization of stable INS1 cell lines. A and B, expression levels of PI3K-C2α and other PI3K isoforms in parental INS1 and stable INS1 cells expressing a scrambled shRNA or shRNAs targeting PI3K-C2α are shown. Equal loading was assessed using an anti-Akt antibody (representative blot). C and D, proliferation (C) and cell cycle profile (D) of the indicated cells is shown. Data are the means ± S.E. from three (C) and five (D) independent experiments.
PI3K-C2α Regulates Insulin Granule Exocytosis
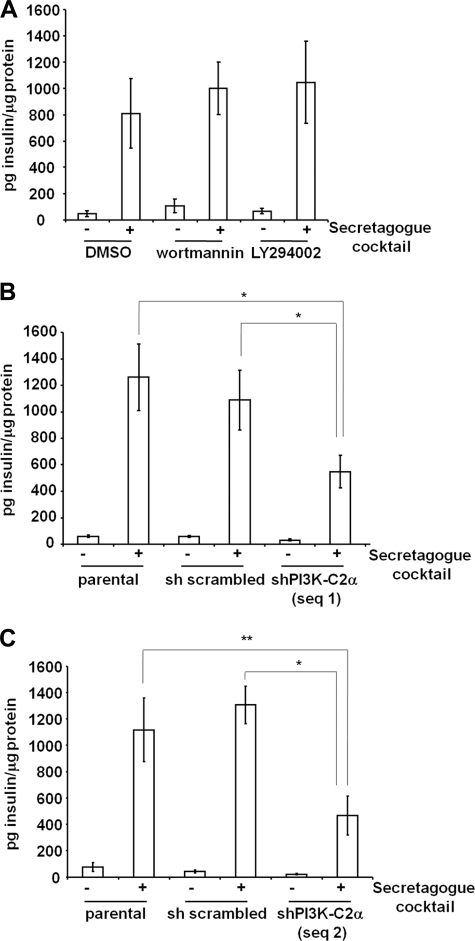
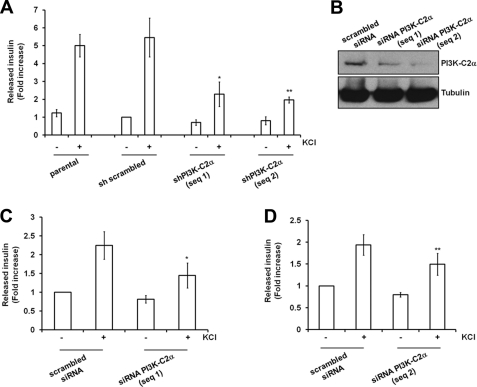
To investigate the potential role of PI3K-C2α in the final steps of insulin granule exocytosis, we stimulated cells with a secretagogue mixture able to induce maximal insulin granule exocytosis. Under these experimental conditions, we observed that treatment of INS1 cells with classical PI3K inhibitors did not reduce insulin secretion (Fig. 2A). Because PI3K-C2α is the only PI3K isoform resistant to treatment with both inhibitors (35), these data suggested a potential involvement of PI3K-C2α in this process. Consistent with this hypothesis, we observed that down-regulation of PI3K-C2α strongly reduced the total amount of insulin secreted upon cellular stimulation in both PI3K-C2α knockdown cell lines (Fig. 2, B and C). No difference in insulin secretion was observed between parental and control sh scrambled cells (Fig. 2, B and C), ruling out nonspecific effects of shRNA expression or of retroviral infection. Similar data were obtained in the stable cell lines when insulin secretion was stimulated by treatment with KCl (Fig. 3A; supplemental Fig. S1A). Consistent with recent reported evidence (14), significant inhibition of glucose-dependent insulin secretion was also detected in INS1 lacking PI3K-C2α (supplemental Fig. S1B). To rule out potential secondary effects due to chronic down-regulation of PI3K-C2α, insulin secretion induced by KCl stimulation was analyzed in cells upon transient down-regulation of the enzyme using siRNAs (Fig. 3B). A clear inhibition of KCl-induced insulin secretion was detected in cells transfected with two specific siRNAs targeting PI3K-C2α compared with cells expressing a control scrambled siRNA (Fig. 3, C and D). Taken together, these data indicate that PI3K-C2α specifically regulates secretion of insulin granules.
FIGURE 2.
Effect of PI3K-C2α down-regulation on insulin secretion induced by a secretagogue mixture. A, INS1 cells were pretreated for 15 min with 100 nm wortmannin, 10 μm LY294002, or vehicle alone (DMSO), and insulin secretion was determined upon stimulation with a secretagogue mixture for 3 h in the presence of the indicated inhibitors. B and C, insulin secretion was determined upon stimulation of the indicated cell lines with a secretagogue mixture for 3 h. Data are the means ± S.E. from 4 (A), 10 (B), and 3 (C) independent experiments performed in duplicate and are expressed as pg of insulin normalized by total protein content. Student's t test: *, p < 0.01; **, p < 0.05. shPI3K-C2α sequence 1 unstimulated, p < 0.01 versus parental or scrambled unstimulated. shPI3K-C2α sequence 2 unstimulated, not significant versus parental unstimulated or scrambled unstimulated.
FIGURE 3.
Effect of PI3K-C2α down-regulation on insulin secretion induced by treatment with KCl. A, insulin secretion was determined upon stimulation of the indicated stable cell lines with KCl for 30 min. Data are the means ± S.E. from 7 independent experiments performed in duplicate except for shPI3K-C2α sequence 2 (n = 6). Total secreted insulin for each sample (pg insulin) was normalized by the total protein content (μg of protein) and then expressed as -fold increase over the unstimulated sh scrambled cells (pg insulin/μg of protein: 62.467 ± 12.154). Student's t test: *, p < 0.01; **, p < 0.05 versus scrambled or parental. B, shown is Western blotting analysis of PI3K-C2α expression in cells transiently transfected with a scrambled siRNA or siRNAs specifically targeting PI3K-C2α. C and D, insulin secretion in cell lines transiently transfected with the indicated siRNA and stimulated with KCl for 30 min normalized to total protein contents is shown. Data are expressed as -fold increase over insulin released by unstimulated cells expressing the scrambled siRNA (pg insulin/μg protein, 68.145 ± 26.263 for C; pg insulin/μg of protein, 65.385 ± 25.561 for D) and are the means ± S.E. from five independent experiments performed in duplicate. Student's t test: *, p < 0.01; **, p < 0.05.
Down-regulation of PI3K-C2α Does Not Affect Insulin Content and Calcium Uptake upon KCl Treatment
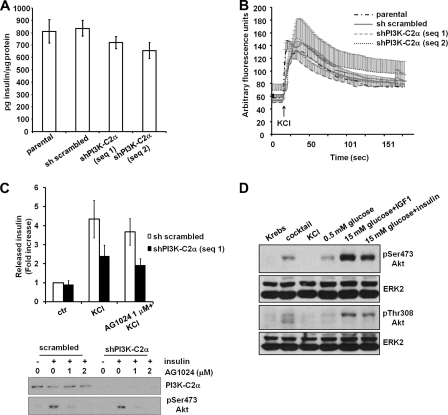
Impairment of insulin secretion did not appear to be the consequence of a reduced insulin synthesis in PI3K-C2α knockdown cells as we observed only a slight reduction in the insulin content in these cells (Fig. 4A). Similar results were obtained by radioimmunoassay (data not shown). Therefore, the defect in insulin secretion detected in cells lacking PI3K-C2α must be due either to an impairment of the signaling pathways regulating insulin secretion or to a defect in the machinery responsible for insulin granule exocytosis.
FIGURE 4.
Investigation of the mechanisms of PI3K-C2α-dependent insulin secretion. A, total insulin content in the indicated cells is shown. Data are the means ± S.E. from nine independent measurements. B, intracellular calcium increase upon treatment of the indicated cells with KCl (in KRB containing 1 mm Ca2+), monitored as specified under “Experimental Procedures,” is shown. Data are the means ± S.E. from 6 independent experiments except for shPI3K-C2α sequence 2 (n = 3). C, cells untreated or pretreated with 1 μm insulin receptor inhibitor AG1024 were left unstimulated or stimulated with KCl for 30 min in the presence or absence of AG1024. Data are the means ± S.E. from five independent experiments performed in duplicate. Total secreted insulin for each sample (pg insulin) was normalized by the total cell number and then expressed as -fold increase over the unstimulated sh scrambled cells (pg insulin/cell: 0.013 ± 0.005). Student's t test KCl-stimulated shPI3K-C2α sequence 1, p < 0.05 versus KCl-stimulated scrambled. AG1024-treated, KCl-stimulated scrambled, not significant versus KCl-stimulated scrambled. AG1024-treated, KCl-stimulated shPI3K-C2α sequence 1, not significant versus KCl-stimulated shPI3K-C2α sequence 1. Blot, cells pretreated for 30 min with the indicated concentrations of AG1024 were stimulated with 100 nm insulin for further 30 min in the presence or absence of AG1024, lysed, and analyzed by Western blot. Akt activation was then determined by analyzing the phosphorylation of its residue Ser-473. D, Western blotting analysis of Akt activation in sh scrambled INS1 was performed as described under “Experimental Procedures.” Equal loading was assessed using an anti-ERK2 antibody.
Because the increase in intracellular calcium upon closure of the K+ATP channel is one of the key signals triggering insulin granule exocytosis, we first investigated whether down-regulation of PI3K-C2α affected the uptake of extracellular calcium upon treatment of cells with KCl. Calcium imaging analysis revealed a clear and rapid increase in intracellular calcium upon KCl stimulation in parental, scrambled, and both PI3K-C2α knockdown cells with no difference between any of the cell lines tested (Fig. 4B).
PI3K-C2α Regulates Insulin Secretion upon KCl Stimulation in a Mechanism Independent of Insulin Receptor and Akt Activation
It has been proposed that an insulin feedback loop involving Akt activation is required for the PI3K-C2α-dependent regulation of insulin secretion upon glucose stimulation (14). To determine whether a similar Akt-dependent mechanism is activated in our experimental conditions, we monitored the effect of chemical blockade of the insulin receptor on KCl-induced insulin secretion. Data revealed that treatment with 1 μm insulin receptor inhibitor AG1024 had no significant effect on KCl-induced secretion (Fig. 4C), although it was able to strongly inhibit the insulin-induced Akt phosphorylation in both scrambled and PI3K-C2α knockdown cells (Fig. 4C). These data indicate that activation of the insulin receptor and Akt do not play a key role in the KCl-dependent insulin secretion in our experimental conditions. Consistent with this, no Akt activation, monitored by analyzing its phosphorylation state, was detected in INS1 treated for 30 min with KCl (Fig. 4E), whereas parallel stimulation with insulin growth factor like 1 or insulin was able to strongly activate Akt in these cells (Fig. 4D). Consistent with this, no tyrosine phosphorylation of insulin receptor substrate 2 (IRS2) was detected in INS1 upon treatment with KCl for 30 min (supplemental Fig. 2A), whereas tyrosine phosphorylation of IRS2 was detected in both sh scrambled and cells lacking PI3K-C2α upon insulin stimulation (supplemental Fig. 2B). These data further suggest that Akt is not involved in the KCl-induced insulin secretion in our experimental conditions. A slight Akt activation (Fig. 4D) and IRS2 tyrosine phosphorylation (supplemental Fig. S2B) was detected in cells treated with the secretagogue mixture. Taken together these results indicate that the reported role of PI3K-C2α in modulating insulin secretion through an insulin feedback loop involving Akt (14) is not the only mechanism by which this enzyme is involved in insulin secretion.
Down-regulation of PI3K-C2α Affects Insulin Granule Fusion
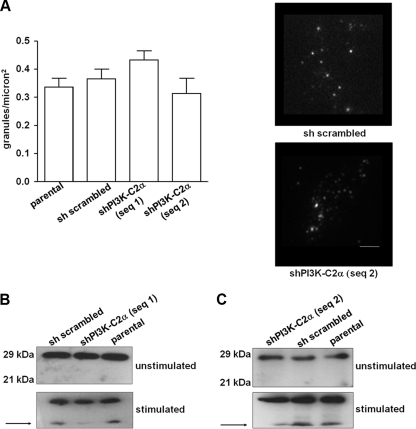
To further investigate the mechanism of PI3K-C2α-dependent insulin secretion, we determined whether stable down-regulation of this enzyme affects the expression level of proteins known to be required for insulin secretion. mRNA was extracted from cells in growing conditions, and gene expression analysis was performed using qPCR. Data revealed that PI3K-C2α does not regulate the mRNA levels of key proteins required for insulin secretion, including the glucose transporter protein GLUT2, the enzyme glucokinase, and several proteins necessary for the exocytosis of insulin granules, including SNAP25, VAMP2, and several synaptotagmins (Fig. 5A). Consistent with data on insulin content, insulin gene expression is also not affected in PI3K-C2α knockdown cells compared with control (sh scrambled) cells (Fig. 5A). No difference in the protein levels of syntaxin 1, syntaxin 4, and VAMP2 was detected between the different cell lines (Fig. 5B). Similarly, confocal microscopy analysis of cells in growing conditions revealed no major difference in the intracellular localization of both syntaxin 1 and VAMP1/2/3 upon down-regulation of PI3K-C2α (supplemental Fig. S3). These data indicate that the reduced insulin secretion detected in cells lacking PI3K-C2α is not due to alteration in the expression levels of key proteins of the secretory pathways. Furthermore, as shown in Fig. 6A, no difference was observed in the number of insulin granules proximal to the plasma membrane in resting conditions, as assessed by TIRF microscopy (29). Taken together these data support the hypothesis that PI3K-C2α is required for the late steps of insulin granule exocytosis, consistent with its role in priming and fusion of neurosecretory granules (18).
FIGURE 5.
Down-regulation of PI3K-C2α does not affect expression levels of key proteins involved in insulin secretion. A, results from qPCR analysis of mRNAs extracted from the indicated cells in growing conditions are shown. Data are the means ± S.E. from six preparations. B, shown is a representative Western blot analysis of the levels of syntaxin 1A, syntaxin 4, and VAMP2 in the indicated cells. Corresponding levels of PI3K-C2α are also shown together with tubulin (as loading control).
FIGURE 6.
PI3K-C2α is required for fusion of the insulin granules to the plasma membrane. A, shown is the number of granules on the cell surface as detected by live cell TIRF microscopy. Data are the means ± S.E. from 3–4 independent experiments (n = 23–30 cells per condition). Representative images are shown (average of 4 × 50-ms exposures). Scale bar = 2 μm. B and C, Western blotting analysis of SNAP25 in the indicated unstimulated cells or cells stimulated with the secretagogue mixture for 3h. Arrows indicate the product of proteolysis induced by cellular stimulation.
To determine whether PI3K-C2α was required for fusion of insulin granules, we analyzed the potential effect of its down-regulation on SNAP25 proteolysis, an event associated with granules fusion (27). In unstimulated cells, down-regulation of PI3K-C2α did not affect the protein levels of SNAP25, which appeared as a band at the expected molecular weight (Fig. 6B), consistent with data on gene expression (Fig. 5A). Similarly, intracellular localization of SNAP25 did not appear to be affected in cells lacking PI3K-C2α compared with control cells (supplemental Fig. S4). Stimulation of parental and control sh scrambled cells with the secretagogue mixture induced SNAP25 proteolysis, as indicated by the appearance of a band at a lower molecular weight (Fig. 6, B and C). Interestingly, a reduced proteolysis of SNAP25 was detected in cell lines lacking PI3K-C2α upon cellular stimulation (Fig. 6, B and C). Taken together these data indicate that PI3K-C2α does not control insulin synthesis and recruitment of insulin granules to the plasma membrane, but it regulates their fusion, possibly by modulating SNAP25 hydrolysis.
PI3K-C2α mRNA May Be Altered in Type 2 Diabetes
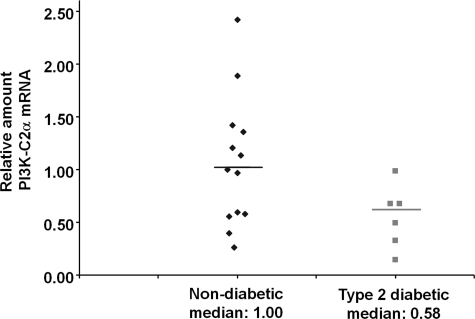
To determine whether alterations in the level of PI3K-C2α occur in type 2 diabetes, we compared the mRNA levels of the class II PI3K isoforms in human islets from non-diabetic and type 2 diabetic individuals. Microarray analysis revealed down-regulation of PI3K-C2α mRNA in human islets from type 2 diabetic compared with non-diabetic individuals, whereas mRNA of the other class II isoforms was not affected (Table 1). Analysis by qPCR performed on samples from 13 non diabetic and 6 type 2 diabetic individuals confirmed a reduction in PI3K-C2α mRNA in the latter samples (Fig. 7), suggesting that alteration of PI3K-C2α mRNA may represent a specific feature of type 2 diabetes.
TABLE 1.
Results from microarray analysis of human islets
RNA was extracted from islets purified from seven non-diabetic and six T2D individuals and treated as described under “Experimental Procedures.” Genes with a p value lower than 0.01 were considered as differentially expressed. FC, fold change.
| Symbol | Description | Gene ID | Reference sequence | FC | p Value |
|---|---|---|---|---|---|
| PIK3-C2A | Phosphoinositide-3-kinase, class 2, α polypeptide | 5286 | NM_002645 | 0.513641 | 0.005046 |
| PIK3-C2B | Phosphoinositide-3-kinase, class 2, β polypeptide | 5287 | NM_002646 | 1.420516 | 0.082541 |
| PIK3-C2G | Phosphoinositide-3-kinase, class 2, γ polypeptide | 5288 | NM_004570 | 1.157347 | 0.199697 |
FIGURE 7.
PI3K-C2α mRNA is altered in islets in type 2 diabetes. Real-time qPCR analysis of PI3K-C2α mRNA levels in human pancreatic islets from non-diabetic (13 samples) compared with type 2 diabetic individuals (6 samples). mRNAs levels were normalized to the reference gene actin. Data are expressed as -fold change relative to the median of the non-diabetic samples. Mann-Whitney's U test one-tailed p < 0.05.
DISCUSSION
PI3Ks play a critical role in several intracellular functions, and it is now well established that deregulation of PI3K-dependent signaling pathways is associated with several diseases, including cancer and diabetes. There is currently a huge interest in understanding the specific functions of distinct PI3K isoforms and determining whether they have redundant or complementary intracellular roles. The importance of these questions is highlighted by the current effort to develop selective PI3K inhibitors to be potentially used as therapeutic drugs in several diseases, including cancer. Among the three classes of PI3Ks, class II isoforms remain the least-well understood, although several lines of evidence indicate that they can have critical roles in many intracellular functions (15). Evidence is also emerging that suggests a role for class II-deregulated pathways in human diseases, including the association of a polymorphism of the gene encoding for PI3K-C2γ with type 2 diabetes, which has been found in a Japanese population (36) and studies indicating an increase in copy number of the gene encoding for PI3K-C2β in human ovarian cancer specimens and glioblastoma (37).
Data from different laboratories including our own have begun to elucidate the roles of some of the class II PI3K isoforms in cell signaling. In particular, data so far indicate that PI3K-C2α may play a role in exocytosis in distinct cellular contexts (see the Introduction). These data together with the observation that PI3K-C2α mRNA is present in human β cells (12, 13) and that the enzyme is detected in human islets (14) strongly suggested that PI3K-C2α might be involved in the complex process of insulin secretion. Indeed, it has been recently reported that PI3K-C2α has a role in glucose-induced insulin secretion in a mechanism involving an insulin-mediated feedback loop that also involves Akt1 activation (14). Questions remained about whether the enzyme can only act downstream of this insulin-dependent loop upon glucose stimulation (14) or whether it could also have a direct role in regulating insulin granule exocytosis by controlling their docking/priming or fusion, as suggested by its role in distinct cellular contexts (17, 18, 20).
To address this question we decided to investigate the effect of PI3K-C2α down-regulation on insulin granule exocytosis induced by either a secretagogue mixture or KCl. In particular, because treatment with KCl is able to induce insulin secretion in the absence of any metabolic component, these experimental conditions could allow us to unveil a distinct role for PI3K-C2α compared with the role already described downstream of glucose stimulation (14).
In this study we observe that down-regulation of PI3K-C2α strongly inhibits insulin granule exocytosis induced by treatment with a secretagogue mixture or KCl. Similar data were obtained using two distinct shRNAs targeting PI3K-C2α and upon stable or transient down-regulation of the enzyme, confirming the specificity of the effect. Furthermore our data clearly indicate that in the context of KCl stimulation, PI3K-C2α regulates insulin secretion through a mechanism that is different from its reported role upon glucose stimulation (14). Indeed we observed that chemical inhibition of the insulin receptor did not affect the KCl-induced insulin secretion, and no Akt activation was detected in KCl-treated cells. Therefore, these experimental conditions highlighted an additional role for PI3K-C2α in insulin secretion, revealing that regulation of glucose-induced insulin secretion downstream of insulin receptor activation is not the only mechanism by which this enzyme can regulate insulin secretion.
Down-regulation of PI3K-C2α does not affect calcium uptake upon KCl stimulation, indicating that the impairment of insulin secretion due to down-regulation of this enzyme occurs in the late steps of insulin secretion. Furthermore, we show that the expression levels of key proteins for insulin secretion (including the SNAREs (soluble N-ethylmaleimide factor attachment proteins) receptor) VAMP2, and syntaxin 1) are not affected in cells lacking PI3K-C2α, further indicating that the inhibition of insulin secretion is not simply due to alteration of the expression pattern of these proteins. These data together with our observation that the number of granules proximal to the plasma membrane is not affected by PI3K-C2α down-regulation support the hypothesis of a specific role for PI3K-C2α in the late steps of exocytosis. Consistent with this hypothesis, we observed that SNAP25 proteolysis, an event that has been associated with granule fusion (27), appears to be reduced in the absence of PI3K-C2α. Taken together our data indicate that, besides the reported role in regulating insulin secretion upon glucose stimulation (14), PI3K-C2α can directly regulate this process by controlling final steps in insulin granule exocytosis.
In this respect it is noteworthy that the protease calpain 10 has been proposed to be involved in Ca2+-induced proteolysis of SNAP25 (27). Notably, this enzyme has also been reported to be required for GLUT4 translocation and glucose uptake in 3T3-L1 adipocytes (38) and in skeletal muscle (39, 40). Because PI3K-C2α is also necessary for these processes (17), the possibility exists that PI3K-C2α may regulate GLUT4 translocation and insulin secretion through a common mechanism, possibly involving calpain 10 activation.
It must be noted that no defect in KCl-induced insulin secretion was previously reported in MIN6 cells lacking PI3K-C2α (14). The different results can be due to the different cellular systems or to the shorter time of KCl stimulation used in the previous study (14) compared with ours.
It is important to remember that the involvement of PI3K-C2α in neurosecretory granule exocytosis (20) and translocation of GLUT4-containing vesicles to the plasma membrane (17) requires synthesis of its lipid product PtdIns3P. Indeed incubation of muscle cells and adipocytes with exogenous PtdIns3P is sufficient to induce GLUT4 translocation (41). Recent studies using reconstituted proteoliposomes have revealed that PtdIns3P can directly support fusion in particular experimental conditions (42) and that this phosphoinositide is part of a minimal set of lipids required for fusion (43). The possibility, therefore, exists that PI3K-C2α may regulate insulin granule fusion directly by maintaining a pool of PtdIns3P necessary for this event. It was previously reported that the basal secreted insulin was responsible for maintaining basal PtdIns3P levels in pancreatic β cells HIT-T15 (44), although whether PI3K-C2α can regulate this basal pool of PtdIns3P is currently unknown. It has been recently suggested that PI3K-C2α stimulation with insulin in MIN6 cells generates the lipid phosphatidylinositol 3,4-bisphosphate and not PtdIns3P (14). The reason for the discrepancy between this finding and our previous data showing that PtdIns3P is the sole in vivo product of PI3K-C2α in muscle cells upon insulin stimulation (17) remains to be addressed. Nevertheless, the possibility still exists that an increase in intracellular Ca2+ stimulation (rather than the secreted insulin) can activate PI3K-C2α and induce PtdIns3P synthesis in β cell, as detected in neurosecretory granules (20). A precise analysis of the phosphoinositide(s) generated through PI3K-C2α activation upon increase in Ca2+ levels after membrane depolarization is still missing.
Our data indicating a role for PI3K-C2α in insulin granule fusion together with the reported role of p110γ in recruitment of granules vesicles to the membrane (11) suggest that class I and class II PI3Ks may play complementary roles in insulin secretion, supporting the hypothesis that these two classes are not redundant and are instead responsible for activation of distinct signaling pathways. It must be noted that down-regulation of PI3K-C2α did not affect the proliferation of INS1 cells in complete medium, and it only slightly increased the subG1 population. This is in contrast with data reporting a critical role for PI3K-C2α in cell proliferation (45) and survival of several cancer cells (46). These data suggest that down-regulation of PI3K-C2α in pancreatic β cells is not likely to be associated with increased basal cell apoptosis, although we cannot rule out the possibility that such a down-regulation can enhance β cell apoptosis in glucotoxic or lipotoxic conditions.
Finally, using microarray and qPCR analysis, we detected a selective down-regulation of PI3K-C2α mRNA in islets of Langerhans from type 2 diabetic compared with non diabetic individuals. None of the other class II PI3K isoforms seems to be down-regulated. These data together with the observation that reduced levels of PI3K-C2α result in impairment of insulin secretion suggest the intriguing possibility that down-regulation of this enzyme may represent a feature of type 2 diabetes. Identification of factors responsible for the selective reduction of PI3K-C2α mRNA levels in type 2 diabetes and its significance will represent an important area for future investigation. Because impaired pancreatic β cell function in type 2 diabetes is the main event responsible for progressive deterioration of glycemic control (47–51) and the onset of type 2 diabetes is associated with decreased β cell mass in both human and rodents models (48, 52–54), these results may have profound implications toward preservation of β cell function.
Supplementary Material
Acknowledgment
We thank Dr. Giampietro Schiavo for kindly providing anti-VAMP2 antibody.
This work was supported by Barts and The London Charity Grant 430/647, Diabetes Research & Wellness Foundation, and Diabetes UK Grant BDA:09/0003971 (all to T. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- GLUT4
- glucose transporter protein 4
- IRS2
- insulin receptor substrate 2
- KRB
- Krebs-Ringer buffer
- PtdIns3P
- phosphatidylinositol 3-phosphate
- SNAP25
- synaptosomal-associated protein of 25 kDa
- TIRF
- total internal reflection fluorescence
- qPCR
- quantitative PCR
- PI3K
- phosphoinositide 3-kinase.
REFERENCES
- 1. Cantley L. C. (2002) Science 296, 1655–1657 [DOI] [PubMed] [Google Scholar]
- 2. Leibiger I. B., Leibiger B., Moede T., Berggren P. O. (1998) Mol. Cell 1, 933–938 [DOI] [PubMed] [Google Scholar]
- 3. Kulkarni R. N., Brüning J. C., Winnay J. N., Postic C., Magnuson M. A., Kahn C. R. (1999) Cell 96, 329–339 [DOI] [PubMed] [Google Scholar]
- 4. Da Silva Xavier G., Qian Q., Cullen P. J., Rutter G. A. (2004) Biochem. J. 377, 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zawalich W. S., Zawalich K. C. (2000) J. Endocrinol. 166, 111–120 [DOI] [PubMed] [Google Scholar]
- 6. Zawalich W. S., Tesz G. J., Zawalich K. C. (2002) J. Endocrinol. 174, 247–258 [DOI] [PubMed] [Google Scholar]
- 7. Hagiwara S., Sakurai T., Tashiro F., Hashimoto Y., Matsuda Y., Nonomura Y., Miyazaki J. (1995) Biochem. Biophys. Res. Commun. 214, 51–59 [DOI] [PubMed] [Google Scholar]
- 8. Turner M. D., Arvan P. (2000) J. Biol. Chem. 275, 14025–14030 [DOI] [PubMed] [Google Scholar]
- 9. MacDonald P. E., Joseph J. W., Yau D., Diao J., Asghar Z., Dai F., Oudit G. Y., Patel M. M., Backx P. H., Wheeler M. B. (2004) Endocrinology 145, 4078–4083 [DOI] [PubMed] [Google Scholar]
- 10. Li L. X., MacDonald P. E., Ahn D. S., Oudit G. Y., Backx P. H., Brubaker P. L. (2006) Endocrinology 147, 3318–3325 [DOI] [PubMed] [Google Scholar]
- 11. Pigeau G. M., Kolic J., Ball B. J., Hoppa M. B., Wang Y. W., Rückle T., Woo M., Manning Fox J. E., MacDonald P. E. (2009) Diabetes 58, 2084–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muller D., Huang G. C., Amiel S., Jones P. M., Persaud S. J. (2006) Diabetes 55, 2835–2842 [DOI] [PubMed] [Google Scholar]
- 13. Muller D., Huang G. C., Amiel S., Jones P. M., Persaud S. J. (2007) Diabetologia 50, 1239–1242 [DOI] [PubMed] [Google Scholar]
- 14. Leibiger B., Moede T., Uhles S., Barker C. J., Creveaux M., Domin J., Berggren P. O., Leibiger I. B. (2010) FASEB J. 24, 1824–1837 [DOI] [PubMed] [Google Scholar]
- 15. Falasca M., Maffucci T. (2007) Biochem. Soc Trans 35, 211–214 [DOI] [PubMed] [Google Scholar]
- 16. Maffucci T., Cooke F. T., Foster F. M., Traer C. J., Fry M. J., Falasca M. (2005) J. Cell Biol. 169, 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falasca M., Hughes W. E., Dominguez V., Sala G., Fostira F., Fang M. Q., Cazzolli R., Shepherd P. R., James D. E., Maffucci T. (2007) J. Biol. Chem. 282, 28226–28236 [DOI] [PubMed] [Google Scholar]
- 18. Meunier F. A., Osborne S. L., Hammond G. R., Cooke F. T., Parker P. J., Domin J., Schiavo G. (2005) Mol. Biol. Cell 16, 4841–4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falasca M., Maffucci T. (2009) Biochim. Biophys. Acta 1793, 1795–1803 [DOI] [PubMed] [Google Scholar]
- 20. Wen P. J., Osborne S. L., Morrow I. C., Parton R. G., Domin J., Meunier F. A. (2008) Mol. Biol. Cell 19, 5593–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rutter G. A. (2001) Mol. Aspects Med. 22, 247–284 [DOI] [PubMed] [Google Scholar]
- 22. Rutter G. A. (2004) Diabetologia 47, 1861–1872 [DOI] [PubMed] [Google Scholar]
- 23. Henquin J. C. (2009) Diabetologia 52, 739–751 [DOI] [PubMed] [Google Scholar]
- 24. Rorsman P., Renström E. (2003) Diabetologia 46, 1029–1045 [DOI] [PubMed] [Google Scholar]
- 25. Iezzi M., Kouri G., Fukuda M., Wollheim C. B. (2004) J. Cell Sci. 117, 3119–3127 [DOI] [PubMed] [Google Scholar]
- 26. Gauthier B. R., Duhamel D. L., Iezzi M., Theander S., Saltel F., Fukuda M., Wehrle-Haller B., Wollheim C. B. (2008) FASEB J. 22, 194–206 [DOI] [PubMed] [Google Scholar]
- 27. Marshall C., Hitman G. A., Partridge C. J., Clark A., Ma H., Shearer T. R., Turner M. D. (2005) Mol. Endocrinol. 19, 213–224 [DOI] [PubMed] [Google Scholar]
- 28. Axelrod D. (1981) J. Cell Biol. 89, 141–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsuboi T., Zhao C., Terakawa S., Rutter G. A. (2000) Curr. Biol. 10, 1307–1310 [DOI] [PubMed] [Google Scholar]
- 30. Tsuboi T., Rutter G. A. (2003) Curr. Biol. 13, 563–567 [DOI] [PubMed] [Google Scholar]
- 31. da Silva Xavier G., Loder M. K., McDonald A., Tarasov A. I., Carzaniga R., Kronenberger K., Barg S., Rutter G. A. (2009) Diabetes 58, 894–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicolson T. J., Bellomo E. A., Wijesekara N., Loder M. K., Baldwin J. M., Gyulkhandanyan A. V., Koshkin V., Tarasov A. I., Carzaniga R., Kronenberger K., Taneja T. K., da Silva Xavier G., Libert S., Froguel P., Scharfmann R., Stetsyuk V., Ravassard P., Parker H., Gribble F. M., Reimann F., Sladek R., Hughes S. J., Johnson P. R., Masseboeuf M., Burcelin R., Baldwin S. A., Liu M., Lara-Lemus R., Arvan P., Schuit F. C., Wheeler M. B., Chimienti F., Rutter G. A. (2009) Diabetes 58, 2070–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marchetti P., Bugliani M., Lupi R., Marselli L., Masini M., Boggi U., Filipponi F., Weir G. C., Eizirik D. L., Cnop M. (2007) Diabetologia 50, 2486–2494 [DOI] [PubMed] [Google Scholar]
- 34. Bugliani M., Masini M., Liechti R., Marselli L., Xenarios I., Boggi U., Filipponi F., Masiello P., Marchetti P. (2009) Islets 1, 106–110 [DOI] [PubMed] [Google Scholar]
- 35. Domin J., Pages F., Volinia S., Rittenhouse S. E., Zvelebil M. J., Stein R. C., Waterfield M. D. (1997) Biochem. J. 326, 139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daimon M., Sato H., Oizumi T., Toriyama S., Saito T., Karasawa S., Jimbu Y., Wada K., Kameda W., Susa S., Yamaguchi H., Emi M., Muramatsu M., Kubota I., Kawata S., Kato T. (2008) Biochem. Biophys. Res. Commun. 365, 466–471 [DOI] [PubMed] [Google Scholar]
- 37. Kok K., Geering B., Vanhaesebroeck B. (2009) Trends Biochem. Sci. 34, 115–127 [DOI] [PubMed] [Google Scholar]
- 38. Paul D. S., Harmon A. W., Winston C. P., Patel Y. M. (2003) Biochem. J. 376, 625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Logie L. J., Brown A. E., Yeaman S. J., Walker M. (2005) Mol. Genet. Metab. 85, 54–60 [DOI] [PubMed] [Google Scholar]
- 40. Brown A. E., Yeaman S. J., Walker M. (2007) Mol Genet. Metab. 91, 318–324 [DOI] [PubMed] [Google Scholar]
- 41. Maffucci T., Brancaccio A., Piccolo E., Stein R. C., Falasca M. (2003) EMBO J. 22, 4178–4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mima J., Wickner W. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 16191–16196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mima J., Wickner W. (2009) J. Biol. Chem. 284, 27114–27122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu J., Berggren P. O., Barker C. J. (2007) Mol. Endocrinol. 21, 2775–2784 [DOI] [PubMed] [Google Scholar]
- 45. Ng S. K., Neo S. Y., Yap Y. W., Karuturi R. K., Loh E. S., Liau K. H., Ren E. C. (2009) Biochem. Biophys. Res. Commun. 387, 310–315 [DOI] [PubMed] [Google Scholar]
- 46. Elis W., Triantafellow E., Wolters N. M., Sian K. R., Caponigro G., Borawski J., Gaither L. A., Murphy L. O., Finan P. M., Mackeigan J. P. (2008) Mol. Cancer Res. 6, 614–623 [DOI] [PubMed] [Google Scholar]
- 47. UK Prospective Diabetes Study (UKPDS) Group (1995) Diabetes 44, 1249–1258 [PubMed] [Google Scholar]
- 48. Weyer C., Tataranni P. A., Bogardus C., Pratley R. E. (2001) Diabetes Care 24, 89–94 [DOI] [PubMed] [Google Scholar]
- 49. Del Prato S., Bianchi C., Marchetti P. (2007) Diabetes Metab. Res. Rev. 23, 518–527 [DOI] [PubMed] [Google Scholar]
- 50. Marchetti P., Dotta F., Lauro D., Purrello F. (2008) Regul. Pept. 146, 4–11 [DOI] [PubMed] [Google Scholar]
- 51. Rutter G. A., Parton L. E. (2008) Front. Horm. Res. 36, 118–134 [DOI] [PubMed] [Google Scholar]
- 52. Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Diabetes 52, 102–110 [DOI] [PubMed] [Google Scholar]
- 53. Del Guerra S., Lupi R., Marselli L., Masini M., Bugliani M., Sbrana S., Torri S., Pollera M., Boggi U., Mosca F., Del Prato S., Marchetti P. (2005) Diabetes 54, 727–735 [DOI] [PubMed] [Google Scholar]
- 54. Rahier J., Guiot Y., Goebbels R. M., Sempoux C., Henquin J. C. (2008) Diabetes Obes. Metab. 10, 32–42 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.