Abstract
The origin of Aboriginal Australians has been a central question of palaeoanthropology since its inception during the 19th Century. Moreover, the idea that Australians could trace their ancestry to a non-modern Pleistocene population such as Homo erectus in Southeast Asia have existed for more than 100 years, being explicitly linked to cranial robusticity. It is argued here that in order to resolve this issue a new program of research should be embraced, one aiming to test the full range of alternative explanations for robust morphology. Recent developments in the morphological sciences, especially relating to the ontogeny of the cranium indicate that character atomisation, an approach underpinning phylogenetic reconstruction, is fraught with difficulties. This leads to the conclusion that phylogenetic-based explanations for robusticity should be reconsidered and a more parsimonious approach to explaining Aboriginal Australian origins taken. One that takes proper account of the complex processes involved in the growth of the human cranium rather than just assuming natural selection to explain every subtle variation seen in past populations. In doing so, the null hypothesis that robusticity might result from phenotypic plasticity alone cannot be rejected, a position at odds with both reticulate and deep-time continuity models of Australian origins.
1. Introduction
The origin of modern humans remains a core topic of palaeoanthropology. Although four major models are presently being debated, only the out-of-Africa suite has received strong support from interpretations of both the fossil record and DNA [1–6]. Yet, the evolutionary origin of Aboriginal Australians remains controversial owing to the presence of considerable variability in cranial morphology during the Pleistocene and interpretations of its possible phylogenetic importance [1, 2, 7–18]. Moreover, there is sharp disagreement about the possible alternative causes of this variation and its significance to a global understanding of the evolutionary history of modern Homo sapiens [1, 2, 11, 12, 17, 18].
Long before a human fossil record was known for Australia, various speculative evolutionary sequences were devised linking nonmodern hominins to Aboriginal Australians. In particular, it was proposed that Pithecanthropus (Homo erectus) or the late-surviving Ngandong population of this species (sometimes referred to H. soloensis [14]) played a role in their origins. This idea has been an enduring theme of palaeoanthropology for more than 100 years: from Klaatsch [19] through to Westaway and Groves [18]. A recent major review of the question of modern human origins [1] identified three major issues for Australian palaeoanthropology to be resolved: (1) the relationship of the first Australians to later inhabitants of the continent, (2) whether late Pleistocene morphological diversity may have been accentuated by the severity of the last glacial maximum, leading to isolation and the forcing of morphological change in some Australian populations, and (3) if archaic populations such as those known from Ngandong did survive into the late Pleistocene, an analogous situation to that in Europe might have existed, raising the possibility of gene flow with dispersing H. sapiens. All three points are clearly interrelated. Regarding the first, the very earliest fossil remains from Australia (e.g., Willandra Lakes Human 3) do seem to fit metrically and morphologically within the range of living Aborigines [8]. Stringer's [1] points 2 and 3 relate to the possible cause(s) of cranial robusticity in some Pleistocene/early Holocene Australians. That is, whether such features arose as a result of natural selection acting on populations within Australia or were brought here by people who evolved from, or hybridised/admixed with, a nonmodern population in Southeast Asia (i.e., the Solo/Ngandong hominins).
Australian palaeoanthropological theory and method continues to be dominated by adaptationist accounts [20] of robusticity and population history. The assumption that the cranium is optimised part by part and that atomising its form into traits assumed to be heritable units, functionally discrete, to have been shaped by natural selection and, therefore, positively associated with reproductive success, remains the core proposition of the field. In the present contribution, it is argued that the failure to fully consider alternative (nonadaptationist) approaches is a major reason why the interrelated issues of firstly, the cause(s) of cranial robusticity and, secondly, its relevance to reconstructing the origins of Aboriginal Australians remain unresolved. This paper commences with a review of the history of ideas regarding cranial robusticity and the origins of Aboriginal Australians. Then, an alternative to atomisation, herein called the ontogenetic approach, is described and some key concepts underpinning it are introduced. Finally, this approach is applied to some characters used to support the Ngandong ancestry model for Aboriginal Australians.
2. History of an Idea
Ever since Blumenbach's De Generis Humani Varietate Nativa of 1795, European scientists have been attempting to understand the affinities and hence origins of Aboriginal Australians. Blumenbach had at hand the skulls of several Australians provided to him by Joseph Banks [21]. However, only with the wider exploration of the continent by Europeans and the beginnings of a global trade in human cranial remains during the 19th Century was a systematic effort made to understand their skeletal morphology [22]. During this period, the deep antiquity of the earth was beginning to be established, including an ancient origin for humankind as presented in Lyell's Antiquity of Man [23]. In Lyell's volume, Huxley's (1863) first comparisons of Aboriginal skulls to Neanderthal remains were noted, indicating various morphological resemblances (not exclusive though, or implying ancestry). As the first anthropologist to study human origins from comparative anatomical and fossil sources, and placing his ideas within the Darwinian evolutionary framework, Huxley can reasonably be considered the founder of the discipline of palaeoanthropology. Moreover, as Aboriginal Australians were central to his ideas, as well as to the early development of this scientific field, Huxley ensured their place at the centre of debate surrounding human origins, a position they have held for close to 150 years.
During the early 20th century, many researchers continued to focus on documenting similarities in cranial form between Australians and the Neanderthals [24–26], inspired by Huxley's earlier and highly influential work (see Figure 1). However, following the discovery of fossil human-like remains in Indonesia by Dubois [27], the view eventually emerged that Australians may actually have descended from a local population within Southeast Asia, either Pithecanthropus [19] or Homo soloensis [28, 29]. Klaatsch [19] found it “impossible to believe that the Australian natives are descended from European palaeolithic man” (page 162) as proposed by Huxley (see other criticisms in [30, 31]). However, the idea of a regional evolutionary sequence between Aboriginal Australians and Pithecanthropus in Southeast Asia seems not to have been in the minds of these workers. Instead, they saw Aboriginal Australians as representatives of the most “primitive type” of living H. sapiens and had in mind a global evolutionary sequence in which “Solo Man” (Ngandong) and “Rhodesian Man” (Kabwe) were examples of “proto-Australians,” belonging together to living humans in H. sapiens [29]. Also, Dubois [28, 29] thought that the Wadjak remains he recovered from Indonesia were “Australoid” although they now seem to be terminal Pleistocene in age and are probably not related to Aboriginal Australians [32].
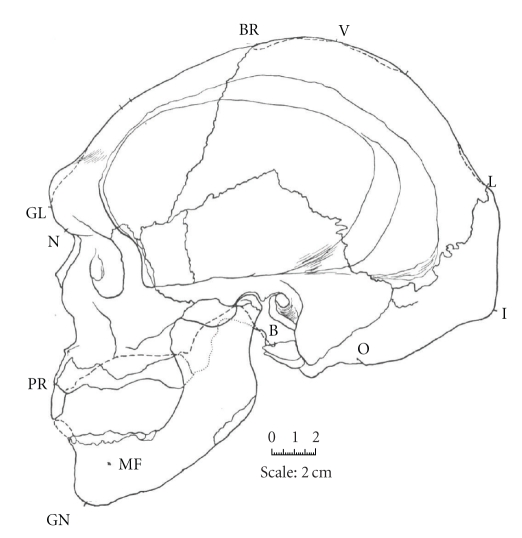
Figure 1.
Lateral line drawing of an Aboriginal skull described in the early 20th century as “Neanderthaloid” [24].
With the ideas of Weidenreich [33, 34] and Coon [35], the “Pithecanthropoid-Australoid” lineage hypothesis become a major feature of palaeoanthropological theorising. Both these workers believed that modern humans around the world had evolved from regional populations of Pithecanthropus (today H. erectus). Thus, the regional lineage from this taxon through to Aboriginal Australians was part of a global process, which Howells [36] dubbed the “Candelabra” hypothesis. Parenthetically, it should be noted that this idea was not universally supported; for example, Hrdlička [37] preferred a Neanderthal stage in human evolution and considered Aborigines to belong to an “old race” of “whites,” borrowing heavily from Huxley, while Birdsell's [38] views are strikingly similar to the contemporary out-of-Africa theory.
The increase in the number of fossilised human remains recovered during controlled excavations from the 1960s onwards [39–43], and demonstration of a Pleistocene occupation of the Australian continent [44, 45] encouraged renewed interest in the Pithecanthropoid-Australoid lineage hypothesis [39–43]. Again, it must be stressed that not all researchers accepted this hypothesis, Macintosh in particular reversing his earlier endorsement [46].
During the 1980s and 1990s, this idea was reformulated, initially as the “Regional Continuity” hypothesis [47] and later as the global “Multiregional” model of modern human origins [48, 49]. At its core was the notion of a deep-time Southeast Asian-Australian clade supported by evidence of morphological continuity in the skull and dentition between lower and upper Pleistocene nonmodern hominins in Indonesia through to recent Aboriginal Australians. As Thorne and Wolpoff [47] stated, “in no other region can a specimen (i.e., Sangiran 17) be found that combines so many features that seem unique or at least of high frequency in Pleistocene Australians” (page 345; words in parenthesis added). It is important to note that many proponents of the multiregional model have since the early 1990s regarded H. erectus to be a junior synonym of H. sapiens [50]; thus, the emergence of modern humans is seen by them as a process occurring within a single, long lasting, and widely distributed evolutionary lineage, or species.
Moreover, Thorne [51–53] proposed that two populations had colonised Australia at different times during the Pleistocene in his “dihybrid” model, the merging of the two giving rise to modern Aborigines. The first population was the “robust” group descended from H. erectus and the second a later arriving “gracile” population originating in Pleistocene China and, like modern East Asians, evolving from East Asian H. erectus. Although the order of arrivals had to be revised (reversed) once it was established that gracile crania like WLH3 were actually geologically much older than any robust remains recovered from various localities [15, 53].
Three recent variants of the regional continuity model have been published. The first emerged from a test of multiregionalism in Southeast Asia-Australia using the WLH50 calvaria (see Figure 2) [54, 55]. It was found that six of the seven Ngandong calvaria examined were phenetically closer to WLH50 than to any other specimens considered, including early modern humans from Skhul and Qafzeh. In this work, it was concluded that the results implied a “dual ancestry” for Aboriginal Australians because “there is no evidence suggesting WLH-50 can be grouped with either Late Pleistocene Africans or Levantines to the exclusion of the Ngandong sample” [55, page 296]. That is, it was argued that Australians are descended from both an ancient regional population (e.g., Solo/Ngandong) and recent modern humans from Africa through a process of reticulation (admixture/hybridisation). The present author has made similar conclusions employing different fossils and an alternative, multivariate, methodology [2]. However, his findings were argued to be consistent with the “assimilation” model of modern human origins [56, 57], a hypothesis receiving stronger support from genetic studies [2]. Weidenreich in a personal communication to Birdsell [58] changed his mind late in life and also thought Australians had a dual ancestry. Oppenheimer [59] has also suggested that interbreeding might account for Australian robusticity. The present author has, however, changed his views and no longer considers interbreeding to provide a parsimonious explanation for Aboriginal Australian morphology or the origins of these people [17].
Figure 2.
Willandra Lakes Human 50 calvaria.
The final recent variant was proposed by Webb [14] and borrows heavily from Birdsell's “trihybrid” hypothesis [60, 61], as have Thorne's later ideas [62]. Webb [14] speculated that the first population to colonise Australia was the species H. soloensis, an upper Pleistocene descendent of Javan H. erectus. He argued that it migrated to the continent as early as 130–150 ka and ultimately adapted to local conditions, founding the robust Australian Pleistocene/early Holocene population. Further, he contended that modern humans entered Australia sometime between 50 ka and 75 ka, tracing their origins back to Africa. These people are argued to have been “Negrito or Negrito-like,” being of small stature, a feature developed “external to Australia” [14, page 239]. The two species (H. soloensis and H. sapiens) are argued to have gradually formed a single population through “genetic mixing,” but the process was dominated by the modern group, “which had the larger population and constantly, albeit slowly, receives fresh genes through a series of migrations arriving throughout the glacial maximum and after, into the early Holocene” [14, page 269].
3. Some Objections to Continuity
Many objections to Southeast Asian-Australian regional continuity and the related dihybrid origins model have been offered over the last 30 years or so. Some opponents have argued that perceived similarities between robust individuals and H. erectus may simply represent the retention of plesiomorphies of later Homo by Aboriginal Australians [11, 63]. Indeed, Larnach [64] found that Aborigines are closest in cranial morphology among living people to H. erectus but stressed “their resemblance is never close” (page 159). He also noted that the Solo/Ngandong population exhibits a number of autapomorphies not found among Aboriginal Australians (see also [65, 66]), and “if Australians are descended from Solo Man, then sometime during that descent these unique traits were lost and their sites reverted to a similar state to that obtaining in Homo erectus” [64, page 170]. Thus, if regional continuity is correct, Pithecanthropus (H. erectus) must have given rise to the robust Pleistocene Australian group in a separate event, the Ngandong population (H. soloensis) being a late surviving and autapomorphic descendent, and unrelated to Australians. Durband [67] has pointed out that the available data for Javan H. erectus especially from the facial skeleton (i.e., n = 1 or Sangiran 17) is too inadequate to make reliable phylogenetic inferences.
Moreover, the dihybrid model and by implication regional continuity has been rejected by most Australian workers on the basis of its failure to take adequate account of spatiotemporal variability in cranial and dental morphology, including size, within a single continental population [8–10, 12, 18, 68–75], or at the least its failure to reject this as a null hypothesis. Moreover, some individual features underpinning regional continuity such as vault thickness have been proposed as selected for in response to violence and cranial trauma [8] (see below for a discussion of epigenetic explanations for some robust traits). Finally, the conceptual underpinnings of the dihybrid/migrationist model have been deemed to be founded in a “palaeontological” (i.e., typological) rather than population-based approach [10], as would be demanded by the synthetic evolutionary paradigm.
4. An Alternative Program
As noted above, most studies of Australian robusticity have assumed that the morphological characters under investigation are genetic (or heritable). This concept is used in its broader sense to mean that the phenotype of an individual is a good predictor of the genotype [76]. However, it has become a truism of developmental biology that “the old and compelling idea that there exist specific genes for virtually every structural detail throughout the craniofacial complex is simply not true” [77, page 230]. The more that is learned about the genes and developmental processes forming the cranium, the less tenable the atomisation of complex organs like the cranium has become [78]. Some concepts described in this section indicate powerfully why this is the case and underpin the need for an alternative paradigm in Australian palaeoanthropology.
Estimates of the heritability for many commonly employed cranial measurements and traits are available (e.g., [79–82]). Although there are some uncertainties surrounding the application of sample-specific heritability estimates to other populations, they nonetheless provide insight into the potential reliability of particular traits/variables as well as a broader understanding of the heritability and integrated nature of cranial ontogeny. Heritabilities vary widely for standard craniometric variables: h2 = 0.000 ± 0.000 to 0.867 ± 0.156 [81]. Moreover, many variables have been found to exhibit heritability estimates that do not differ from zero (i.e., h2 = 0) [79, 81]. In a recent investigation, only two commonly employed measurements have been found to exhibit high heritability values: palate breadth (MAB) and nasal height (NLH) [81]. Moreover, major length (GOL), breadth (XCB), and height (BBH) dimensions exhibited low to moderate, but statistically significant heritability estimates [81]. However, many important regional measurements such as frontal chord (FRC), bifrontal breadth (FMB), biasterionic breadth (ASB), bizygomatic breadth (ZYB), and nasal breadth (NLB) are characterised by low and nonsignificant heritability estimates [81]. Additionally, these estimates show a spatial pattern whereby lower estimates tend to be associated with the face. In many cases, they are associated with measurements for areas involving attachment sites for the muscles of mastication [81] suggesting an important role for epigenesis in their ontogeny.
Endocranial dimensions show moderate to high heritability estimates including important measures of human basicranial size and angulation [80]. These results highlight the reasonably high heritability and considerable ontogenetic integration (correlation among dimensions) of human endocranial form, but contrast with low-moderate heritability for measurements on the ectocranial surface [81].
In a recent study of the heritability of cranial dimensions employing a 3D approach, Martínez-Abadías et al. [82] found a broadly similar pattern to previous (2D) studies [79–81]. Overall, dimensions showed low-moderate heritability with about 72% being significant (i.e., h2 > 0). Interestingly, they found the face to be the region with the highest number of significantly heritable traits and highest mean heritability, followed by the cranial base and the neurocranium [82]. Within regions they found the orbit, nasal part, neurocranial vault, and basicranium to be characterised by low to moderate heritability, while the masticatory apparatus exhibited low heritability [82], a finding consistent with Carson's [81] results. Between cranial regions, they also found low-moderate heritability confirming the concept of ontogenetic integration of the cranium. The findings of Martínez-Abadías et al. [82] show that the cranial base, neurocranium, and face are characterised by similar levels of heritability and also strongly point to an important role for epigenesis in ontogeny (see also [80, 81, 83–86]).
Epigenesis is defined as the developmental interactions among cells, tissues, and their environments [84]. It can translate localised developmental alterations into integrated and widespread morphological changes and provide a fundamental mechanism for introducing flexibility into developmental programs including phenotypic plasticity [83–86]. Lieberman et al. [87] have discussed three types of epigenetic interactions during ontogeny: (1) primary interactions, occurring at the cellular level, (2) secondary interactions, involving those between adjacent tissues during growth, and (3) tertiary interactions, in which interactions occur throughout ontogeny between cells within a unit (e.g., via hormones) and the rest of the organism as well as the environment.
One important and widely discussed concept underpinning epigenesis in the ontogeny of the cranium is the functional matrix hypothesis (FMH) of Moss [88]. He described the FMH in the following way: “The developmental origin of all cranial skeletal elements (e.g., skeletal units) and all their subsequent changes in size and shape (e.g., form) and location, as well as their maintenance in being, are always, without exception, secondary, compensatory, and mechanically obligatory responses to the temporally and operationally prior demands of their related cephalic nonskeletal cells, tissues, organs, and operational volumes (e.g., the functional matrices)” [88, page 9]. The FMH explains many aspects of cranial development and is widely regarded to be an important concept surrounding secondary epigenetic interactions during cranial ontogeny (e.g., [77, 89]).
An important example of tertiary epigenetic interactions is the widely discussed “Wolff's” law of bone transformation [90]. This “law” is actually a poorly defined and frequently criticised term usually applied as a “catch-all” concept to refer to the adaptation of bone to mechanical stimuli [77, 78, 91, 92]. Put simply, Wolff's law is, “an extension of the old and trusted idea that form is interrelated with and inseparable from function… that bone grows and develops in such a manner that the composite of physiologic forces exerted on it are accommodated by the bone's developmental processes” [77, page 233]. Historically, one problem with this concept's use has been that it was frequently invoked to argue for uniform responses such as bone deposition in the face of biomechanical stress particular from muscle, when very often the opposite response, resorption, had occurred [77, 91]. Thus, while in its general form this law remains valid and useful, it is now widely acknowledged that just how bone responds to mechanical stress is complex and varies according to its location and whether stress is direct or is mediated by other tissue. The reader is refereed to some recent and more detailed reviews of this concept [77, 78, 92].
The external environment through tertiary epigenetic interactions has long been argued to be an important determinant of cranial form. For example, the shapes of the neurocranium and nose have long been linked to climatic adaptation [93–100] and facial form to diet or masticatory practices (see [101–104]: see below for a more detailed discussion of this idea). A 3D study of cranial morphology compared to neutral genetic population distances [105] found that the human cranium does preserve a “signal” of population history. However, historical signatures are not equal across cranial regions and seem to be largely concentrated in the temporal bone and neurocranium [105].
The final concepts to be briefly considered here are those of modularity and integration. Modularity is a general property of biological systems, from molecular to ecosystemic levels of interaction [86]. In ontogeny, it represents the observation that morphological features do not vary independently but are integrated with each other, reflecting coordination in development, function, and evolution. They might be thought of as forming modules or “complexes that are highly integrated internally but are relatively independent of each other” [86, page 628]. The morphological characters within modules are characterised by three major properties [106]: (1) they collectively serve a common functional role, (2) they are tightly integrated by pleiotropic effects of genetic variation, and (3) they are relatively independent of other modules. This independence among structures (modules) allows unrelated components to vary and evolve separately, but the integration within the units maintains functionally necessary relationships among traits [85–87, 106–113]. Morphological integration assumes that developmentally/functionally related traits are coinherited and will produce coordinated responses in evolution [85–87, 106–113].
The cranium is divided along ontogenetic lines into three regions: the cranial base, neurocranium, and face. As the cranial base is in an evolutionary sense the oldest structure, it is phylogenetically highly conserved [112] and believed to be subject to stronger genetic influence in ontogeny than the neurocranium and face [77, 113–116]. Moreover, it widely is assumed that the face is the most sensitive region to epigenetic factors as it stops growing later in ontogeny than either the cranial base or neurocranium [77, 114, 115]. Thus, it is subject to greater influence from mechanical loading during mastication and from various environmental factors during ontogeny [116–119]. There is, however, considerable evidence that the face and neurocranium are characterised by both integration between regions and considerable modularity, or region-specific integration [77, 80–83, 85, 87, 115, 120–123]. Moreover, this pattern seems to characterise humans, chimpanzees, and gorillas [106, 120, 124, 125] and is known to be phylogenetically conserved in mammals [107, 126]. Cranial shape patterns appear early in ontogeny and remain from early childhood until adulthood [127], including in depository and resorptive cranial growth fields [115, 128]. Although this is somewhat simplistic as endocranial shape continues to change in humans through adolescence, well after brain growth it has ceased [129].
A range of studies aiming to test and develop Enlow's ideas about facial and cranial growth [77, 115, 130] have been published over the past two decades and employed a range of techniques including 3D morphometrics [119, 122, 123, 130–144]. Many studies have focussed on interactions between the cranial base, neurocranium, and facial form, and some major findings include that larger relative brain size is associated with a larger basicranial angle, while large faces may produce the opposite situation, although the influence of facial size seems to be weaker than for brain size [140]. Lieberman et al. [122, 123] also found that cranial proportions (neurocranial shape, or degree of brachycephaly versus dolichocephaly) depend on interactions between cranial base width and brain volume. This has been confirmed by 3D studies showing that changes in cranial base width play an important role in cranial shape variation, including facial width [127]. Moreover, studies of the heritability of cranial regions confirm strong covariation between the breadth measures of major developmental regions of the skull [82]. Additionally, independent of age and size, an important proportion of cranial shape variability seems to be traceable to differences in the position and orientation of the face and masticatory system relative to the braincase [127].
Experimental research using mice suggests that integration in the mammalian skull is highly structured following a hierarchical scheme dominated by covariation between the widths of the neurocranium and the basicranium and, to a lesser extent, also the face [85]. Thus, the cranial base can be thought of as the “skull's central integrator” [122, 123]. It strongly influences overall cranial shape, constraining facial breadth, height, and length, and neurocranial breadth and length, helping prevent the “different regions from evolving independently and would preserve the functional and architectural requirements of the skull” [82, page 29].
5. Nonadaptationist Explanations and Australian Robusticity
The above review of some ontogenetic concepts and recent research findings provide the context for a reconsideration of the possible causes of cranial robusticity among Australians. There have been very few synthetic attempts to explain it using nonphylogenetic or nonadaptationist approaches. One important, but overlooked, example is that of Howells [145] who proposed that robusticity may have been “a phenotypic plastic response to some regional and transient environmental stimulus. That is, the phenomenon would be, not adaptation and selection, resulting in genetic change in the post-Mungo population, but a reversible phenotypic shift, on an unchanged genetic basis, toward larger size and related allometric effects on the face and mandible particularly, producing in some relatively smaller-brained individuals the special flattened and narrow frontal which is so striking in Cohuna and some of the Kow Swamp skulls” (pages 646-647). This explanation is broadly consistent with the ontogenetic approach outlined above, being in large part a putative example of tertiary epigenetic interactions.
The cultural practice of artificial deformation, another example of tertiary interactions, has been argued to have produced or exaggerated the acute angle of the frontal squama, angulation of the parietals, and aspects of occipital squama morphology in a small number of robust Australian crania such as Kow Swamp 5, Cohuna, some Coobool Creek crania and Nacurrie 1 [9, 146–151]. Other epigenetic factors have been proposed to explain vault thickness, including, increased levels of growth hormone [8], nutritional phosphorous deficiency [8], and inherited anaemia [13, 14, 152, 153]. The latter (pathology) hypothesis has been examined to some extent by Stuart-Macadam [154], Curnoe and Thorne [16], Westaway [155], and Curnoe [16] but requires further scrutiny (see below).
Lahr and Wright [156] using multivariate statistics considered spatial (or architectural) aspects of cranial form and proposed that the “superstructures” (see below) characteristic of cranial robusticity may be integrated and covary allometrically. For Australian Aborigines, they concluded that like other human populations the expression of robust characters was likely a response to some functional complex, possibly mastication. They also suggested that the “distinctive anatomical combination present in Australian crania of a very narrow vault and pronounced robusticity, does not represent a plesiomorphic state” inherited from either early modern humans or the Solo/Ngandong population [156, page 184]. Moreover, they proposed that allometry in combination with this regional morphology might explain extreme robusticity seen in the Pleistocene of Australia. However, in attempting to explain the origins of this cranial morphology, they turned again to an explanation involving natural selection during the settlement of Australia.
In recent research by the present author [17], the ontogenetic framework was adopted in an attempt to explore nonphylogenetic explanations for cranial robusticity among Pleistocene/early Holocene Australians. It was concluded that robusticity among these individuals may have been the result of the complex developmental and functional interplay between (1) a large neurocranium, (2) narrow cranial base, (3) large viscerocranium with considerable high midfacial projection, and (4) large dentition, especially the posterior teeth, with their resultant large jaws (mandible and maxilla) and high volume of masticatory muscles. While it was suggested that these features were probably heritable to some extent, other factors such as body size, advanced physiological age, environmental effects from the physical demands of a hunter-gatherer lifestyle in an arid zone, dietary factors including food abrasiveness and limited preparation of food, and the use of teeth as tools may all have been factors affecting (exaggerating) the expression of robusticity in the Australian context.
5.1. Cranial Thickening as Pathology in WLH50
As noted above, pathological processes are suggested to have greatly enlarged the already thick vault bones of WLH50 (Figure 2). Webb [13, 14, 152, 153] has listed three indicators of pathology in this individual: (1) uniform vault thickness, (2) identification of the “hair-on-end” sign on lateral radiographs of the calvaria, and (3) a thin vault cortex.
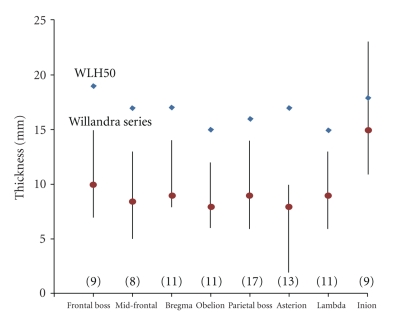
Figure 3 compares vault thickness at eight locations in WLH50 with the median and range for the remaining Willandra Lakes series, as measured by Webb [13]. The large absolute thickness of the WLH50 vault is striking although thickness at one landmark (inion) does lie well within the range of the remaining Willandra Lakes sample. It is also very clear that vault thickness in WLH50 is far from uniform, as it varies between locations in a similar magnitude to the medians for the Willandra Lakes series (Figure 3). The magnitude of change (difference) between each of eight locations was calculated for WLH50 and the rest of the Willandra Lakes series, and the median value is actually larger in this individual (2 mm versus 1 mm). Moreover, a nonparametric Mann-Whitney U-test showed that the sample medians are statistical indistinguishable (n = 7/54, U = 187.5, P = .98; Monte Carlo P = .98). Thus, WLH50 does not possess a vault of uniform thickness when compared to the rest of the Willandra Lakes Human sample.
Figure 3.
Thickness in Willandra Lakes Human (WLH) 50 compared with the sample median and range for the remaining Willandra Lakes series (NB: numbers in parenthesis are sample size).
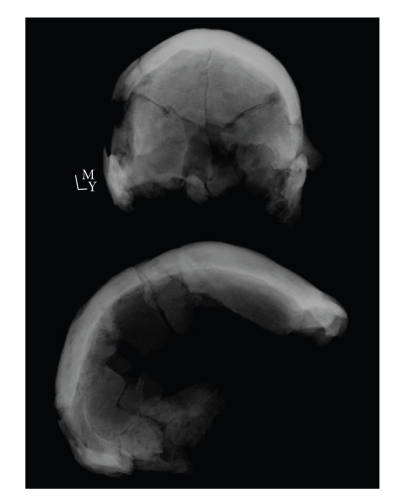
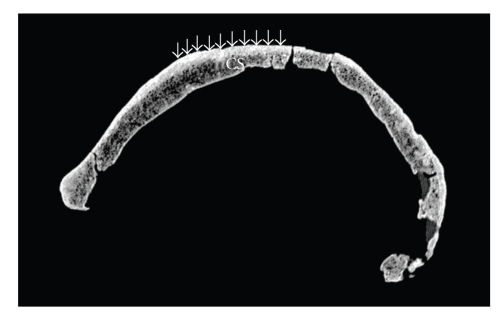
Figure 4 is a lateral radiograph of WLH50 taken in 2006 by the author and A. Thorne. After careful inspection, no indication of the hair-on-end sign can be seen. Figure 5 is a lateral slice of a CT-scan of WLH50 also taken by the author and A. Thorne in 2006. Again, in this and every slice inspected by the present author (245 slices in three planes, or 735 images), no evidence of the hair-on-end sign can be found. There is, however, evidence for abrasion on the external cortex around bregma (Figure 5), which may have been mistaken by Webb for the vertical spiculations sometimes associated with porotic hyperostosis.
Figure 4.
Lateral plain film X-ray of Willandra Lakes Human 50.
Figure 5.
Sagittal CT-scan of Willandra Lakes Human 50 (lateral to the MSP). Arrows indicate surface abrasion; “CS” denotes coronal suture.
It is also clear in Figures 4 and 5 that the external table of WLH50 is far from thin. To assess this quantitatively, the ratio of diploe to external table thickness was calculated from thickness measurements made on CT-scans just lateral to the median sagittal plane by the author. External table thickness was chosen because along with the diploë this vault component is altered in cases of porotic hyperostosis [157]. The median of this ratio for WLH50 measured at nine locations shows the diploë to be 1.4 times as thick as the external table, while in WLH3 the median shows it to be 0.9 times as thick. While WLH50 certainly does exhibit relatively thinner external table, it is far from thin. Finally, the results of a Mann-Whitney U-test showed the median difference between WLH3 and WLH50 to be statistically nonsignificant (n = 9/23, U = 74, P = .22; Monte Carlo P = .23).
5.2. Ontogenetic Examination of Some Phylogenetic Characters
Table 1 lists 16 morphological traits for WLH50 used to support a role for the Solo/Ngandong population in the evolution of Aboriginal Australians (i.e., a reticulate or dual ancestry model) [54, 55]. These “nonmetric” traits were originally selected in order to avoid “duplicating features that seemed to reflect the consequences of the same anatomical variation” [55, page 294]. While somewhat ambiguous, this statement is understood by the present author to imply that traits were employed that were believed to be developmentally and functionally nonintegrated and, therefore, to be characterised by weak or absent covariation. This assumption is crucial to an evaluation of the phyletic valency of cranial robusticity in the Australian context and the implicit assumption that characters shared by Australian and Solo/Ngandong crania are homologous. (The reader is referred also to Bräuer et al. [158] who provided a critical evaluation of the character coding and statistical methods employed by Hawks et al. [54] and Wolpoff et al. [55].)
Table 1.
Cranial traits used in phenetic studies of WLH50 (characters from [55]).
| Character | Developmental subunit* |
|---|---|
| (1) Angular torus | Vault |
| (2) Coronal keel | Vault |
| (3) Sagittal keel on frontal | Vault |
| (4) Lateral frontal trigone | Vault |
| (5) Linea obliquus strongly developed | Vault |
| (6) Mastoid crest | Vault |
| (7) Sagittal keel on parietal | Vault |
| (8) Postlambdoidal eminence | Vault |
| (9) Prebregmatic eminence | Vault |
| (10) Projecting inion | Vault |
| (11) Sulcus dividing the medial and lateral elements of the supraorbital torus or superciliary arches | Orbit |
| (12) Superior margin of the orbit blunt (as opposed to sharp) | Orbit |
| (13) Suprainiac fossa | Vault |
| (14) Supramastoid crest | Vault |
| (15) Temporal line forms a ridge | Vault |
| (16) Transversely extensive nuchal torus | Vault |
*After Ackermann and Cheverud [120].
All of the characters shown in Table 1 belong to a single cranial (developmental) unit, namely, the neurocranium. A small part of the lateral cranial base is preserved while the facial skeleton is missing save a fragment of zygomatic bone. Moreover, these traits represent two subunits of the neurocranium most of them belong to the cranial vault (14), with the remaining (2) belonging to the orbit subunit (Table 1). Given the now well-established finding of ontogenetic modularity and integration of the neurocranium (e.g., [77, 80–83, 85–89, 106–144]), there are good reasons to be suspicious about the use of the atomisation in this instance.
According to the functional matrix hypothesis [88], the cranium comprises various functional cranial components (FCC), which are ontogenetically integrated. One FCC contains the brain, cerebellum, and ocular globe, sharing a common embryological origin in the neural tube [77]. This FCC develops in an integrated way being subjected to common heritable and epigenetic factors. The neurocranium largely develops as a result of the passive displacement of the vault bones, occurring within a connective tissue stroma, which is enlarged by the growing brain [77]. As a result, all three parts of this FCC follow a common growth trajectory, reaching 90–96% of their adult size by about age 6-7 [77, 89, 115, 129]. However, the neurocranium also forms an integrated unit with the cranial base, the “neurobasicranial complex,” and its form is strongly influenced by factors such as basicranial flexion, while the developing face also epigenetically “fine-tunes” basicranial morphology, indirectly altering the form of the cranial vault [122, 123, 131, 132, 136, 138–144].
Additionally, all 16 characters are cranial “superstructures” as originally defined by Weidenreich [159]. He suggested that superstructures develop in response to tension (“pull”) from muscles, a hypothesis subsequently verified experimentally with respect to some cranial crests [77, 78, 91, 92, 115, 160, 161], implying that they “belong to, or sit only upon, the outer bony table” [91, page 208]. However, this situation is not universal for superstructures and their development is complex.
As Enlow has shown [77, 91, 115, 161], the external surface of a bone is frequently shifted from an endosteal position. This means that developmental changes within the endocranium as well as epigenetic factors acting on the external table determine the morphology of the vault. Moreover, different processes may be involved in remodelling the neurocranium in spatially adjacent regions, and different regions of the cranium may exert influence over the growth of a single structure at different ontogenetic stages. For example, about midway up the frontal squama is a reversal line from resorptive (inferiorly) to depositional (superiorly) remodelling [77]. Once the frontal lobes have largely completed their enlargement, the internal table stops growing, while the outer table continues to remodel, progressively separating the inner and outer tables, and replacing through resorption cancellous bone to form the frontal sinus. However, this continued growth now results directly from the anterior remodelling of the upper face or nasomaxillary complex [77]. Further, the orbit (subunit) is contained within this region, and the inner table of its roof (endocranial side) is resorptive while the external (orbital plate) is depository [77]. As the orbital roof continues to remodel inferiorly along with the growth of the frontal lobes of the brain, the orbit must grow anteriorly to provide sufficient room for the growing eye; it is displaced outwards with other bones of the orbit which form part of the face according to the V principle [77].
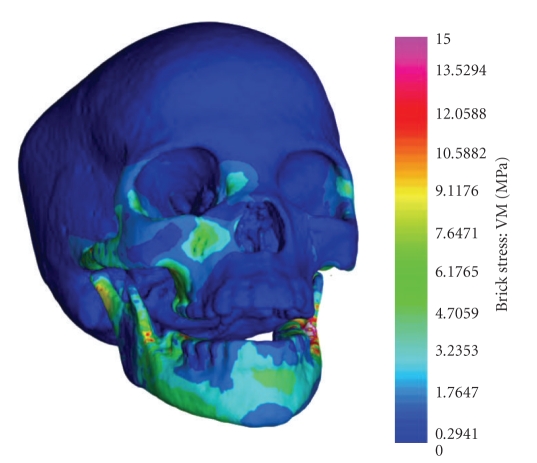
In the case of the supraorbital torus, also considered to be a superstructure by Weidenreich [159], there is now abundant evidence that this feature develops epigenetically as a result of the spatial relationships among the face, neurocranium, and cranial base rather than biomechanical stresses during mastication [77, 115, 162–167], contra [168]. For example, Figure 6 is a Holocene San skull indicating stress gradients from masticatory stress as analysed with the finite element method [166]. The supraorbital region is unaffected by strain in this homogenous 3D model (but see similar results using a heterogeneous model [165]).
Figure 6.
Stress (von Mises) distributions in a high-resolution three-dimensional computer simulation of cranial mechanics in a Holocene San skull (modified from [166]).
Thus, while 14 characters might be regarded as superstructures of the cranial vault, developing to provide muscle attachments and remodelling in response to mechanical stress, research into the supraorbital torus makes clear that the two orbital roof features lack epigenetic influences from the muscles of mastication. The supraorbital develops as a result of a complex interplay between the shape of the neurocranium and breadth of the basicranium, growth of the frontal lobes of the brain, development of the frontal sinus, remodelling of the orbit to accommodate the growing eye, and growth of the nasal region, which develops in response to lung enlargement and in particular affects the superior orbital rim, and the anteroposterior length of the face seems also to be a determinant of its position relative to the frontal squama and, therefore, the degree of frontal recession seen [77, 115, 122, 123, 137, 162–171]. Moreover, crania with high midfacial projection exhibit a long anterior sphenoid resulting in a viscerocranium positioned anterior to the neurocranium [133, 134, 170, 171] and in a prominent supraorbital. Finally, the development of the supraorbital region may even be epigenetically influenced by exercise through the effects of hormones seemingly independently from (but commonly with) other structures such as thickened vault bones (see [172, 173]; see also [8]).
As noted above, Mitteroecker and Bookstein [106] have found four common cranial factors or trajectories across a range of primates including humans, which are integrated through genetic effects such as pleiotropy and linkage, as well as epigenetic processes.
Factor 1 —
An enlarged and prognathic maxilla, relatively small cranial capsule, cranial crests, and enlarged zygomatic arches are features epigenetically associated in primates mainly as a result of masticatory muscle action. These are all characteristics of cranial robusticity and while they can be observed as a package in better-preserved crania (e.g., Cohuna, Kow Swamp 1), some of them are observable in incomplete remains such as WLH50 (Table 1, Figures 2 and 7) [2, 8–14, 16–18, 41–43, 46–52, 54, 55, 64, 72, 151].
Figure 7.
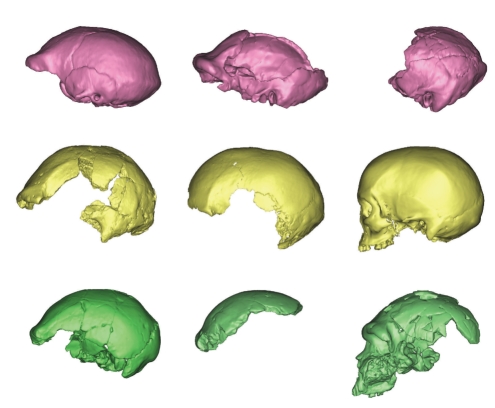
Three-dimensional models of various fossil crania made from CT-scans, left lateral view: (top to bottom, left to right) H. erectus Sangiran 2, OH9, and Sangiran 4; Pleistocene Australians WLH50 and WLH3; African Holocene San; premodern Middle Pleistocene Africans LH18, Florisbad, and Bodo. Models scaled to approximately the same A-P length.
Factor 2 —
This pattern contrasts broad and short crania with narrow and long crania (brachycephalic versus dolichocephalic crania), including both the face and the neurocranium, and also involves changes in the overall size of the face relative to the neurocranium. All robust Australians (e.g., Cohuna, KS1, WLH50) exhibit narrow and long crania (Figures 2 and 7) [2, 8–14, 16–18, 41–43, 46–52, 64, 72, 151]. Overall neurocranial length, breadth, and height are characterised by moderate but significant levels of heritability, suggesting a large amount of variation in these features determined by epigenetic forces [79, 81, 82]. Moreover, while their faces are absolutely long and consistent with this factor (e.g., KS1, KS5, Cohuna), they differ in exhibiting absolutely and relatively broad faces [2, 8, 9, 11–14, 17, 18, 41–43, 47–49, 51, 52, 151]. However, facial shape has been found to exhibit weaker correlations with neurocranial and basicranial shape than these regions have with each other, perhaps explaining why the face is the most variable part of the skull in humans [85]. Additionally, the dimensions of facial length, breadth, and height are characterised by moderate heritability indicating that additive genetic variation accounts for approximately 30% of the phenotypic variation [79, 81, 82]. Further, facial breadth is well known to be under strong ontogenetic influence from the muscles of mastication [171, 174–178]. A positive correlation between total masticatory muscle size (cross-sectional area) and body size (stature and weight) has also been found [171, 174–178]. The body size of the WLH50 individual, like other Pleistocene Aboriginal Australians, would have been large by later Holocene and contemporary standards [8, 17]. In fact, metrical dimensions of size and endocranial volume reveal it to be one of the largest Pleistocene modern humans found anywhere in the world and comparable in size to early African crania such as from Herto, and larger than many nonmodern individuals (compare with data in White et al. [179]), including H. erectus calvarii [17].
Factor 3 —
Encompassing relative size of the midface and neurocranial globularity, two characteristics that are tightly associated during postnatal ontogeny. While sample size disallows a proper comparison, robust Australian individuals such as KS1 are certainly characterised by a relatively narrow midface (ZMB/NPH = c66%) compared with the gracile Keilor cranium (c76%) (data from [151]). Moreover, these and other crania are strongly contrasted in their degree of neurocranial globularity; robust crania exhibiting highly angulated vaults, with receding frontal squamae (Table 1, Figure 2) [2, 8, 9, 11–14, 17, 18, 41–43, 47–49, 51, 52, 151]. In contrast, individuals such as WLH1, WLH3, and Keilor possess more globular crania (see Figure 7) [2, 9, 13, 14, 17, 41, 42, 51–53, 71, 72, 151].
Factor 4 —
A roundish and relatively short and high neurocranium to elongated, ellipsoidal crania. Exaggeration of the latter generates an occipital bun and lambdoid flattening along with large browridges. Robust Australian crania all exhibit ellipsoidal crania (superior outline); many have an occipital bun and lambdoid flattening, and all exhibit prominent browridges by modern human standards (see Figures 2 and 7) [2, 8, 9, 11–14, 17, 18, 41–43, 47–49, 51, 52, 151]. Although the WLH50 calvaria is actually relatively tall in addition to being very long, this probably results from the combination of large endocranial volume and very narrow cranial base (see [17, 156]; see also [180, 181]). A range of other factors interacting in a complex manner are also known to influence the development of the supraorbital part, cranial shape being one of them (see above).
It is interesting also to note that Mitteroecker and Bookstein [106] found that their Factors 1 and 2 were the most highly conserved among humans, chimpanzees, and gorillas, with all three sharing a single ontogenetic trajectory owing to the presence of tight integration during development. Many of the traits listed in Table 1 can be explained by variation within these two highly constrained modules. Trajectories for Factors 3 and 4 were found to deviate considerably in humans compared to chimpanzees and gorillas suggesting that certain human cranial features have decoupled during evolution [106]. They opined that this difference was best explained by local developmental factors unique to humans such as relative brain size, posture, and locomotion (Factor 3), and facial shape, mainly involving the browridge (Factor 4). In the end, this research indicates that selection does not act on individual features, but instead cranial traits form part of an integrated and heritable set of factors or trajectories. Australian cranial robusticity lies on the trajectory for all four factors but is never as extreme in its expression as seen among nonmodern hominins.
Figure 7 presents 3D models (scaled to the same length) constructed by the author from CT-scans of WLH3 and WLH50, a Holocene African hunter-gatherer and various Pleistocene (nonmodern) hominins. Compared to WLH3 and the Holocene San individual, WLH50 does exhibit a receding frontal squama, prominent browridge, and some projection of the occipital. However, compared to all nonmodern, middle Pleistocene calvarii, WLH50 is tall, has a more upright frontal squama, which bulges anteriorly (prebregmatic eminence), and has relatively rounded parietal and occipital profiles and mild lambdoidal flattening. In fact, it closely resembles WLH3 in all these respects. Moreover, it lacks the strongly projecting browridge and a posttoral shelf so evident in all middle Pleistocene remains, whether belonging to H. erectus, H. heidlebergensis/rhodesiensis, or archaic H. sapiens/H. helmei. Its profile including the frontal squama is quite rounded (globular) compared to nonmodern crania. Thus, the purported similarities between WLH50 and H. erectus seem to have been greatly exaggerated, particularly when a comparison involving Middle Pleistocene African crania, the putative ancestors of all modern humans [1], is made. Instead, WLH50 simply presents as a more rugged version of the WLH3 morphology, the two resembling each other in their angle of the posterior part of the frontal squama and profile of the parietals and occipital; when compared with the San cranium, and indicating regional-specific features for Australian crania. Moreover, the comparatively modest differences in shape between WLH3 and WLH50 seem to be explicable in terms of the ontogenetic trajectories described by Mitteroecker and Bookstein [106].
Another important recent study of cranial robusticity with strong bearing on the morphology of WLH50 and other Pleistocene/early Holocene Australians is that of Baab et al. [182]. They tested for integration/correlation among various robusticity traits in a global sample of humans including recent Aboriginal Australians. Overall, they found that crania with more prognathic faces, expanded glabellar and occipital regions, and longer skulls were more robust than those with rounder vaults and more orthognathic faces. This supports the findings of Lahr and Wright [156] and, more broadly, the results of Mitteroecker and Bookstein [106].
Baab et al. [182] also found evidence for significant positive but weak coexpression among all robust traits (frontal trigone, sagittal keel, infraglabellar notch, supraorbital torus, zygomaxillary tubercle, and prebregmatic eminence) with the exception of the occipital torus. They did, however, find that the supraorbital torus is strongly and significantly correlated with an infraglabellar notch and moderately and significantly correlated with a zygomaxillary tubercle and a zygomatic trigone. Moreover, moderate and significant correlations were found between the zygomaxillary tubercle and bregmatic eminence, inferolateral rounding of the orbit, infraglabellar notch, and zygomatic trigone; between a bregmatic eminence and inferolateral rounding of the orbit; infraglabellar notch and zygomatic trigone. They concluded that epigenesis through masticatory function was the most likely cause of the development of these traits.
One feature studied by Baab et al. [182] failed to show correlation with the other robust traits they examined, namely, the occipital torus. Again this seems to be explicable in terms of epigenetic forces during ontogeny. For example, Perez et al. [183] have described a positive association between occipital muscle attachment areas and head shape (dolichocephaly) among males in a sample of South American hunter-gatherer crania. Thus, it may simply be an epigenetic consequence of an elongated cranium, in-line with other changes such as a protruding occipital and formation of an occipital bun [106, 122, 123, 180, 181]. A related character is the suprainiac fossa in WLH50 (Table 1). Functionally, this feature is suggested to provide attachment for nuchal ligament [184] or to represent an area of resorptive bone developing in response to bending forces along the nuchal torus [185]. Irrespective of its cause, this feature is actually found in both WLH3 and WLH50 (personal observation) and cannot, therefore, be considered a defining trait of Australian robusticity nor evidence bearing on their phylogenetic history.
The issue of vault ridges (or keeling) has not been explicitly addressed in most ontogenetic studies of the human cranium. Although Baab et al. [182] did find statistically significant moderate correlations between a sagittal keel and bregmatic eminence, both features are associated with cranial vault sutures. Their development may, therefore, be part of the integrated process of ontogeny and result from epigenetic factors relating to cranial shape and mechanical forces.
The research of Baab et al. [182] also highlights that while integration and modularity are fundamental to understanding cranial ontogeny, certain features sometimes show lower than expected correlation and covariation than expected. Similar findings have been made with respect to the endocranium [186–188].
A number of researchers have specifically addressed the issue of the mechanical effects of mastication on cranial sutures experimentally. In vivo studies [188] found that strain (tension or compression) along sutures was not the result of torsion but rather the localised effects of masticatory actions. Strain was found to be significant along all of the major vault sutures, and its effects varied depending upon the muscle involved. For example, while masseter contraction tensed the coronal suture, the temporal caused compression at this region. Moreover, an earlier study [189] found that even a relatively small bite force was sufficient to cause separation of the sagittal suture in juvenile monkeys, from which they concluded that “such separation might act as a major factor in the local control of osteogenesis of the sutures” [189, page 907]. Additionally, Sun et al. [190] confirmed earlier findings of significant masticatory strain along vault sutures and further found that synostosis (normal fusion) resulted in increased suture strain and enhanced bone growth on the ectocranial surface leading to thicker bones in adults. Finally, the sutures have also been suggested to play a role in dissipating masticatory strain [191], while the diploë may also play a similar role [165]; although these hypotheses require further testing.
Clinical interest in cranial ridges along the major vault sutures stems from understanding the causes and treatment of abnormal head shape as result of congenital or acquired conditions (such as birth injuries) or pathophysiological processes leading to clinical craniosynostosis (premature suture closure). Midline ridges are found within the clinical spectrum of such cranial deformities [192]. However, in the absence of changes to vault or facial shape, they are considered normal variants [193]. The metopic ridge can arise in an infant (before two years of age) from premature closure of the metopic suture, while a midline bony ridge over vertex from front to back may develop following premature fusion of the sagittal suture in adults prior to 40 years of age [192]. Coronal bony ridges can also form in cases of premature craniosynostosis, again arising any time before normal closure of the coronal suture (typically around 40 years of age [192]).
Premature synostosis of one or more sutures is accompanied by compensatory growth, both in other sutures, and by remodelling (appositional growth) of other parts of the skull [194]. Premature closure of sutures prevents separation of the bones and affects skull growth in a direction perpendicular to the affected suture, leading to skull deformations [194, 195]. Much later in life (i.e., around 40 years of age), premature craniosynostosis of the sagittal and coronal sutures can occur and leads in mild cases to the development of sagittal and coronal ridges. Among robust Australian crania, the parietals are relatively long and the lower occipital scale (nuchal plane) is unusually relatively short [151]. Moreover, premature craniosynostosis of the sagittal suture at around 40 years of age can result in dolicocephaly, accompanied by a narrow head with bitemporal widening, sometimes frontal and occipital bossing, and a midline bony ridge over the vertex from front to back [192].
The current clinical incidence of synostosis is 1 in 2,000–3,000 newborns although syndromic craniofacial malformations involving more than one suture occur in about 15% of cases [192]. Sagittal craniosynostosis is rarer, with an incidence of about 1 in 5,000 adults [192]. The aetiology of craniosynostosis is heterogeneous: hereditary, mechanical, teratogenic, and idiopathic [195]. Multiple genetic and environmental causes have been identified. Among the latter are rickets caused by vitamin D deficiency or resistance, chronic renal failure, hypothyroidism and hypophosphatasia, and multiple causes of abnormal fetal positioning in utero leading to constraint of the fetal skull [192]. Rickets is documented among human remains from the Willandra Lakes [153]. Moreover, synostosis may have been more common in the past among some populations. For example, White [196] found an incidence of 3% in a sample of normal Mayan skulls, rising to 31% among those from the precontact period; and in 41% of them premature synostosis was apparently explained by artificial deformation. This lends support to suggestions that artificial deformation may be an exaggerating influence in the robusticity of Pleistocene/Holocene Australians [9, 146–150].
6. Conclusions
The idea that Aboriginal Australians posses an unusual and phylogenetically informative pattern of cranial robusticity has been important in palaeoanthropology for about 150 years. There has, however, been a wide range of views about specific aspects of their evolutionary history. Particularly, whether or not Pleistocene nonmodern populations in Southeast Asia (i.e., Pithecanthropus, H. soloensis, or H. erectus) played a role in their evolutionary history. Irrespective of whether the multiregional, assimilation, or Out-of-Africa model has been supported, most investigations have taken the human cranium to be optimised part by part, and atomised its morphology into traits assumed to be heritable units, functionally discrete, shaped by natural selection and, therefore, to be positively associated with reproductive success in order to make phylogenetic inferences. Yet, a large body of research from the morphological sciences, including anatomy and embryology, pathology, genetics, and evolutionary biology makes it clear that these assumptions may be unrealistic and that atomisation should be used with great care in phylogenetic reconstruction.
The human (vertebrate) cranium is characterised by ontogenetic and functional modularity, with much integration/covariation among the elements within each module, as well as between them. Moreover, epigenesis is a powerful, pervasive, and very complex force, its influences being felt in all levels of organisation during ontogeny, from cellular interactions to those among adjacent tissues to interactions with the external environment of an organism. Additionally, while many aspects of cranial size and shape are heritable, epigenesis may determine 70% or more of variation seen within populations with well-established pedigrees [79–82]. Finally, many of the traits designated “robust” are also characterised by some ontogenetic integration and are likely to derive from a set of common epigenetic factors, which includes the spatial effects/constraints of size and shape, for example, central integrating features such as the cranial base and mechanical effects from the muscles of mastication.
While there is clearly a strong message in this research for palaeoanthropology in general, its implications for studies of Australian Aboriginal robusticity are particularly acute. Phylogenetic reconstruction across all organisms and employing all methods involves a set of important assumptions about the traits used. For example, Lieberman [197] lists seven such assumptions, the first four being relatively uncontroversial, with the remaining three particularly relevant here although hardest to satisfy; (5) characters should reflect independent units of information about phylogenetic relationships, (6) they must be heritable if they are to provide reliable information on ancestry and descent, and (7) they should be equivalent units or homologous structures. The ontogenetic approach outlined here suggests that these assumptions probably cannot be met in the case of phylogenetic reconstructions involving robust Australian crania when using atomised traits. Most characters are unlikely to be independent units, being integrated in their development and influenced by a complicated set of epigenetic interactions [77, 83–89, 106–144, 160–167, 169–178, 180–183, 188–197]. They also show mostly low to moderate heritability [79–82] and are in some instances clearly not homologous in modern humans and nonmodern hominins.
For example, a character used widely in studies of the evolutionary origins of Aboriginal Australians is the possession of a flat frontal squama among robust individuals [2, 8, 9, 11–14, 16–18, 39–43, 47–49, 51, 52, 63, 72, 151]. Putting aside epigenetic explanations involving pseudopathology [17, 146–150], this trait fails to satisfy all three assumptions of phylogenetic reconstruction. The size and shape of the growing brain in accordance with the functional matrix hypothesis [77, 88, 114, 115] and its epigenetic interactions with the developing cranial base largely determine the size and shape of the vault. Moreover, there are also influences from the growing face although, they are more subtle than those of the basicranium [122, 123, 131, 132, 136, 138–144]. The anteroposterior length of the face seems also to be a determinant of its position relative to the frontal squama and, therefore, the degree of frontal recession present; crania with high midfacial projection exhibit a long anterior sphenoid, resulting in a viscerocranium positioned anterior to the neurocranium [122, 123, 131–134].
In nonmodern hominins like H. erectus, a flat frontal squama results from a complex combination of a small frontal brain lobe, broad cranial base, combined high midfacial projection and long anterior sphenoid. However, in anatomically modern humans with our large brains, including relatively broad and steep frontal lobe [198], angulation of the frontal squama results from the brain being epigenetically forced to grow excessively posteriorly by a narrow cranial base resulting in a long and tall cranium [17, 156, 180, 181]. Moreover, a flat frontal squama is associated with a browridge, the orbital roof being forced to grow forward to accommodate the growing eye, and an occipital bun, the result of the posterior growth of the brain within a narrow vault. Additionally, because the brain and its frontal lobe are relatively broader and more globular (taller) in modern humans and our face characterised by significant retraction [137, 170], the brow is never as prominent in modern humans as it is in nonmodern hominins including in WLH50 (see Figure 7) [122, 123, 181, 199]. As noted above, WLH50 is among the largest modern humans found in the fossil record globally and is comparable in size to the earliest modern remains such as from Herto (see data in [179]), and larger than many nonmodern crania including H. erectus. While large cranial size and a narrow cranial base, as seen in WLH50, are certainly plesiomorphic traits, they are not shared with H. erectus or other nonmodern hominins but are plesiomorphies of anatomically modern H. sapiens [17, 122, 123]. Moreover, they seem to have been important influences on the form of this calvaria and other Australian remains.
To conclude, there is now ample evidence that the atomisation of cranial robusticity has provided a misleading picture of the evolutionary history of Aboriginal Australians. Moreover, models suggesting that living Australians as demonstrated by their robust cranial morphology can trace their origins to the nonmodern hominins of Pleistocene Southeast Asia should be reconsidered in light of major developments in the morphological sciences. In 1976, Howells [145] issued a challenge to Australian palaeoanthropology to reject a null hypothesis that differences between gracile and robust crania can be explained by phenotypic plasticity alone. So far, this challenge has been ignored. It is time to reconsider the adaptationist program and to take a more parsimonious approach to explaining Aboriginal Australian origins, one that takes account of the complex processes involved in the ontogeny of the human cranium rather than just assuming that natural selection explains every subtle variation seen in past populations.
Acknowledgments
The author wishes to express his deepest gratitude to Alan Thorne for allowing him access to fossilised human remains from the Willandra Lakes in his care and for encouraging critical thought over a decade of friendship and collaboration. He would also like to acknowledge the traditional owners of the Willandra Lakes region who have continued to support scientific research about their history and on the remains of their ancestors. Thanks is extended to Rob Lindemann, Ella Onikul, and the staff of the Radiology Section of the Children's Hospital Westmead for undertaking X-rays and CT-scans of WLH3 and WLH50 during 2006. James Brink is acknowledged for allowing the author to study fossils in his care and them to be CT-scanned. CT-scans of Florisbad and Holocene San remains were undertaken in 2008 at the Bloemfontein MediClinic; thanks to Leanne Carver, other radiographers, and the nursing staff at Drs Van Dyk and Partners. Fred Spoor is thanked for providing CT-scans of the Sangiran crania. CT-scans for other fossil hominins were purchased from the European Virtual Anthropology Network. The comments of two referees (including Emiliano Bruner) improved the paper. This research was funded by the Australian Research Council under nos. DP0877603 and DP0987985 and by the University of New South Wales.
References
- 1.Stringer C. Modern human origins: progress and prospects. Philosophical Transactions of the Royal Society B. 2002;357(1420):563–579. doi: 10.1098/rstb.2001.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curnoe D. Modern human origins in Australasia: testing the predictions of competing models. Homo—Journal of Comparative Human Biology. 2007;58(2):117–157. doi: 10.1016/j.jchb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Bräuer G. The origin of modern anatomy: by speciation or intraspecific evolution? Evolutionary Anthropology. 2008;17(1):22–37. [Google Scholar]
- 4.Endicott P, Ho SYW, Stringer C. Using genetic evidence to evaluate four palaeoanthropological hypotheses for the timing of Neanderthal and modern human origins. Journal of Human Evolution. 2010;59(1):87–95. doi: 10.1016/j.jhevol.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Green RE, Krause J, Briggs AW, et al. A draft sequence of the neandertal genome. Science. 2010;328(5979):710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SC, Miller W, Ratan A, et al. Complete khoisan and bantu genomes from Southern Africa. Nature. 2010;463(7283):943–947. doi: 10.1038/nature08795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorne AG. The longest link: human evolution in Southeast Asia and the settlement of Australia. In: Fox JJ, Garnaut AG, McCawley PT, Maukie JAC, editors. Indonesia: Australian Perspective. Canberra, Australia: Australian National University; 1980. pp. 35–43. [Google Scholar]
- 8.Brown P. Pleistocene homogeneity and Holocene size reduction: the Australian human skeletal evidence. Archaeology in Oceania. 1987;22:41–67. [Google Scholar]
- 9.Brown P. Coobool Creek. Canberra, Australia: Department of Prehistory, Research School of Pacific Studies, The Australian National University; 1989. [Google Scholar]
- 10.Pardoe C. Competing paradigms and ancient human remains: the state of the discipline. Archaeology in Oceania. 1991;26:79–85. [Google Scholar]
- 11.Groves CP. A regional approach to the problem of the origin of modern humans in Australasia. In: Mellars P, Stringer CB, editors. The Human Revolution. Edinburgh, Scotland: Edinburgh University Press; 1989. pp. 274–285. [Google Scholar]
- 12.Habgood PJ. The origin of anatomically-modern humans in Australasia. In: Mellars P, Stringer CB, editors. The Human Revolution. Edinburgh University Press; 1989. pp. 245–273. [Google Scholar]
- 13.Webb SG. The Willandra Lakes Hominids. Canberra, Australia: Department of Prehistory, Research School of Pacific Studies, the Australian National University; 1989. [Google Scholar]
- 14.Webb SG. The First Boat People. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 15.Thorne A, Grün R, Mortimer G, et al. Australia’s oldest human remains: age of the lake Mungo 3 skeleton. Journal of Human Evolution. 1999;36(6):591–612. doi: 10.1006/jhev.1999.0305. [DOI] [PubMed] [Google Scholar]
- 16.Curnoe D, Thorne A. The question of cranial robusticity. Before Farming, article 2, 2006.
- 17.Curnoe D. Possible causes and significance of cranial robusticity among Pleistocene-Early Holocene Australians. Journal of Archaeological Science. 2009;36(4):980–990. [Google Scholar]
- 18.Westaway MC, Groves CP. The mark of Ancient Java is on none of them. Archaeology in Oceania. 1989;44(2):84–95. [Google Scholar]
- 19.Klaatsch H. Report from the Pathological Laboratory of the Lunacy Department. Vol. 1. NSW; 1908. The skull of the Australian aboriginal; pp. 43–167. [Google Scholar]
- 20.Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proceedings of the Royal Society of London. 1979;205(1161):581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 21.Turner W. Report on the human crania and other bones of the skeleton collected during the voyages of the HMS Challenger, in the years 1873-1876. Part 1: The Crania. Report of the Scientific Results of the Exploring Voyage of the HMS Challenger 1873-1876. Zoology. 1884;10:1–130. [Google Scholar]
- 22.Mulvaney DJ. Fact, fancy and Aboriginal Australian ethnic origins. Mankind. 1966;6:299–305. [Google Scholar]
- 23.Lyell C. The Antiquity of Man. London, UK: J.M. Dent and Sons; 1863. [Google Scholar]
- 24.Burkitt AN, Hunter JI. The description of a Neanderthaloid Australian skull, with remarks on the production of the facial characteristics of Australian skulls in general. Journal of Anatomy. 1922;57:31–54. [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham DJ. The head of an Aboriginal Australian. The Journal of the Royal Anthropological Institute of Great Britain and Ireland. 1907;37:47–57. [Google Scholar]
- 26.Jones FW. Contrasting types of Australian skulls. Journal of Anatomy London. 1934;68:323–330. [PMC free article] [PubMed] [Google Scholar]
- 27.Dubois E. Pithecanthropus erectus, eine menschenaehnliche Uebergangs form aus Java. Batavia, NY, USA, 1894.
- 28.Dubois E. The proto-Australian man of Wadjak, Java. Koninklke Akademie van Wetenschappen te Amsterdam B. 1922;23:1012–1051. [Google Scholar]
- 29.Dubois E. On the fossil human skulls recently discovered in Java and Pithecanthropus Erectus. Man. 1937;37:1–7. [Google Scholar]
- 30.Morant GM. A Study of the Australian and Tasmanian skulls, based on previously published measurements. Biometrika. 1927;19:417–440. [Google Scholar]
- 31.Howells WW. Fossil Man and the origin of races. American Anthropologist. 1942;44:182–193. [Google Scholar]
- 32.Schutler R, Head JM, Donahue DJ, et al. Modern Quaternary Research in Southeast Asia. Vol. 18. Leiden, the Netherlands: Balkema; 2004. AMS radiocarbon dates on bone from cave sites in southeast Java, Indonesia, including Wadjak; pp. 89–94. [Google Scholar]
- 33.Weidenreich F. (Palaeontologica Sinica New Series).The Skull of Sinanthropus Pekinensis; A Comparative Study on a Primitive Hominid Skull . 1943;10 [Google Scholar]
- 34.Weidenreich F. (Anthropological papers of the American Museum of Natural History).Morphology of Solo Man. 1951;43 [Google Scholar]
- 35.Coon CS. The Origin of Races. New York, NY, USA: Alfred A. Knopf; 1962. [Google Scholar]
- 36.Howells WW. Mankind in the Making. New York, NY, USA: Doubleday; 1959. [Google Scholar]
- 37.Hrdlička A. The peopling of the Earth. Proceedings of the American Philosophical Society. 1926;65(3):150–156. [Google Scholar]
- 38.Birdsell JB. The origin of human races. The Quarterly Review of Biology. 1963;38:178–185. [Google Scholar]
- 39.Macintosh NWG. Origin and physical differentiation of the Australian Aborigines. Australian Natural History. 1963;14:248–252. [Google Scholar]
- 40.Macintosh NWG. The physical aspect of Man in Australia. In: Berndt RM, Berndt CH, editors. Aboriginal Man in Australia. Sydney, Australia: Angus & Robertson; 1965. [Google Scholar]
- 41.Thorne AG. Mungo and kow swamp: morphological variation in Pleistocene Australians. Mankind. 1971;8:p. 85. [Google Scholar]
- 42.Thorne AG, Macumber PG. Discoveries of late Pleistocene man at Kow Swamp, Australia. Nature. 1972;238(5363):316–319. doi: 10.1038/238316a0. [DOI] [PubMed] [Google Scholar]
- 43.Freedman L, Lofgren M. Human skeletal remains from Lake Tandou, New South Wales. Archaeology in Oceania. 1983;18:98–105. [Google Scholar]
- 44.Macintosh NWG. Radiocarbon dating as a pointer in time to the arrival and history of man in Australia and islands to the north west. In: Proceedings of the International Conference on Radiocarbon Dating; 1972; Wellington, New Zealand. [Google Scholar]
- 45.Bowler JM. Recent developments in reconstructing Late Quaternary environments in Australia. In: Kirk RL, Thorne AG, editors. The Origin of the Australians. Vol. 6. Canberra, Australia: Australian Institute of Aboriginal Studies; 1976. pp. 57–77. (Human Biology Series). [Google Scholar]
- 46.Macintosh NWG, Larnach SL. The persistence of Homo erectus traits in Australian Aboriginal crania. Archaeology and Physical Anthropology in Oceania. 1972;7:1–7. [Google Scholar]
- 47.Thorne AG, Wolpoff MH. Regional continuity in Australasian Pleistocene hominid evolution. American Journal of Physical Anthropology. 1981;55(3):337–349. doi: 10.1002/ajpa.1330550308. [DOI] [PubMed] [Google Scholar]
- 48.Wolpoff WH, Wu XZ, Thorne AG. Modern Homo sapiens origins: a general theory of hominid evolution involving the fossil evidence from East Asia. In: Smith F, Spencer F, editors. The Origins of Modern Humans: A World Survey of the Fossil Evidence. Vol. 6. New York, NY, USA: Alan. R. Liss; 1984. pp. 411–483. [Google Scholar]
- 49.Frayer DW, Wolpoff MH, Thorne AG, Smith FH, Pope GG. Theories of modern human origins: the paleontological test. American Anthropologist. 1993;95(1):14–50. [Google Scholar]
- 50.Wolpoff MH, Thorne AG, Jelinek J, Yinyun Z. The case for sinking Homo erectus : 100 years of Pithecanthropus is enough. Courier Forshungs-Institute Senckenberg. 1994;171 [Google Scholar]
- 51.Thorne AG. Morphological contrasts in Pleistocene Australia. In: Kirk RL, Thorne AG, editors. The Origin of the Australians. Vol. 6. Canberra, Australia: Australian Institute of Aboriginal Studies; 1976. pp. 95–112. (Human Biology Series). [Google Scholar]
- 52.Thorne AG. The longest link: human evolution in Southeast Asia and the settlement of Australia. In: Fox JJ, Garnaut AG, McCawley PT, Maukie JAC, editors. Indonesia: Australian Perspective. Canberra, Australia: Australian National University; 1980. pp. 35–43. [Google Scholar]
- 53.Thorne A, Curnoe D. Sex and significance of lake Mungo 3: reply to brown "Australian Pleistocene variation and the sex of lake mungo 3". Journal of Human Evolution. 2000;39(6):587–600. doi: 10.1006/jhev.2000.0442. [DOI] [PubMed] [Google Scholar]
- 54.Hawks J, Oh S, Hunley K, et al. An Australasian test of the recent African origin theory using the WLH-50 calvarium. Journal of Human Evolution. 2000;39(1):1–22. doi: 10.1006/jhev.1999.0384. [DOI] [PubMed] [Google Scholar]
- 55.Wolpoff MH, Hawks J, Frayer DW, Hunley K. Modern human ancestry at the peripheries: a test of the replacement theory. Science. 2001;291(5502):293–297. doi: 10.1126/science.291.5502.293. [DOI] [PubMed] [Google Scholar]
- 56.Smith FH, Falsetti AB, Donnelly SM. Modern human origins. Yearbook of Physical Anthropology. 1989;32:35–68. [Google Scholar]
- 57.Trinkaus E. Early modern humans. Annual Review of Anthropology. 2005;34:207–230. [Google Scholar]
- 58.Birdsell JH. The recalibration of a paradigm for the first peolpling of Greater Australia. In: Allen J, Golson J, Jones R, editors. Sunda and Sahul. London, UK: Academic Press; 1967. pp. 113–168. [Google Scholar]
- 59.Oppenheimer S. Out of Eden, the Peopling of the World. London, UK: Constable and Robinson; 2004. [Google Scholar]
- 60.Birdsell JB. The racial origin of the extinct Tasmanians. Records of the Queen Victoria Museum. 1949;2:105–122. [Google Scholar]
- 61.Birdsell JB. Preliminary data on the trihybrid origins of the Australian Aborigines. Archaeology and Physical Anthopology of Oceania. 1967;2:100–155. [Google Scholar]
- 62.Thorne A. Migration and prehistory. In: Arthur W, Morphy H, editors. Macquarie Atlas of Indigenous Australia. Sydney, Australia: Macquarie/Macmillan; 1967. [Google Scholar]
- 63.Groves CP, Lahr MM. A bush not a ladder: speciation and replacement in human evolution. Perspectives in Human Biology. 1994;4:1–11. [Google Scholar]
- 64.Larnach SL. Australian aboriginal craniology. 21. 1978. (The Oceania Monographs). [Google Scholar]
- 65.Antón SC. The natural history of Homo erectus. Yearbook of Physical Anthropology. 2003;46:126–170. doi: 10.1002/ajpa.10399. [DOI] [PubMed] [Google Scholar]
- 66.Kaifu Y, Aziz F, Indriati E, Jacob T, Kurniawan I, Baba H. Cranial morphology of Javanese Homo erectus: new evidence for continuous evolution, specialization, and terminal extinction. Journal of Human Evolution. 2008;55(4):551–580. doi: 10.1016/j.jhevol.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Durband AC. Southeast Asian and Australian paleoanthropology: a review of the last century. Journal of Anthropological Sciences. 2009;87:7–31. [PubMed] [Google Scholar]
- 68.Wright RVS. Evolutionary process and semantics: Australian prehistoric tooth size as a local adjustment. In: Kirk RL, Thorne AG, editors. The Origin of the Australians. Canberra, Australia: Australian Institute of Aboriginal Studies; 1967. pp. 265–276. [Google Scholar]
- 69.Pardoe C. Becoming Australian: evolutionary processes and biological variation from ancient to modern times. Before Farming 2006. article 4, 2006. [Google Scholar]
- 70.Pietrusewsky M. Craniofacial variation in Australasian and Pacific populations. American Journal of Physical Anthropology. 1990;82(3):319–340. doi: 10.1002/ajpa.1330820309. [DOI] [PubMed] [Google Scholar]
- 71.Brown P. Australian Pleistocene variation and the sex of lake Mungo 3. Journal of Human Evolution. 2000;38(5):743–749. doi: 10.1006/jhev.1999.0400. [DOI] [PubMed] [Google Scholar]
- 72.Bulbeck D. Robust and gracile Australian crania: the tale of the Willandra Lakes. In: Simanjuntak T, Handini R, editors. Sangiran: Man, Culture and Environment in Pleistocene Times. Jakarta , Indonesia: Yayasan Obor Indonesia/The National Research Centre of Archaeology/École Francaise d’Extrême-Orient; 2001. pp. 60–106. [Google Scholar]
- 73.Stone T, Cupper ML. Last Glacial Maximum ages for robust humans at Kow Swamp, southern Australia. Journal of Human Evolution. 2003;45(2):99–111. doi: 10.1016/s0047-2484(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 74.Cameron DW, Groves CP. Bones, Stones and Molecules. London, UK: Elsevier; 2004. [Google Scholar]
- 75.Lahr MM. The Evolution of Modern Human Diversity. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- 76.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era—concepts and misconceptions. Nature Reviews Genetics. 2008;9(4):255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 77.Enlow DH, Hans MG. Essentials of Facial Growth. 2nd edition. Ann Arbor, Mich, USA: Needham; 2008. [Google Scholar]
- 78.Pearson OM, Lieberman DE. The aging of Wolff’s "law": ontogeny and responses to mechanical loading in cortical bone. Yearbook of Physical Anthropology. 2004;47:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 79.Sjøvold T. A report on the heritability of some cranial measurements and non-metric traits. In: van Vark GN GN, Howells WW, editors. Multivariate Statistical Methods in Physical Anthropology. Dordrecht, the Netherlands: D. Reidel Publishing; 1984. pp. 223–246. [Google Scholar]
- 80.Sherwood RJ, Duren DL, Demerath EW, Czerwinski SA, Siervogel RM, Towne B. Quantitative genetics of modern human cranial variation. Journal of Human Evolution. 2008;54(6):909–914. doi: 10.1016/j.jhevol.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carson EA. Maximum likelihood estimation of human craniometric heritabilities. American Journal of Physical Anthropology. 2006;131(2):169–180. doi: 10.1002/ajpa.20424. [DOI] [PubMed] [Google Scholar]
- 82.Martínez-Abadías N, Esparza M, Sjøvold T, González-José R, Santos M, Hernández M. Heritability of human cranial dimensions: comparing the evolvability of different cranial regions. Journal of Anatomy. 2009;214(1):19–35. doi: 10.1111/j.1469-7580.2008.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheverud JM. Developmental integration and the evolution of pleiotropy. American Zoologist. 1996;36(1):44–50. [Google Scholar]
- 84.Jamniczky HA, Boughner JC, Rolian C, et al. Rediscovering Waddington in the post-genomic age: operationalising Waddington’s epigenetics reveals new ways to investigate the generation and modulation of phenotypic variation. Bioessays. 2010;32(7):553–558. doi: 10.1002/bies.200900189. [DOI] [PubMed] [Google Scholar]
- 85.Hallgrímsson B, Lieberman DE, Liu W, Ford-Hutchinson AF, Jirik FR. Epigenetic interactions and the structure of phenotypic variation in the cranium. Evolution and Development. 1997;9(1):76–91. doi: 10.1111/j.1525-142X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 86.Klingenberg CP. Evolution and development of shape: integrating quantitative approaches. Nature Reviews Genetics. 2010;11(9):623–635. doi: 10.1038/nrg2829. [DOI] [PubMed] [Google Scholar]
- 87.Lieberman DE, Krovitz GE, Mcbratney-Owen B. Testing hypotheses about tinkering in the fossil record: the case of the human skull. Journal of Experimental Zoology Part B. 2004;302(3):284–301. doi: 10.1002/jez.b.21004. [DOI] [PubMed] [Google Scholar]
- 88.Moss ML. The functional matrix hypothesis revisited. 1. The role of mechanotransduction. American Journal of Orthodontics and Dentofacial Orthopedics. 1997;112(1):8–11. doi: 10.1016/s0889-5406(97)70267-1. [DOI] [PubMed] [Google Scholar]
- 89.Sardi ML. A cross-sectional study of human craniofacial growth. Annals of Human Biology. 2005;32(3):390–396. doi: 10.1080/03014460400027441. [DOI] [PubMed] [Google Scholar]
- 90.Wolff J. The Law of Bone Remodeling. Berlin, Germany: Springer; 1986. translated from the 1892 original, Das Gesetz der Transformation der Knochen, by P. Maquet and R. Furlong. [Google Scholar]
- 91.Hoyte DA, Enlow DH. Wolff’s law and the problem of muscle attachment on resorptive surfaces of bone. American Journal of Physical Anthropology. 1966;24(2):205–213. doi: 10.1002/ajpa.1330240209. [DOI] [PubMed] [Google Scholar]
- 92.Ruff C, Holt B, Trinkaus E. Who’s afraid of the big bad Wolff?: "Wolff’s law" and bone functional adaptation. American Journal of Physical Anthropology. 2006;129(4):484–498. doi: 10.1002/ajpa.20371. [DOI] [PubMed] [Google Scholar]
- 93.Coon CS, Garn SM, Birdsell JB. Races: A Study of the Problems of Race Formation in Man. Springfield, Ill, USA: Charles C. Thomas; 1950. [Google Scholar]
- 94.Carey JW, Steegmann ATAT. Human nasal protrusion, latitude, and climate. American Journal of Physical Anthropology. 1981;56(3):313–319. doi: 10.1002/ajpa.1330560312. [DOI] [PubMed] [Google Scholar]
- 95.Franciscus RG, Long JC. Variation in human nasal height and breadth. American Journal of Physical Anthropology. 1991;85(4):419–427. doi: 10.1002/ajpa.1330850406. [DOI] [PubMed] [Google Scholar]
- 96.Roseman CC. Detecting interregionally diversifying natural selection on modern human cranial form by using matched molecular and morphometric data. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(35):12824–12829. doi: 10.1073/pnas.0402637101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roseman CC, Weaver TD. Multivariate apportionment of global human craniometric diversity. American Journal of Physical Anthropology. 2004;125(3):257–263. doi: 10.1002/ajpa.10424. [DOI] [PubMed] [Google Scholar]
- 98.Nicholson E, Harvati K. Quantitative analysis of human mandibular shape using three-dimensional geometric morphometrics. American Journal of Physical Anthropology. 2006;131(3):368–383. doi: 10.1002/ajpa.20425. [DOI] [PubMed] [Google Scholar]
- 99.Beals KL, Smith CL, Dodd SM. Climate and the evolution of brachycephalization. American Journal of Physical Anthropology. 1983;62(4):425–437. doi: 10.1002/ajpa.1330620407. [DOI] [PubMed] [Google Scholar]
- 100.Hublin J-J. Climatic changes, paleogeography, and the evolution of the Neandertals. In: Akazawa T, Aoki K, Bar-Yosef O, editors. Neandertals and Modern Humans in Western Asia. New York, NY, USA: Plenum Press; 1998. pp. 295–310. [Google Scholar]
- 101.Hylander WL. The adaptive significance of Eskimo craniofacial morphology. In: Dahlberg AA, Graber TM, editors. Orofacial Growth and Development. Chicago, Ill, USA: Mouton; 1977. pp. 129–170. [Google Scholar]
- 102.Skelton RR. Evolutionary relationships among early hominids. Journal of Human Evolution. 1992;23(4):309–349. [Google Scholar]
- 103.Lieberman DE, Krovitz GE, Yates FW, Devlin M, St. Claire M. M. Effects of food processing on masticatory strain and craniofacial growth in a retrognathic face. Journal of Human Evolution. 2004;46(6):655–677. doi: 10.1016/j.jhevol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 104.Sardi ML, Novellino PS, Pucciarelli HM. Craniofacial morphology in the Argentine Center-West: consequences of the transition to food production. American Journal of Physical Anthropology. 2006;130(3):333–343. doi: 10.1002/ajpa.20379. [DOI] [PubMed] [Google Scholar]
- 105.Harvati K, Weaver TD. Human cranial anatomy and the differential preservation of population history and climate signatures. Anatomical Record A. 2006;288(12):1225–1233. doi: 10.1002/ar.a.20395. [DOI] [PubMed] [Google Scholar]
- 106.Mitteroecker P, Bookstein F. The evolutionary role of modularity and integration in the hominoid cranium. Evolution. 2008;62(4):943–958. doi: 10.1111/j.1558-5646.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- 107.Goswami A. Cranial modularity shifts during mammalian evolution. American Naturalist. 2006;168(2):270–280. doi: 10.1086/505758. [DOI] [PubMed] [Google Scholar]
- 108.Olson EC, Miller RL. Morphological Integration. Chicago, Ill, USA: University of Chicago Press; 1958. [Google Scholar]
- 109.Cheverud JM. Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution. 1982;36:499–516. doi: 10.1111/j.1558-5646.1982.tb05070.x. [DOI] [PubMed] [Google Scholar]
- 110.Cheverud JM. Quantitative genetics and developmental constraints on evolution by selection. Journal of Theoretical Biology. 1984;110(2):155–171. doi: 10.1016/s0022-5193(84)80050-8. [DOI] [PubMed] [Google Scholar]
- 111.Cheverud JM. Morphological integration in the saddle-back tamarin (Saguinus fuscicollis) cranium. American Naturalist. 1995;145(1):63–89. [Google Scholar]
- 112.Carlson BM. Human Embryology and Developmental Biology. Philadelphia, Pa, USA: Mosby Elsevier; 1999. [Google Scholar]
- 113.Schilling TF, Thorogood P. Development and evolution of the vertebrate skull. In: O’Higgins P, Cohn M, editors. Development, Growth and Evolution. Implications for the Study of the Hominid Skeleton. London, UK: Academic Press; 2001. pp. 57–83. [Google Scholar]
- 114.Sperber GH. Craniofacial Development. London, UK: BC Decker; 2001. [Google Scholar]
- 115.Enlow DH. Facial Growth. 3rd edition. Philadelphia, Pa, USA: W. B. Saunders; 1990. [Google Scholar]
- 116.Kohn LPA. The role of genetics in craniofacial morphology and growth. Annual Review of Anthropology. 1991;20:261–278. [Google Scholar]
- 117.Siebert JR, Swindler DR. Evolutionary changes in the midface and mandible: establishing the primate form. In: Mooney MP, Siegel MI, editors. Understanding Craniofacial Anomalies. The Ethiopathogenesis of Craniosynostoses and Facial Clefting. New York, NY, USA: Wiley-Liss; 2002. pp. 345–376. [Google Scholar]
- 118.Vioarsdóttir US, O’Higgins P, Stringer C. A geometric morphometric study of regional differences in the ontogeny of the modern human facial skeleton. Journal of Anatomy. 2002;201(3):211–229. doi: 10.1046/j.1469-7580.2002.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bastir M, Rosas A. Facial heights: evolutionary relevance of postnatal ontogeny for facial orientation and skull morphology in humans and chimpanzees. Journal of Human Evolution. 2004;47(5):359–381. doi: 10.1016/j.jhevol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 120.Ackermann RR, Cheverud JM. Morphological integration in primate evolution. In: Pigliucci M, Preston K, editors. Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes. Oxford, UK: Oxford University Press; 2004. pp. 302–319. [Google Scholar]
- 121.Larsen CS. Bioarcheology: Interpreting Behavior from the Human Skeleton. New York, NY, USA: Cambridge University Press; 1997. [Google Scholar]
- 122.Lieberman DE, Pearson OM, Mowbray KM. Basicranial influence on overall cranial shape. Journal of Human Evolution. 2000;38(2):291–315. doi: 10.1006/jhev.1999.0335. [DOI] [PubMed] [Google Scholar]
- 123.Lieberman DE, Ross CF, Ravosa MJ. The primate cranial base: ontogeny, function, and integration. Yearbook of Physical Anthropology. 2000;43:117–169. doi: 10.1002/1096-8644(2000)43:31+<117::aid-ajpa5>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 124.Ackermann RR. Patterns of covariation in the hominoid craniofacial skeleton: implications for paleoantropological models. Journal of Human Evolution. 2002;43(2):167–187. doi: 10.1006/jhev.2002.0569. [DOI] [PubMed] [Google Scholar]
- 125.Ackermann RR. Ontogenetic integration of the hominoid face. Journal of Human Evolution. 2005;48(2):175–197. doi: 10.1016/j.jhevol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 126.Morriss-Kay GM. Derivation of the mammalian skull vault. Journal of Anatomy. 2001;199(1-2):143–151. doi: 10.1046/j.1469-7580.2001.19910143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zollikofer CPE, Ponce de León MS. Visualizing patterns of craniofacial shape variation in Homo sapiens. Proceedings of the Royal Society B. 2002;269(1493):801–807. doi: 10.1098/rspb.2002.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O’Higgins P, Jones N. Facial growth in Cercocebus torquatus: an application of three-dimensional geometric morphometric techniques to the study of morphological variation. Journal of Anatomy. 1998;193(2):251–272. doi: 10.1046/j.1469-7580.1998.19320251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Neubauer S, Gunz P, Hublin J-J. Endocranial shape changes during growth in chimpanzees and humans: a morphometric analysis of unique and shared aspects. Journal of Human Evolution. 2010;59(5):555–566. doi: 10.1016/j.jhevol.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 130.Bastir M. A systems-model for the morphological analysis of integration and modularity in human craniofacial evolution. Journal of Anthropological Sciences. 2008;86:37–58. [PubMed] [Google Scholar]
- 131.Ross CF, Ravosa MJ. Basicranial flexion, relative brain size, and facial kyphosis in nonhuman primates. American Journal of Physical Anthropology. 1993;91(3):305–324. doi: 10.1002/ajpa.1330910306. [DOI] [PubMed] [Google Scholar]
- 132.Ross C, Henneberg M. Basicranial flexion, relative brain size, and facial kyphosis in Homo sapiens and some fossil hominids. American Journal of Physical Anthropology. 1995;98(4):575–593. doi: 10.1002/ajpa.1330980413. [DOI] [PubMed] [Google Scholar]
- 133.Spoor F, O’Higgins P, Dean C, Lieberman DE. Anterior sphenoid in modern humans. Nature. 1999;18:p. 72. doi: 10.1038/17505. [DOI] [PubMed] [Google Scholar]
- 134.Lieberman DE. Shortening and the evolution of modern human cranial shape. Nature. 1998;14:158–162. doi: 10.1038/30227. [DOI] [PubMed] [Google Scholar]
- 135.Lieberman DE. Ontogeny, homology, and phylogeny in the hominid craniofacial skeleton: the problem of the browridge. In: O’Higgins P, Cohn M, editors. Development, Growth and Evolution: Implications for the Study of Hominid Skeletal Evolution. London, UK: Academic Press; 2002. pp. 85–122. [Google Scholar]
- 136.Lieberman DE, McCarthy RC. The ontogeny of cranial base angulation in humans and chimpanzees and its implications for reconstructing pharyngeal dimensions. Journal of Human Evolution. 1999;36(5):487–517. doi: 10.1006/jhev.1998.0287. [DOI] [PubMed] [Google Scholar]
- 137.Lieberman DE, McBratney BM, Krovitz G. The evolution and development of cranial form in Homo sapiens. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(3):1134–1139. doi: 10.1073/pnas.022440799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jeffery N, Spoor F. Brain size and the human cranial base: a prenatal perspective. American Journal of Physical Anthropology. 2002;118(4):324–340. doi: 10.1002/ajpa.10040. [DOI] [PubMed] [Google Scholar]
- 139.Ross CF, Henneberg M, Ravosa MJ, Richard S. Curvilinear, geometric and phylogenetic modeling of basicranial flexion: is it adaptive, is it constrained? Journal of Human Evolution. 2004;46(2):185–213. doi: 10.1016/j.jhevol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 140.Bastir M, Rosas A, Lieberman DE, O’Higgins P. Middle cranial fossa anatomy and the origin of modern humans. Anatomical Record. 2008;291(2):130–140. doi: 10.1002/ar.20636. [DOI] [PubMed] [Google Scholar]
- 141.Bastir M, Rosas A, Stringer C, et al. Effects of brain and facial size on basicranial form in human and primate evolution. Journal of Human Evolution. 2010;58(5):424–431. doi: 10.1016/j.jhevol.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 142.Bastir M, Rosas A. Hierarchical nature of morphological integration and modularity in the human posterior face. American Journal of Physical Anthropology. 2005;128(1):26–34. doi: 10.1002/ajpa.20191. [DOI] [PubMed] [Google Scholar]
- 143.Bastir M, Rosas A. Correlated variation between the lateral basicranium and the face: a geometric morphometric study in different human groups. Archives of Oral Biology. 2006;51(9):814–824. doi: 10.1016/j.archoralbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 144.Bastir M, Rosas A. Mosaic evolution of the basicranium in Homo and its relation to modular development. Evolutionary Biology. 2009;36(1):57–70. [Google Scholar]
- 145.Howells WW. Physical variation and history in Melanesia and Australia. American Journal of Physical Anthropology. 1976;45(3):641–649. doi: 10.1002/ajpa.1330450330. [DOI] [PubMed] [Google Scholar]
- 146.Brothwell D. Possible evidence of a cultural practice affecting head growth in some late pleistocene east Asian and Australasian populations. Journal of Archaeological Science. 1975;2(1):75–77. [Google Scholar]
- 147.Brown P. Artificial cranial deformation: a component in the variation in Pleistocene Australian Aboriginal crania. Archaeology in Oceania. 1981;16:156–167. [Google Scholar]
- 148.Brown P. Nacurrie 1: mark of ancient Java, or a caring mother’s hands, in terminal Pleistocene Australia? Journal of Human Evolution. 2010;59:168–187. doi: 10.1016/j.jhevol.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 149.Anton SC, Weinstein KJ. Artificial cranial deformation and fossil Australians revisited. Journal of Human Evolution. 1999;36(2):195–209. doi: 10.1006/jhev.1998.0266. [DOI] [PubMed] [Google Scholar]
- 150.Clark JL, Dobson SD, Antón SC, Hawks J, Hunley KL, Wolpoff MH. Identifying artificially deformed crania. International Journal of Osteoarchaeology. 2007;17(6):596–607. [Google Scholar]
- 151.Curnoe D, Thorne A. Human origins in Australia: the skeletal evidence. Before Farming 2006. article 5, 2006. [Google Scholar]
- 152.Webb S. Cranial thickening in an Australian Hominid as a possible palaeoepidemiological indicator. American Journal of Physical Anthropology. 1990;82(4):403–411. doi: 10.1002/ajpa.1330820402. [DOI] [PubMed] [Google Scholar]
- 153.Webb SG. Paleopathology of Aboriginal Australians. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- 154.Stuart-Macadam P. Cranial thickening and anemia: reply to Dr. Webb. American Journal of Physical Anthropology. 1992;88(1):109–110. doi: 10.1002/ajpa.1330880110. [DOI] [PubMed] [Google Scholar]
- 155.Westaway MC. The Pleistocene human remains collection from the Willandra Lakes World Heritage Area, Australia, and its role in understanding modern human origins. In: Tomida Y, editor. In: Proceedings of the 7th and 8th Symposia on Collection Building and Natural History Studies in Asia and the Pacific Rim, vol. 34; 2006; Tokyo, Japan. National Science Museum Monographs; pp. 127–138. [Google Scholar]
- 156.Lahr MM, Wright RVS. The question of robusticity and the relationship between cranial size and shape in Homo sapiens. Journal of Human Evolution. 1996;31(2):157–191. [Google Scholar]
- 157.Stuart-Macadam P. A radiographic study of porotic hyperostosis. American Journal of Physical Anthropology. 1987;74(4):511–520. doi: 10.1002/ajpa.1330740409. [DOI] [PubMed] [Google Scholar]
- 158.Bräuer G, Collard M, Stringer C. On the reliability of recent tests of the out of Africa hypothesis for modern human origins. Anatomical Record A. 2004;279A(2):701–707. doi: 10.1002/ar.a.20064. [DOI] [PubMed] [Google Scholar]
- 159.Weidenreich F. The brain and its role in the phylogenetic transformation of the human skull. Transactions of the American Philosophical Society. 1941;31:320–442. [Google Scholar]
- 160.Washburn SL. The relation of the temporal muscle to the form of the skull. The Anatomical Record. 1947;99:239–248. doi: 10.1002/ar.1090990303. [DOI] [PubMed] [Google Scholar]
- 161.Enlow DH. Principles of Bone Remodeling. Springfield, Ill, USA: Charles C. Thomas; 1963. [Google Scholar]
- 162.Moss ML, Young RW. A functional approach to craniology. American journal of physical anthropology. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- 163.Ravosa MJ. Ontogenetic perspective on mechanical and nonmechanical models of primate circumorbital morphology. American Journal of Physical Anthropology. 1991;85(1):95–112. doi: 10.1002/ajpa.1330850111. [DOI] [PubMed] [Google Scholar]
- 164.Hylander WL, Picq PG, Johnson KR. Function of the supraorbital region of primates. Archives of Oral Biology. 1991;36(4):273–281. doi: 10.1016/0003-9969(91)90097-e. [DOI] [PubMed] [Google Scholar]
- 165.Wroe S, Moreno K, Clausen P, Mchenry C, Curnoe D. High-resolution three-dimensional computer simulation of hominid cranial mechanics. Anatomical Record. 2007;290(10):1248–1255. doi: 10.1002/ar.20594. [DOI] [PubMed] [Google Scholar]
- 166.Wroe S, Ferrara TL, McHenry CR, Curnoe D, Chamoli U. The craniomandibular mechanics of being human. Proceedings of the Royal Society B. 2010;277(1700):3579–3586. doi: 10.1098/rspb.2010.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kupczik K, Dobson CA, Crompton RH, et al. Masticatory loading and bone adaptation in the supraorbital torus of developing macaques. American Journal of Physical Anthropology. 2009;139(2):193–203. doi: 10.1002/ajpa.20972. [DOI] [PubMed] [Google Scholar]
- 168.Endo B. Experimental studies on the mechanical significance of the form of the human facial skeleton. Journal of the Faculty of Science, University of Tokyo, Section V, Anthropology. 1966;3:1–106. [Google Scholar]
- 169.Shea BT. On aspects of skull form in African apes and orangutans, with implications for hominoid evolution. American Journal of Physical Anthropology. 1985;68(3):329–342. doi: 10.1002/ajpa.1330680304. [DOI] [PubMed] [Google Scholar]
- 170.Lieberman DE. Speculations about the selective basis for modern human craniofacial form. Evolutionary Anthropology. 2008;17(1):55–68. [Google Scholar]
- 171.Weijs WA, Hillen B. Relationships between masticatory muscle cross-section and skull shape. Journal of Dental Research. 1984;63(9):1154–1157. doi: 10.1177/00220345840630091201. [DOI] [PubMed] [Google Scholar]
- 172.Churchill SE. Cold adaptation, heterochrony, and Neandertals. Evolutionary Anthropology. 1998;7(2):46–61. [Google Scholar]
- 173.Lieberman DE. How and why humans grow thin skulls: experimental evidence for systemic cortical robusticity. American Journal of Physical Anthropology. 1996;101(2):217–236. doi: 10.1002/(SICI)1096-8644(199610)101:2<217::AID-AJPA7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 174.Weijs WA, Hillen B. Correlations between the cross-sectional area of the jaw muscles and craniofacial size and shape. American Journal of Physical Anthropology. 1986;70(4):423–431. doi: 10.1002/ajpa.1330700403. [DOI] [PubMed] [Google Scholar]
- 175.Raadsheer MC, Kiliaridis S, Van Eijden TMGJ, Van Ginkel FC, Prahl-Andersen B. Masseter muscle thickness in growing individuals and its relation to facial morphology. Archives of Oral Biology. 1996;41(4):323–332. doi: 10.1016/0003-9969(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 176.Raadsheer MC, Van Eijden TMGJ, Van Ginkel FC, Prahl-Andersen B. Contribution of jaw muscle size and craniofacial morphology to human bite force magnitude. Journal of Dental Research. 1999;78(1):31–42. doi: 10.1177/00220345990780010301. [DOI] [PubMed] [Google Scholar]
- 177.Van Spronsen PH, Koolstra JH, Van Ginkel FC, Weijs WA, Valk J, Prahl-Andersen B. Relationships between the orientation and moment arms of the human jaw muscles and normal craniofacial morphology. European Journal of Orthodontics. 1997;19(3):313–328. doi: 10.1093/ejo/19.3.313. [DOI] [PubMed] [Google Scholar]
- 178.Şatiroğlu F, Arun T, Işik F. Comparative data on facial morphology and muscle thickness using ultrasonography. European Journal of Orthodontics. 2005;27(6):562–567. doi: 10.1093/ejo/cji052. [DOI] [PubMed] [Google Scholar]
- 179.White TD, Asfaw B, DeGusta D, et al. Pleistocene Homo sapiens from Middle Awash, Ethiopia. Nature. 2003;423(6941):742–747. doi: 10.1038/nature01669. [DOI] [PubMed] [Google Scholar]
- 180.Trinkaus E, LeMay M. Occipital bunning among later Pleistocene hominids. American Journal of Physical Anthropology. 1982;57(1):27–35. doi: 10.1002/ajpa.1330570106. [DOI] [PubMed] [Google Scholar]
- 181.Lieberman DE. Testing hypotheses about recent human evolution from skulls: integrating morphology, function, phylogeny and development. Current Anthropology. 1995;36:159–197. [Google Scholar]
- 182.Baab KL, Freidline SE, Wang SL, Hanson T. Relationship of cranial robusticity to cranial form, geography and climate in Homo sapiens. American Journal of Physical Anthropology. 2010;141(1):97–115. doi: 10.1002/ajpa.21120. [DOI] [PubMed] [Google Scholar]
- 183.Perez SI, Bernal V, Gonzalez PN. Morphological differentiation of aboriginal human populations from Tierra del Fuego (Patagonia): implications for South American peopling. American Journal of Physical Anthropology. 2007;133(4):1067–1079. doi: 10.1002/ajpa.20633. [DOI] [PubMed] [Google Scholar]
- 184.Cartmill M, Smith FH. The Human Lineage. Hoboken, NJ, USA: Wiley-Blackwell; 2009. [Google Scholar]
- 185.Caspari R. The evolution of the posterior cranial vault in the central European Upper Pleistocene. University of Michigan; 1991. Ph.D. thesis. [Google Scholar]
- 186.Bruner E, Ripani M. A quantitative and descriptive approach to morphological variation of the endocranial base in modern humans. American Journal of Physical Anthropology. 2008;137(1):30–40. doi: 10.1002/ajpa.20837. [DOI] [PubMed] [Google Scholar]
- 187.Bastir M, Rosas A, Lieberman DE, O’Higgins P. Middle cranial fossa anatomy and the origin of modern humans. Anatomical Record. 2008;291(2):130–140. doi: 10.1002/ar.20636. [DOI] [PubMed] [Google Scholar]
- 188.Herring SW, Teng S. Strain in the braincase and its sutures during function. American Journal of Physical Anthropology. 2000;112(4):575–593. doi: 10.1002/1096-8644(200008)112:4<575::AID-AJPA10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Behrents RG, Carlson DS, Abdelnour T. In vivo analysis of bone strain about the sagittal suture in Macaca mulatta during masticatory movements. Journal of Dental Research. 1978;57(9-10):904–908. doi: 10.1177/00220345780570091401. [DOI] [PubMed] [Google Scholar]
- 190.Sun Z, Lee E, Herring SW. Cranial sutures and bones: growth and fusion in relation to masticatory strain. Anatomical Record A. 2004;276(2):150–161. doi: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rayfield EJ. Finite element analysis and understanding the biomechanics and evolution of living and fossil organisms. Annual Review of Earth and Planetary Sciences. 2007;35:541–576. [Google Scholar]
- 192.Williams H. Lumps, bumps and funny shaped heads. Archives of Disease in Childhood: Education and Practice Edition. 2008;93(4):120–128. doi: 10.1136/adc.2008.145532. [DOI] [PubMed] [Google Scholar]
- 193.Huang MHS, Mouradian WE, Cohen SR, Gruss JS. The differential diagnosis of abnormal head shapes: separating craniosynostosis from positional deformities and normal variants. Cleft Palate-Craniofacial Journal. 1998;35(3):204–211. doi: 10.1597/1545-1569_1998_035_0204_tddoah_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 194.Morriss-Kay GM, Wilkie AOM. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. Journal of Anatomy. 2005;207(5):637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aleck K. Craniosynostosis syndromes in the genomic era. Seminars in Pediatric Neurology. 2004;11(4):256–261. doi: 10.1016/j.spen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 196.White CD. Sutural effects of fronto-occipital cranial modification. American Journal of Physical Anthropology. 1996;100:397–410. doi: 10.1002/(SICI)1096-8644(199607)100:3<397::AID-AJPA7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 197.Lieberman DE. Homology and hominid phylogeny: problems and potential solutions. Evolutionary Anthropology. 1999;7(4):142–151. [Google Scholar]
- 198.Bruner E, Holloway RL. A bivariate approach to the widening of the frontal lobes in the genus Homo. Journal of Human Evolution. 2010;58(2):138–146. doi: 10.1016/j.jhevol.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 199.Bruner E. Cranial shape and size variation in human evolution: structural and functional perspectives. Child’s Nervous System. 2007;23(12):1357–1365. doi: 10.1007/s00381-007-0434-2. [DOI] [PubMed] [Google Scholar]