Abstract
With increasing access to antiretroviral therapy for children infected with HIV, especially in sub-Saharan Africa, better understanding of the development and maintenance of memory T- and B-cell responses to pathogens after immune reconstitution is needed to assess the risk of infection. Knowledge of long-term immune responses after starting HAART is of particular importance for policies on revaccination of HIV-infected children, who may lose protective immunity to prior infections and immunizations. We review normal development of T- and B-cell memory responses to viruses and vaccines against viral pathogens, and contrast the immunological effects of perinatal HIV transmission with HIV infection acquired later in life. We then explore the potential benefits of antiretroviral therapy and revaccination, using measles virus as a model.
Keywords: antiretroviral therapy, children, HIV, immunological memory, vaccines
An estimated 33 million individuals are infected with HIV worldwide and two-thirds of this burden is in sub-Saharan Africa [201]. Of the estimated two million HIV-infected children, 90% reside in sub-Saharan Africa, where high rates of morbidity and mortality occur due to poor nutrition, limited access to healthcare and high risk of exposure to pathogens [1,2, 201]. Regardless of geographical location, untreated HIV infection reduces the capacity of a child’s immune system to respond to infections and vaccines, resulting in high mortality rates in early childhood. Recently, access to HAART has increased due to funding from the US President’s Emergency Plan for AIDS Relief (PEPFAR), the Global Fund to Fight AIDS, Tuberculosis, and Malaria, and several other sources [202,203]. As of September 2009, PEPFAR alone supplied HAART to almost 2.5 million individuals [203]. Children have benefited from the rapid scale-up of HAART, with several studies reporting increased survival rates among HAART-treated children [3]. As mortality due to HIV-related conditions diminishes, an increasing number of children will be living with HIV, necessitating an increased understanding of how HAART affects immunity to vaccine-preventable infections.
Infection with HIV produces dysfunctional humoral and cellular immunity, resulting in an inability to mount effective immune responses after antigen exposure through vaccination or infection. With the initiation of HAART, aberrant immune stimulation declines in response to decreasing HIV viral load, and naive and memory B- and T-cell populations reconstitute. The magnitude and quality of immune reconstitution determine the risk of infection in persons receiving HAART but may not ensure protection against vaccine antigens received prior to treatment. To better understand the immunological basis for revaccination of HIV-infected children receiving HAART, we review normal development of immunological memory with an emphasis on children, the effects of HIV and HAART on memory T and B cells, and the potential public health impact on vaccine-preventable viral infections. We then describe immune responses to measles vaccination as a model system for elucidating immune memory dysfunction and reconstitution in HIV-infected children.
Normal development of immunological memory responses to viral antigens
T cells
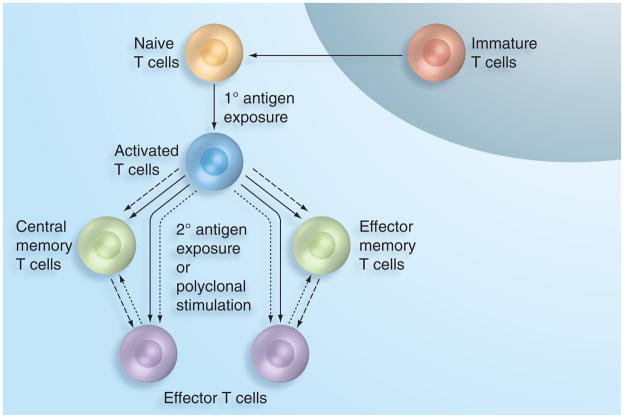
Naive T cells emigrate from the thymus to the peripheral circulation, express the cell surface marker CD45RA and are activated via interaction with viral peptides displayed by MHC molecules on antigen-presenting cells [4,5]. Activated cells develop into either memory phenotypes, which expand upon subsequent antigen exposure, or effector cells that perform immediate protective functions such as stimulating further lymphocyte activation and proliferation or inducing cytolysis of virus-infected cells. The pathways of memory T-cell development are currently a subject of intense research as varying hypotheses have been proposed (Figure 1). One study concluded that two memory subsets, effector memory and central memory, develop independently and are distinguished by expression of the lymph node-homing marker CCR7 [6]. Another indicated that a single naive cell may give rise to both effector and memory daughter cells, suggesting that the CCR7 phenotype may not be predetermined [7]. Other studies suggest that T cells transition through an effector phase before developing into a memory phenotype [8–10]. These hypotheses may not be mutually exclusive; for example, effector memory cells may develop through an effector stage while central memory cells may be produced in concert with effectors. Nevertheless, CCR7 remains an established marker for differentiating central memory (CCR7+) from effector memory (CCR7−) cells.
Figure 1. Three proposed T-cell differentiation pathways.
Effector cell development via memory phenotypes (dashed arrows), memory cell development via effector phenotypes (dotted arrows), or independent memory and effector cell development (solid arrows).
Among individuals as old as 18 years of age, T cells (CD3+) constitute approximately 65–73% of the peripheral blood lymphocyte pool [11], but the proportion of phenotypic subsets among the total T-cell population varies between individuals and with age [12–14]. The proportions of CCR7+CD45RA+ naive T cells diminish with age (Table 1), suggesting differentiation and immune maturation [11,15–17], as well as decreased T-cell emigration from the thymus. A high proportion of naive T cells were observed among 6–12-month-old infants, with medians of 83% and 72% of the CD4+ and CD8+ T-cell populations, respectively, which subsequently decreased with age [11]. Levels of recent thymic emigrants, as measured by T-cell receptor excision circles (TRECs), also decreased with increasing age [18,19]. A model of naive T-cell emigration from the thymus using TRECs and Ki67, markers of T-cell turnover and proliferation, from infancy to young adulthood demonstrated that thymopoesis occurs at a very rapid rate from birth to 1 year of age, decreases profoundly until 8 years old, and then declines more slowly until 20 years of age [20], supporting reports that thymopoesis declines in a biphasic manner over the lifespan with an increasing rate of decline occurring before middle age followed by a slower rate of decline for the remainder of life [19].
Table 1.
The effects of age, HIV infection and HAART initiation stratified by time of infection (perinatal vs adolescent or adult infection) on T- and B-cell subsets.
| Cell subset | Effect of age | Effect of HIV | Effect of HAART | |
|---|---|---|---|---|
| Perinatal infection | Adolescent/adult infection | |||
| T cells (CD3+) | ||||
| Naive (CD45RA+CCR7+) | ↓ [14] | ↓ [52,53] | ↑ [78,82,83] | ↔/↑ [80,84] |
| Central memory (CD45RA+CCR7−) | ↑ [19,20,24] | ↓ [52] | ↔/↑ CD4+† [78,82] ↔/↓ CD8+† [78,82] |
↑ [84] |
| Effector memory (CD45RA−CCR7+) | ↑ [19,20,24] | ↑ [53] | ↔/↑ CD4+;† [78,82] ↔/↓ CD8+† [78,82] |
? |
| Effector (CD45RA−CCR7−) | ↔/↑ [19,24] | ↑ [53] | ? | ↓ [84] |
| B cells (CD19+CD20+) | ||||
| Naive (CD21+CD27−) | ↔/↓ [29,31,35] | ↑ [34,35] | ? | ↔/↑ [31,91] |
| Resting memory (CD21+CD27+) | ↑ [28,29,31,34,36] | ↓ [29] | ↑ [35,93] | ↑ [31,91,92] |
Arrows indicate the change in each cell subset as an increase (↑), decrease (↓), no change (↔) or conflicting evidence (↔/↓ or ↔/↑).
Memory T-cell subsets were not evaluated based on CCR7 expression and were classified as total CD45RO+ memory cells.
In contrast to naive T cells, the proportion of CCR7+CD45RA−, or central memory T cells, within the T-cell pool increases with age (Table 1). Few studies have assessed this T-cell subset in healthy individuals over broad age ranges, especially among young children, but induction of CD8+ central memory T cells are particularly indicative of successful vaccination against viral pathogens [21]. Ono et al. measured T-cell subsets in cord blood and peripheral blood of 12-month old infants, and Saule et al. measured T-cell subsets in 5–96-year-old individuals [16,22]. Central memory T cells represented approximately 9–16% of CD4+ T cells and 5% of CD8+ T cells in cord blood and peripheral blood during the first year of life [22]. These proportions remained relatively stable through 10 years of age but increased to adulthood [16], indicating an increased capacity for memory responses to antigenic challenge.
Although a majority of effector memory (CCR7−CD45RA+) T cells remain in lymphoid tissue [6], an increasing proportion of these cells are detected in the peripheral circulation with age. However, the magnitude of the change across different ages is not consistent among studies. While Ono et al. observed effector memory proportions of 3% for CD4+ T cells and 4% for CD8+ T cells in cord blood rising to 10% and 24% at 12-months of age, respectively, Saule et al. observed lower levels of CD8+ cells in 5–10-year-olds and 10–20-year-olds (10–90th percentiles of 4–14% and 5–19%, respectively). Nevertheless, an increasing trend with age was observed, with 20–40-year-old adults having 19% of CD4+ and 17% of CD8+ effector memory cells in peripheral circulation [16,22].
Finally, CD4+ effector T cells, or terminally differentiated (CCR7−CD45RA−) cells, remain relatively stable throughout the lifespan at levels of approximately 2–5% in peripheral blood. By contrast, CD8+ effector cells increase with age from around 12% in cord blood to 18% in 5–10-year-old children, increasing in adulthood to approximately 40% [16,22]. In summary, the T-cell pool matures over the lifespan, resulting in a substantial reduction in the proportion of naive T cells with concomitant increases in central memory, effector memory and CD8+ effector subsets that react and proliferate in response to antigenic challenge.
B cells
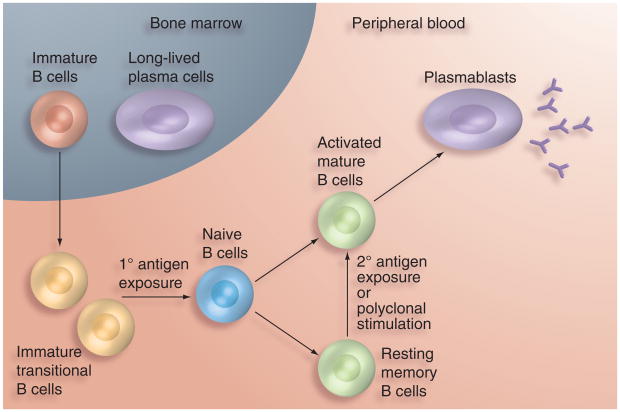
Broadly, the B-cell population is composed of immature, immature transitional, naive mature, activated mature and resting memory B cells, as well as terminally differentiated antibody-secreting plasmablasts and plasma cells (Figure 2). Upon primary antigen exposure, immature transitional B cells undergo rapid transformation, resulting in three subpopulations of highly specific memory B cells – resting memory B cells, plasmablasts and long-lived plasma cells [23] – and few data are available on normal ranges of B-cell subsets in healthy individuals, particularly young children. B cells are typically defined by the expression of CD19 or CD20 [23] and make up only 15–25% of the circulating lymphocyte pool in children and adolescents [11]. Interaction between the CD154 (CD40L) molecule on T cells and CD40 expressed by activated B cells induces the differentiation and expansion of naive B cells into memory B cells [24]. Resting memory B cells constitute 1–10% of the total B-cell population in the peripheral blood of children younger than 12 months of age and 19–42% in healthy adults (Table 1) [25–31], and are capable of generating a rapid anamnestic response upon re-exposure to cognate antigens [32].
Figure 2. B-cell differentiation pathway.
As immature B cells emigrate from the bone marrow, antigen exposure yields either memory B cells or plasmablasts. Plasma cells return to the bone marrow and secrete low levels of antibody.
Memory B cells are commonly defined by expression of the CD27 surface molecule [33,34], which binds CD70 on activated T cells and initiates B-cell terminal differentiation into plasma cells [35]. Circulating plasma cells downregulate CD20 but further upregulate CD27 and CD38 expression, creating a distinct subset of CD19+CD20− CD21LOCD27++CD38++ cells [36]. As plasmablasts and plasma cells migrate through peripheral blood from germinal centers to the bone marrow, tonsils and other sites of sequestration [37], they constitute only 0.14% (range: 0.03–2.39%) of circulating peripheral blood mononuclear cells among healthy adults. This proportion increased to 0.3–0.8% after vaccination with trivalent influenza vaccine among healthy adults and, at its peak, vaccine-specific antibody-secreting cells represented 63% of the total IgG secreting cells as measured by enzyme-linked immunospot assay [38]. Few studies assessed whether antibody-secreting cell proportions differ significantly between children and adults, particularly after vaccination. Among healthy controls participating in a study of systemic lupus erythematosus (SLE), no differences were observed in the proportions of circulating plasma cell proportions between 14 adults and 14 5–16-year-old children [39].
Long-term serological memory relies on production of pathogen-specific antibodies and is dependent on the development and maintenance of memory B-cell and plasma cell populations [40]. Maintenance of protective antibody levels in healthy individuals was shown to be a function of three mechanisms: first, transient, antigen-dependent stimulation of memory B cells following re-exposure to cognate antigens, resulting in rapid proliferation and differentiation of antibody-secreting plasma cells; second, ongoing, antigen-independent B-cell expansion due to polyclonal stimuli resulting in continual, low-level proliferation and differentiation of plasmablasts and; third, constant low-level production of antibodies by long-lived plasma cells residing in the bone marrow [41].
The dominant mechanism by which antibodies are produced appears to depend on the antigen, as indicated by heterogeneities in the quality, magnitude and duration of humoral immune responses [42]. Longer antibody half-lives were demonstrated in response to acute viral infections like measles and rubella viruses compared with nonreplicating proteins such as diphtheria and tetanus toxoids [42]. In a longitudinal study of patients with SLE treated with rituximab, which depletes circulating CD20+ B cells but does not affect cells in the bone marrow, antibodies to measles virus remained relatively stable after treatment but antibodies to tetanus toxoid declined in most patients [43]. These findings suggest that a large proportion of antibodies to measles virus may be produced by long-lived plasma cells residing in the bone marrow, whereas antibodies to tetanus toxoid may require continual, low-level B-cell differentiation. Other viral infections, such as influenza and smallpox viruses, also induce long-lived protective antibody responses as indicated by the detection of antibodies to the 1918 influenza strain among individuals born before 1916 [44] and the correlations between IgG antibody levels against vaccinia virus and neutralization capabilities [32]. Thus, resting memory B cells, plasmablasts, long-lived plasma cells and pathogen-specific IgG antibodies represent critical aspects of the humoral immune response to infection and are responsible for long-term protective immunity following infection and vaccination.
Effects of HIV on T & B cells & serologic memory
T cells
The distribution of T-cell subsets is disrupted by HIV infection through decreased emigration of naive cells, increased immune activation and exhaustion, and increased cellular turnover, all of which must be considered in the context of age at HIV exposure and infection (Table 1). In a cohort of 20 Italian children with a median age of 9.8 years, those with more severe disease had a significantly lower median proportion of naive CD4+ T cells (9.3%) compared with children with less severe disease (57.8%) [45]. As the thymus involutes with age and naive T-cell production decreases [46,47], HIV may further accelerate the decreasing rates of naive T-cell emigration. Thymic volumes in 18–30-year-old HIV-infected adults were smaller in comparison to uninfected adults of the same age [48], and TRECs were substantially decreased among 38–42-year-old HIV-infected adults compared with uninfected adults of the same age [18], suggesting that naive T-cell emigration is partially suppressed in HIV-infected adults.
As naive T cells enter the circulation during HIV infection, albeit at slower rates, immune activation by HIV and other antigens stimulate these cells to differentiate into effector memory and effector phenotypes while central memory phenotypes are depleted [49,50]. Continual stimulation by HIV has been shown to produce an exhausted T-cell phenotype with reduced proliferative and functional capacities [51]. As evidence of increased T-cell activation by HIV, approximately 12% of CD4+ and 40% of CD8+ T cells expressed the activation markers HLA-DR and CD38 in 1–10-year-old HIV-infected Ugandan children compared with 6 and 20% of CD4+ and CD8+ T cells, respectively, in uninfected children [52]. Among a cohort of Italian adults, HIV-infected individuals consistently showed higher proportions of activated CD4+ and CD8+ T cells, as measured by the expression of CD38 and HLA-DR, in comparison to uninfected adults of the same age [53]. Positive correlations were observed between the expression of CD38 and HLA-DR and apoptosis among CD4+ and CD8+ T cells, suggesting T-cell turnover was associated with immune activation [53].
Naive CD4+ and CD8+ T cells are not only activated at higher levels but emigrate into the peripheral circulation at higher rates in HIV-infected individuals with higher viral loads [54,55]. Increased levels of activation markers were observed among both naive CD4+ and CD8+ T cells in HIV-infected individuals with higher viral loads [56] and, as CD4+ T cells were depleted by HIV [49], increasing rates of CD4+ T-cell emigration occurred via homeostatic mechanisms involving IL-7 [56]. High levels of IL-7 were demonstrated in HIV-infected adults in association with increased CD4+ T-cell depletion [57], but decreased levels of IL-7 were recently reported among HIV-infected children [58]. As circulating IL-7 levels are believed to be regulated by T-cell consumption [59], increased rates of IL-7 consumption by newly emigrated T cells may result in decreased IL-7 levels in younger individuals [58]. Thus, immune activation as well as T-cell homeostasis contributes to T-cell turnover and the recruitment of naive T cells into the peripheral circulation in HIV-infected children.
As T-cell turnover occurs at higher rates in HIV-infected individuals compared with uninfected individuals, chronic immune activation may inhibit the development of protective responses following co-infection and vaccination. A recent review suggested antigenic load and duration of exposure may play a role in T-cell phenotypic development [8]. Exposure to antigens yields immunological memory to a diverse array of pathogens [16], yet the magnitude and duration of antigenic stimulation induced by HIV and other chronic viral infections (e.g., cytomegalovirus [CMV] or Epstein-Barr virus, for example) led to increased clonal expansion of effector and memory cells specific for these viral antigens while expansion of other pathogen-specific T cells are out-competed for space and resources in immunologic survival niches. For example, proportions of CMV-specific memory and effector T cells in HIV-infected adults were three times higher than in uninfected adults, while mumps virus-specific T cells in HIV-infected persons were lower than in uninfected individuals [60]. As prior exposure to CMV infection was detected in all study participants and only one HIV-infected participant demonstrated clinically apparent CMV-associated disease (retinitis), this suggested HIV-infected individuals generate an exaggerated cellular immune response to CMV at the expense of the response to mumps virus. This study indicates that HIV viral load may have a direct impact on T-cell phenotypic development in response to HIV and other viral pathogens. Moreover, when children and adults with low CD4+ T-cell counts demonstrate a higher proportion of activated CD4+ T cells [52,61], fewer CD4+ cells may be available for differentiation into memory cells upon exposure to novel antigenic stimuli such as vaccines. Whether the antigenic burden accompanying vaccination would skew vaccine-specific T cells towards an effector phenotype is unknown.
B cells
Although HIV directly binds mature B cells, this is not considered a major pathway for destruction or differentiation of B cells [62]. However, several studies have demonstrated an association between levels of viremia in HIV-infected adults and B-cell death through increased expression of activation markers on B cells, such as bcl-2, LAIR-1, Fas and Fas-L [27,29,30,62–65]. Increased activation-induced B-cell death is consistent with lower levels of CD27+ B cells in HIV-infected adults compared with uninfected adults [30,65]. For example, Italian and Swedish adults infected with HIV at least 7 years earlier had a median of 19% memory B cells compared with healthy adults with a median of 33.8% memory B cells [65]. In addition, expression of CD70 on the surface of T cells in HIV-infected adults induced memory B cells to differentiate into plasma cells upon binding CD27 [34]. HIV-infected Japanese adults had a mean of 5% of T cells that expressed CD70 while only 0.6% of T cells expressed this marker in uninfected adults [66]. Thus, activation of CD27+ B cells by HIV promotes terminal differentiation into antibody-secreting plasmablasts, as well as B-cell death.
Contrary to T-cell populations, HIV-infected individuals have a higher proportion of naive (CD27−) B cells among the total B-cell population compared with uninfected individuals, as demonstrated by lack of CD27 expression on 88.1% of the total B-cell population among HIV-infected Japanese adults compared with 68.6% CD27− B cells in uninfected adults [63]. Among children, 85% of the B-cell population in HIV-infected German children with a mean age of 9 years were naive while only 73% were naive in uninfected controls [30]. Interestingly, CD154 expression on CD4+ T cells, which stimulates memory B-cell development [34], was similar among HIV-infected and uninfected adults but memory B-cell proliferation in response to activated CD4+ T cells was lower among HIV-infected adults [67]. This lower rate of memory B-cell proliferation was attributed to decreased responsiveness of activated memory B cells to IL-2 in HIV-infected persons. Furthermore, CD4+ T cells from HIV-infected adults secreted less IL-2 during periods of viremia, and IL-2 levels increased with decreasing viral load [68]. Thus, while the memory B-cell pool is depleted through activation into plasmablasts, activated B cells differentiate at a slower rate due to decreased B-cell responsiveness and lower IL-2 levels, creating a deficit of memory B cells. Furthermore, high levels of immune activation during HIV infection have been associated with an exhausted memory B-cell phenotype and increased levels of B-cell apoptosis [26,64].
Differentiation of resting memory B cells results in hypergammaglobulinemia in HIV-infected adults [66,69], which may adversely affect vaccine-specific memory cells and the ability to appropriately respond to antigenic challenge. This also suggests an over-abundance of antibody-secreting plasmablasts. Hypergammaglobulinemia and a decreased proportion of measles-specific memory B cells were observed in Italian and Swedish adults with recently acquired HIV infection compared with uninfected adults (0.2% and 1.01%, respectively) [65]. Additionally, a mean antimeasles virus IgG titer of 4.9 IU/ml in adults with chronic HIV infection was significantly lower than that of uninfected adults (8.9 IU/ml) [65]. Interestingly, while adults with chronic HIV infection demonstrated decreased levels of antimeasles virus IgG produced by antibody-secreting cells in response to stimulation compared with healthy controls, adults with primary HIV infection had an even poorer response [65].
Antibody avidity may also be impaired by HIV infection. Significantly lower antibody avidity was observed 3 months following revaccination against measles virus and 3 months after natural measles virus infection in HIV-infected children compared with uninfected children [70]. Whether a decrease in the level of antigen-specific antibodies and antibody binding strength represents destruction of circulating plasmablasts and memory B cells, dysfunctional terminal differentiation of B cells or a combination of these mechanisms is unknown, but these observations indicate a reduced capacity of antibody-secreting plasma cells to maintain protective immune responses in HIV-infected individuals.
Effects of HAART
T cells
Evidence for CD4+ T-cell reconstitution after bone marrow transplantation and chemotherapy suggests that both naive T-cell emigration and memory T-cell expansion contribute to immune reconstitution and the contribution by each of these mechanisms is influenced by an individual’s age (Table 1) [71–73]. While several studies of immune reconstitution after HAART initiation observed correlations between age and naive T-cell emigration or memory T-cell expansion [18,74–77], the relationship between age and the dominant mechanism of reconstitution after HAART is not well characterized in terms of when naive T-cell emigration wanes and memory T-cell expansion waxes. These mechanisms most likely constitute a spectrum and depend in part on the memory cell repertoire (Figure 3). The sequence in which an individual acquires HIV infection and memory immune responses to pathogens likely has important implications for the principal immune reconstitution mechanism. Thus, a perinatally infected child may reconstitute differently after HAART initiation than an adolescent or adult who acquired immunologic memory prior to HIV infection.
Figure 3. Causal diagram of cellular reconstitution in the context of age.
Initiation of HAART in infancy results in immune reconstitution with naive T cells. By contrast, immune reconstitution at an older age relies on expansion of memory T cells.
Thymic size in HIV-infected Italian children ranging in age from 6 to 15 years was positively correlated with increases in naive T-cell levels after 12 months of HAART [45], indicating that the effects of HIV on thymopoesis can be thwarted by effective therapy. Naive, memory and total CD4+ T-cell counts increased by 7, 4 and 12 cells/μl/day, respectively, in three children younger than 3 years after 3 months of HAART, which were significantly higher than 0.4, 0.2 and 0.6 cells/μl/day among ten children who were 5–16 years old [78]. In a cohort of HIV-infected French children aged 0.6–17 years, an initial increase in naive and memory CD4+ T-cell percentages occurred after 3 months of HAART, but only naive cells continued to increase over a 1-year period from approximately 6 to 16% [74]. This pattern of an initial increase in both naive and memory CD4+ T cells followed by sustained increases in naive CD4+ T cells was also observed in HIV-infected European children who demonstrated a 12% increase in naive CD4+ T-cell percentage 48 weeks post-HAART but no significant changes in memory cell percentage [79]. Unfortunately, studies characterizing CD4+ or CD8+ T-cell subsets in children receiving HAART frequently do not characterize age-related changes in uninfected children or measure these cell populations prior to HAART initiation, making the effects of HAART difficult to quantify. Nevertheless, current evidence suggests that T-cell reconstitution following HAART is more heavily skewed toward naive T-cell emigration at younger ages.
Suppression of HIV viral load following HAART has been associated with rapid increases in TRECs among adults 22–63 years old [18] but, in another study, naive CD4+ T-cell counts did not change significantly after HAART initiation despite increases in total CD4+ T-cell counts [76]. In South African adults, memory CD4+ T-cell levels showed a sustained and statistically significant mean increase from 34.2% at baseline to 47.8% after 3 months of HAART, but an 8.9% increase in naive CD4+ T-cell levels only occurred after 9 months of therapy [80]. These studies suggest that while initial increases in both naive and memory cell subsets occur in adults after HAART initiation, memory cell expansion plays a larger role in older individuals than naive T-cell emigration.
Immune reconstitution following HAART appears to mirror the accumulation of memory T cells over the life span, such that young children with relatively few memory cells predominantly reconstitute with naive T cells, adolescents or young adults reconstitute through a combination of naive T-cell emigration and memory T-cell expansion, and older adults reconstitute with the expansion of memory T cells. As thymopoesis continues to occur into late adulthood, even in HIV-infected adults [81,82], naive T cells will be detectable in most individuals regardless of age; however, the contribution of these cells to immune reconstitution declines with increasing age of HAART initiation (Figure 3). Furthermore, immune activation rapidly declines after HAART initiation in both children and adults, as demonstrated by decreases in activation markers such as CD95 (Fas), CD38 and HLA-DR, as well as declines in the proportion of cells undergoing apoptosis [53,76,83–85].
Despite increased levels of memory T-cell expansion in adults, proliferation of memory cells in response to viral vaccine antigens are relatively weak. Decreased proliferation of vaccinia-specific central memory CD4+ T cells was observed in 30–59-year-old adults treated with HAART and vaccinated against smallpox during childhood compared with uninfected individuals of the same age [86]. When tested against another strain of smallpox (NYVAC), HIV-infected individuals demonstrated a complete lack of memory T-cell proliferation, whereas T cells from healthy controls proliferated at normal levels. Similar failure of memory T-cell proliferation was shown in response to mumps and influenza viruses in 39–66-year-old HIV-infected adults despite normalized CD4+ T-cell counts and viral load suppression while receiving HAART [87].
B cells
In contrast to T cells, fewer studies have assessed the effects of HAART on B-cell subsets. A mean increase of 101 naive B cells/μl but only 20 memory B cells/μl were observed in French adults after 18 months of HAART [88], indicating a predominant increase in naive B cells. Among Japanese adults, naive B-cell frequencies were not significantly different between drug-naive and HAART-treated HIV-infected and uninfected adults, but therapy-naive HIV-infected adults had a mean of 11.9% memory B cells, significantly lower than the 16.1 and 31.4% of HAART-treated adults who had achieved undetectable HIV viral loads and uninfected controls, respectively [29]. These findings suggest decreased rates of memory B-cell apoptosis after beginning HAART or increased differentiation of naive cells. One longitudinal study of the effect of HAART on B-cell populations among HIV-infected American adults demonstrated a significant increase in memory B cells from 8.8 to 17% following a decline in HIV viral load, suggesting a partial restoration of this cell population [89]. Additionally, a decrease in Fas expression on B cells from Italian adults occurred within 6 months of HAART initiation [26] and higher levels of Fas expression were seen among the drug-naive, HIV-infected Japanese adults described above compared with HAART-treated and uninfected individuals [29].
Very few studies have reported the effects of HAART on B cells in children. In a study investigating the timing of HAART initiation among Italian children perinatally infected with HIV, comparable memory B-cell percentages were observed between healthy, uninfected children and HIV-infected children who initiated ART during the first year of life [90]. Conversely, children who began HAART after the first year of life had significantly lower percentages of memory B cells compared with healthy, uninfected control children (16 vs 21%, respectively). Although the proportion of memory B cells appears to increase after ART initiation in adults and children, whether emigration and expansion of naive and memory B cells is similar to that of T-cell reconstitution remains unclear. Moreover, HIV-infected children who begin HAART early in life appear to develop normal memory B-cell responses, although this observation requires confirmation.
Immune reconstitution, memory responses & revaccination against measles virus
Persistence of protective immunity to measles virus represents a model for studying immunological memory, the immune dysfunction that accompanies HIV infection, and immune reconstitution with HAART as exposure results in lifelong immunity due to the maintenance of protective IgG antibody levels [204]. In general, low levels of immunity are observed among HIV-infected children in response to vaccines received prior to the initiation of HAART [91]. Untreated Zambian and Thai children infected with HIV demonstrated similar peak antibody levels as those in healthy children, but significant waning of antibody levels occurred over time, potentially creating a cohort of measles-susceptible children as survival is increased in children treated with ART or HAART [92,93]. A mathematical model demonstrated how high mortality rates among untreated, HIV-infected children counterbalanced the impact of waning immunity on the build-up of susceptible children, mitigating the effect of secondary vaccine failure on measles virus transmission dynamics [94]. Upon HAART initiation, however, HIV-related mortality was assumed to decrease and measles virus susceptibility and transmission increased, suggesting a need to revaccinate HIV-infected children if HAART does not restore protective immune responses.
The optimal timing of revaccination in relation to HAART is an important policy issue. Several studies directly addressed whether HIV-infected children who lost protective antibody levels can be effectively revaccinated against measles virus after HAART initiation and approximately 60–83% of revaccinated children seroconverted to measles virus [95–97]. Two of these studies specifically assessed revaccination after achieving normal CD4+ T-cell percentages and reported the highest seroconversion rates of 82 and 83% [96,97]. This proportion is similar to the approximately 85% of healthy children who seroconvert after receiving one dose of measles vaccine at 9 months of age [204], suggesting that immune reconstitution as measured by CD4+ T-cell percentage could be an effective indicator for the timing of measles revaccination. Furthermore, as CD4+ T-cell percentage is commonly measured, this would provide an efficient clinical marker for healthcare workers in less developed settings.
Most studies indicate that if adults are infected or vaccinated prior to HIV infection, measles antibodies persist [98,99], and therefore revaccination of most HIV-infected adults is likely not warranted. However, development of antibodies following vaccination of six measles-unexposed American adults after HIV acquisition occurred in only two individuals [100], suggesting that HIV infection prior to vaccination reduces the likelihood of an antibody response. In a study of measles revaccination in ART-treated Mexican adults who achieved greater than 200 CD4+ T cells/μl, only 34% maintained measles virus antibodies 12 months after revaccination compared with 80% of uninfected individuals [98]. Thus, identifying immunological risk factors associated with the loss of measles antibodies after vaccination may help elucidate the underlying mechanisms involved in cellular and serologic memory formation in both children and adults.
Some expansion of memory T cells occurs after HAART initiation in both children and adults. Whether a similar expansion occurs with B cells has implications for reconstitution of serological memory following HAART. Antibody levels against vaccines in HAART-treated children are inconsistent [91]. For example, 40% of Kenyan children previously vaccinated against measles but measles IgG negative at the time of HAART initiation developed positive measles IgG responses after 6 months of HAART; however, 57% of children who were initially seropositive became measles IgG negative over the same time [95,101]. Others have also observed measles seroreversion in HAART-treated children [101] but the underlying immunologic mechanisms are not clear.
Conclusion
Memory T- and B-cell populations increase throughout an individual’s lifetime due to accumulated antigenic exposure to both natural infections and vaccinations. These exposures result in the development of protective antibodies that are maintained through several mechanisms. Infection with HIV produces aberrant immune activation that accelerates cellular turnover and potentially impedes proper memory cell development. In children infected with HIV in the perinatal period, this dysfunction produces poor long-term immune responses to vaccines, as well as natural infections, that are not reversed in many children following immune reconstitution with HAART. These children likely require revaccination. Immunologic memory in adults may be disrupted after HIV infection but HAART initiation results in the expansion of pathogen-specific memory cells. As antiretro-viral therapy becomes more widely available, revaccination of children who successfully achieve immune reconstitution and virologic suppression while receiving HAART may help restore individual and population immunity.
Future perspective
The characteristics of immunological memory after vaccination of HIV-infected individuals remain incompletely defined and recommendations for revaccination after immune reconstitution require immunologic and epidemiologic evidence. Of particular note is the paucity of data on infants and young children, particularly from geographically diverse settings, which limits inferences on the characterization of immunologic changes during HIV infection and after HAART initiation. Therefore, gaps in knowledge about when to revaccinate remain. More children are expected to initiate HAART in early infancy and early HAART may result in near normal immunological responses to vaccines. Longitudinal studies measuring both T- and B-cell subsets at multiple time points are needed to more fully understand the effects of HAART on immunological memory. B- and T-cell subsets are infrequently measured concurrently but their interactions are critical to the development of immunological memory. In addition to the classical interaction of CD4+ T cells ‘helping’ B cells [4], regulatory B cells have been identified and may affect T-cell responses [102] and require more investigation. In addition, parallels have been drawn between memory B- and T-cell development [103] that may further understanding of the immunologic changes resulting from HIV and HAART.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Kaitlin Rainwater-Lovett and William J Moss were supported by a grant from the National Institute of Allergy and Infectious Diseases (AI070018). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Amadi B, Kelly P, Mwiya M, et al. Intestinal and systemic infection, HIV, and mortality in Zambian children with persistent diarrhea and malnutrition. J Pediatr Gastroenterol Nutr. 2001;32:550–554. doi: 10.1097/00005176-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesh KK, de Bruyn G, Marinda E, et al. Morbidity and mortality among infants born to HIV-infected women in South Africa: implications for child health in resource-limited settings. J Trop Pediatr. 2010 doi: 10.1093/tropej/fmq061. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA, Travers P, Walport M, Schlomchik M. Immunobiology: the immune system in health and disease. 6. Churchill Livingstone; Philadelphia, PA, USA: 2005. [Google Scholar]
- 5.Clement LT. Isoforms of the CD45 common leukocyte antigen family: markers for human T-cell differentiation. J Clin Immunol. 1992;12:1–10. doi: 10.1007/BF00918266. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 7.Stemberger C, Huster KM, Koffler M, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 8••.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. Discusses multifunctional T cells, how to measure these cell populations and their importance in immunologic responses after vaccination. [DOI] [PubMed] [Google Scholar]
- 9.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 10.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. Counts and proportions of T-cell subsets according to age category from 0 to 18 years of age. The three age categories of 0–3 months, 3–6 months and 6–12 months are of particular interest. [DOI] [PubMed] [Google Scholar]
- 12.Kam KM, Leung WL, Wong KH, Lee SS, Hung MY, Kwok MY. Maturational changes in peripheral lymphocyte subsets pertinent to monitoring human immunodeficiency virus-infected Chinese pediatric patients. Clin Diagn Lab Immunol. 2001;8:926–931. doi: 10.1128/CDLI.8.5.926-931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heldrup J, Kalm O, Prellner K. Blood T and B lymphocyte subpopulations in healthy infants and children. Acta Paediatr. 1992;81:125–132. doi: 10.1111/j.1651-2227.1992.tb12187.x. [DOI] [PubMed] [Google Scholar]
- 14.van Gent R, van Tilburg CM, Nibbelke EE, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009;133:95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Tsegaye A, Wolday D, Otto S, et al. Immunophenotyping of blood lymphocytes at birth, during childhood, and during adulthood in HIV-1-uninfected Ethiopians. Clin Immunol. 2003;109:338–346. doi: 10.1016/j.clim.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol. 2004;72:203–212. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- 18.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 19.Hartwig M, Steinmann G. On a causal mechanism of chronic thymic involution in man. Mech Ageing Dev. 1994;75:151–156. doi: 10.1016/0047-6374(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 20.Bains I, Thiebaut R, Yates AJ, Callard R. Quantifying thymic export: combining models of naive T cell proliferation and TCR excision circle dynamics gives an explicit measure of thymic output. J Immunol. 2009;183:4329–4336. doi: 10.4049/jimmunol.0900743. [DOI] [PubMed] [Google Scholar]
- 21•.Zanetti M, Franchini G. T cell memory and protective immunity by vaccination: is more better? Trends Immunol. 2006;27:511–517. doi: 10.1016/j.it.2006.09.004. Describes T-cell responses after vaccination and guidelines for the types of immune responses vaccines should stimulate. [DOI] [PubMed] [Google Scholar]
- 22•.Ono E, Nunes dos Santos AM, de Menezes Succi RC, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res. 2008;41:700–708. doi: 10.1590/s0100-879x2008000800011. Describes proportions of T-cell subsets in cord blood and 12-month-old infants comparing children born to uninfected mothers to uninfected children born to HIV-infected mothers. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SM, Tomayko MM, Shlomchik MJ. Intrinsic properties of human and murine memory B cells. Immunol Rev. 2006;211:280–294. doi: 10.1111/j.0105-2896.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 24.Stuber E, Strober W. The T cell–B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J Exp Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Orsogna LJ, Krueger RG, McKinnon EJ, French MA. Circulating memory B-cell subpopulations are affected differently by HIV infection and antiretroviral therapy. AIDS. 2007;21:1747–1752. doi: 10.1097/QAD.0b013e32828642c7. [DOI] [PubMed] [Google Scholar]
- 26.Titanji K, Chiodi F, Bellocco R, et al. Primary HIV-1 infection sets the stage for important B lymphocyte dysfunctions. AIDS. 2005;19:1947–1955. doi: 10.1097/01.aids.0000191231.54170.89. [DOI] [PubMed] [Google Scholar]
- 27.Bukowska-Strakova K, Kowalczyk D, Baran J, Siedlar M, Kobylarz K, Zembala M. The B-cell compartment in the peripheral blood of children with different types of primary humoral immunodeficiency. Pediatr Res. 2009;66:28–34. doi: 10.1203/PDR.0b013e3181a7b0a2. [DOI] [PubMed] [Google Scholar]
- 28.De Milito A, Morch C, Sonnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS. 2001;15(8):957–964. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 29.Chong Y, Ikematsu H, Kikuchi K, et al. Selective CD27+ (memory) B cell reduction and characteristic B cell alteration in drug-naive and HAART-treated HIV type 1-infected patients. AIDS Res Hum Retroviruses. 2004;20:219–226. doi: 10.1089/088922204773004941. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, Feyen O, Jebran AF, et al. Memory B cell function in HIV-infected children-decreased memory B cells despite ART. Pediatr Res. 2009;66:185–190. doi: 10.1203/PDR.0b013e3181aa057d. [DOI] [PubMed] [Google Scholar]
- 31.Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 33.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–206. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 35.Agematsu K, Nagumo H, Oguchi Y, et al. Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood. 1998;91:173–180. [PubMed] [Google Scholar]
- 36.Avery DT, Ellyard JI, Mackay F, Corcoran LM, Hodgkin PD, Tangye SG. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J Immunol. 2005;174:4034–4042. doi: 10.4049/jimmunol.174.7.4034. [DOI] [PubMed] [Google Scholar]
- 37.Hauser AE, Debes GF, Arce S, et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002;169:1277–1282. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- 38.Halliley JL, Kyu S, Kobie JJ, et al. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–3587. doi: 10.1016/j.vaccine.2010.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odendahl M, Keitzer R, Wahn U, et al. Perturbations of peripheral B lymphocyte homoeostasis in children with systemic lupus erythematosus. Ann Rheum Dis. 2003;62:851–858. doi: 10.1136/ard.62.9.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 41.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 42•.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. Longitudinal measurement of antibody levels against common vaccine pathogens over several decades. [DOI] [PubMed] [Google Scholar]
- 43.Vallerskog T, Gunnarsson I, Widhe M, et al. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol. 2007;122:62–74. doi: 10.1016/j.clim.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Yu X, Tsibane T, McGraw PA, et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455:532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vigano A, Vella S, Saresella M, et al. Early immune reconstitution after potent antiretroviral therapy in HIV-infected children correlates with the increase in thymus volume. AIDS. 2000;14:251–261. doi: 10.1097/00002030-200002180-00007. [DOI] [PubMed] [Google Scholar]
- 46.Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 47•.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. Thymic changes throughout the lifespan and its effects on circulating T-cell populations. [DOI] [PubMed] [Google Scholar]
- 48.Kalayjian RC, Landay A, Pollard RB, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis. 2003;187:1924–1933. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 49.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 50.Ladell K, Hellerstein MK, Cesar D, Busch R, Boban D, McCune JM. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J Immunol. 2008;180:7907–7918. doi: 10.4049/jimmunol.180.12.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 52.Ssewanyana I, Elrefaei M, Dorsey G, et al. Profile of T cell immune responses in HIV-infected children from Uganda. J Infect Dis. 2007;196:1667–1670. doi: 10.1086/522013. [DOI] [PubMed] [Google Scholar]
- 53.Ensoli F, Fiorelli V, Alario C, et al. Decreased T cell apoptosis and T cell recovery during highly active antiretroviral therapy (HAART) Clin Immunol. 2000;97:9–20. doi: 10.1006/clim.2000.4915. [DOI] [PubMed] [Google Scholar]
- 54.Di Mascio M, Sereti I, Matthews LT, et al. Naive T-cell dynamics in human immunodeficiency virus type 1 infection: effects of highly active antiretroviral therapy provide insights into the mechanisms of naive T-cell depletion. J Virol. 2006;80:2665–2674. doi: 10.1128/JVI.80.6.2665-2674.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 56.Catalfamo M, Di MM, Hu Z, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci USA. 2008;105:19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malaspina A, Moir S, Ho J, et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci USA. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cagigi A, Palma P, Nilsson A, et al. The impact of active HIV-1 replication on the physiological age-related decline of immature-transitional B-cells in HIV-1 infected children. AIDS. 2010;24:2075–2080. doi: 10.1097/QAD.0b013e32833c3298. [DOI] [PubMed] [Google Scholar]
- 59.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 60.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eggena MP, Barugahare B, Okello M, et al. T cell activation in HIV-seropositive Ugandans: differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis. 2005;191:694–701. doi: 10.1086/427516. [DOI] [PubMed] [Google Scholar]
- 62.Moir S, Malaspina A, Pickeral OK, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–599. [PubMed] [Google Scholar]
- 63.Chong Y, Ikematsu H, Yamamoto M, et al. Increased frequency of CD27− (naive) B cells and their phenotypic alteration in HIV type 1-infected patients. AIDS Res Hum Retroviruses. 2004;20:621–629. doi: 10.1089/0889222041217455. [DOI] [PubMed] [Google Scholar]
- 64.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Titanji K, De Milito A, Cagigi A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 66.Nagase H, Agematsu K, Kitano K, et al. Mechanism of hypergammaglobulinemia by HIV infection: circulating memory B-cell reduction with plasmacytosis. Clin Immunol. 2001;100:250–259. doi: 10.1006/clim.2001.5054. [DOI] [PubMed] [Google Scholar]
- 67.Moir S, Ogwaro KM, Malaspina A, et al. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc Natl Acad Sci USA. 2003;100:6057–6062. doi: 10.1073/pnas.0730819100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Younes SA, Yassine-Diab B, Dumont AR, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Milito A, Nilsson A, Titanji K, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 70.Nair N, Moss WJ, Scott S, et al. HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection. J Infect Dis. 2009;200:1031–1038. doi: 10.1086/605648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16:413–425. [PubMed] [Google Scholar]
- 72.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 73.Mackall CL, Granger L, Sheard MA, Cepeda R, Gress RE. T-cell regeneration after bone marrow transplantation: differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood. 1993;82:2585–2594. [PubMed] [Google Scholar]
- 74.Hainaut M, Ducarme M, Schandene L, et al. Age-related immune reconstitution during highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 2003;22:62–69. doi: 10.1097/00006454-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 75.Essajee SM, Kim M, Gonzalez C, et al. Immunologic and virologic responses to HAART in severely immunocompromised HIV-1-infected children. AIDS. 1999;13:2523–2532. doi: 10.1097/00002030-199912240-00005. [DOI] [PubMed] [Google Scholar]
- 76.Evans TG, Bonnez W, Soucier HR, Fitzgerald T, Gibbons DC, Reichman RC. Highly active antiretroviral therapy results in a decrease in CD8+ T cell activation and preferential reconstitution of the peripheral CD4+ T cell population with memory rather than naive cells. Antiviral Res. 1998;39:163–173. doi: 10.1016/s0166-3542(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 77.Lederman MM, McKinnis R, Kelleher D, et al. Cellular restoration in HIV infected persons treated with abacavir and a protease inhibitor: age inversely predicts naive CD4 cell count increase. AIDS. 2000;14:2635–2642. doi: 10.1097/00002030-200012010-00002. [DOI] [PubMed] [Google Scholar]
- 78.Cohen Stuart JW, Slieker WA, Rijkers GT, et al. Early recovery of CD4+ T lymphocytes in children on highly active antiretroviral therapy Dutch study group for children with HIV infections. AIDS. 1998;12:2155–2159. doi: 10.1097/00002030-199816000-00010. [DOI] [PubMed] [Google Scholar]
- 79.Gibb DM, Newberry A, Klein N, De RA, Grosch-Woerner I, Babiker A. Immune repopulation after HAART in previously untreated HIV-1-infected children. Paediatric European Network for Treatment of AIDS (PENTA) Steering Committee. Lancet. 2000;355:1331–1332. doi: 10.1016/s0140-6736(00)02117-6. [DOI] [PubMed] [Google Scholar]
- 80.Wilkinson KA, Seldon R, Meintjes G, et al. Dissection of regenerating T-cell responses against tuberculosis in HIV-infected adults sensitized by Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2009;180:674–683. doi: 10.1164/rccm.200904-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Markert ML, Alvarez-McLeod AP, Sempowski GD, et al. Thymopoiesis in HIV-infected adults after highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2001;17:1635–1643. doi: 10.1089/088922201753342040. [DOI] [PubMed] [Google Scholar]
- 82.Smith KY, Valdez H, Landay A, et al. Thymic size and lymphocyte restoration in patients with human immunodeficiency virus infection after 48 weeks of zidovudine, lamivudine, and ritonavir therapy. J Infect Dis. 2000;181:141–147. doi: 10.1086/315169. [DOI] [PubMed] [Google Scholar]
- 83.Bohler T, Walcher J, Holzl-Wenig G, et al. Early effects of antiretroviral combination therapy on activation, apoptosis and regeneration of T cells in HIV-1-infected children and adolescents. AIDS. 1999;13:779–789. doi: 10.1097/00002030-199905070-00006. [DOI] [PubMed] [Google Scholar]
- 84.Sondergaard SR, Aladdin H, Ullum H, Gerstoft J, Skinhoj P, Pedersen BK. Immune function and phenotype before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 1999;21:376–383. [PubMed] [Google Scholar]
- 85.Resino S, Galan I, Bellon JM, Navarro ML, Leon JA, Munoz-Fernandez MA. Characterizing the immune system after long-term undetectable viral load in HIV-1-infected children. J Clin Immunol. 2003;23:279–289. doi: 10.1023/a:1024536816684. [DOI] [PubMed] [Google Scholar]
- 86.Puissant-Lubrano B, Combadiere B, Duffy D, et al. Influence of antigen exposure on the loss of long-term memory to childhood vaccines in HIV-infected patients. Vaccine. 2009;27:3576–3583. doi: 10.1016/j.vaccine.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 87.Elrefaei M, McElroy MD, Preas CP, et al. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol. 2004;173:2184–2189. doi: 10.4049/jimmunol.173.3.2184. [DOI] [PubMed] [Google Scholar]
- 88.Le Guillou-Guillemette H, Renier G, Vielle B, et al. Immune restoration under HAART in patients chronically infected with HIV-1: diversity of T, B, and NK immune responses. Viral Immunol. 2006;19:267–276. doi: 10.1089/vim.2006.19.267. [DOI] [PubMed] [Google Scholar]
- 89.Moir S, Malaspina A, Ho J, et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis. 2008;197:572–579. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 90.Pensieroso S, Cagigi A, Palma P, et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci USA. 2009;106:7939–7944. doi: 10.1073/pnas.0901702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91••.Sutcliffe CG, Moss WJ. Do children infected with HIV receiving HAART need to be revaccinated? Lancet Infect Dis. 2010;10:630–642. doi: 10.1016/S1473-3099(10)70116-X. Low antibody levels in HIV-infected children demonstrating the lack of protection after HAART initiation. [DOI] [PubMed] [Google Scholar]
- 92.Moss WJ, Scott S, Mugala N, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196:347–355. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 93.Aurpibul L, Puthanakit T, Siriaksorn S, Sirisanthana T, Sirisanthana V. Prevalence of protective antibody against measles in HIV-infected children with immune recovery after highly active antiretroviral therapy. HIV Med. 2006;7:467–470. doi: 10.1111/j.1468-1293.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 94•.Scott S, Mossong J, Moss WJ, Cutts FT, Cousens S. Predicted impact of the HIV-1 epidemic on measles in developing countries: results from a dynamic age-structured model. Int J Epidemiol. 2008;37:356–367. doi: 10.1093/ije/dyn007. Mathematical model demonstrating the effects of increased survival of HIV-infected children after HAART initiation and the increase in measles transmission due to the loss of protective immunity in the population. [DOI] [PubMed] [Google Scholar]
- 95.Farquhar C, Wamalwa D, Selig S, et al. Immune responses to measles and tetanus vaccines among Kenyan human immunodeficiency virus type 1 (HIV-1)-infected children pre- and post-highly active antiretroviral therapy and revaccination. Pediatr Infect Dis J. 2009;28:295–299. doi: 10.1097/INF.0b013e3181903ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96••.Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthana V. Persistence of measles, mumps, and rubella protective antibodies 3 years after revaccination in HIV-infected children receiving antiretroviral therapy. Clin Infect Dis. 2010;50:1415–1418. doi: 10.1086/652150. Study demonstrating the development and maintenance of protective measles antibody levels after revaccination in HIV-infected children on HAART. [DOI] [PubMed] [Google Scholar]
- 97.Melvin AJ, Mohan KM. Response to immunization with measles, tetanus, and Haemophilus influenzae type b vaccines in children who have human immunodeficiency virus type 1 infection and are treated with highly active antiretroviral therapy. Pediatrics. 2003;111(6 Pt 1):E641–E644. doi: 10.1542/peds.111.6.e641. [DOI] [PubMed] [Google Scholar]
- 98.Wallace MR, Hooper DG, Graves SJ, Malone JL. Measles seroprevalence and vaccine response in HIV-infected adults. Vaccine. 1994;12(13):1222–1224. doi: 10.1016/0264-410x(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 99.Sha BE, Harris AA, Benson CA, et al. Prevalence of measles antibodies in asymptomatic human immunodeficiency virus-infected adults. J Infect Dis. 1991;164:973–975. doi: 10.1093/infdis/164.5.973. [DOI] [PubMed] [Google Scholar]
- 100.Belaunzaran-Zamudio PF, Garcia-Leon ML, Wong-Chew RM, et al. Early loss of measles antibodies after MMR vaccine among HIV-infected adults receiving HAART. Vaccine. 2009;2327:7059–7064. doi: 10.1016/j.vaccine.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 101.Bekker V, Scherpbier H, Pajkrt D, Jurriaans S, Zaaijer H, Kuijpers TW. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118:E315–E322. doi: 10.1542/peds.2005-2616. [DOI] [PubMed] [Google Scholar]
- 102.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–264. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
Websites
- 201.UNAIDS. Report of the global AIDS epidemic. 2008 www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/
- 202.The Global Fund to FIght AIDS, Tuberculosis and Malaria. Results Summary. 2010 www.theglobalfund.org/en/publications/progressreports/2010/
- 203.The U.S. President’s Emergency Plan for AIDS Relief 2010. Annual Report to Congress on PEPFAR Program Results. 2010 www.pepfar.gov/press/sixth_annual_report.
- 204.World Health Organization. Vaccine and Biologicals. Immunological Basis for Immunization Series, Module 7: Measles. Geneva: World Health Organization; 2009. www.who.int/immunization/documents/ISBN9789241597869/en/index.html. [Google Scholar]