Abstract
Conventional measurement of antibody responses to vaccines largely relies on serum antibodies, which are primarily produced by bone marrow plasma cells and may not represent the entire vaccine-induced B cell repertoire, including important functional components such as those targeted to mucosal sites. After immunization or infection, activated B cells differentiate into plasmablasts in local lymphoid organs, then traffic through circulation to the target sites where they further develop into plasma cells. On day 7 after influenza vaccination, a burst of plasmablasts, highly enriched for vaccine-specific antibody secreting cells, appears in the peripheral blood. This provides a unique window to the overall B cell response to the vaccine, without interference of pre-existing cross-reactive serum antibody. In this study we isolated B cells from volunteers on day 7 after immunization with the inactivated influenza vaccine and cultured them ex vivo to collect plasmablast-derived polyclonal antibodies (PPAb). The PPAb contained secreted IgG and IgA, which was approximately 0.2 ng per antibody secreting cell. Influenza-specific IgG and IgA binding activity was detected in PPAb at dilutions up to 105 by ELISA. The ratio of the titers of influenza-specific IgA to IgG by ELISA was 4-fold higher in PPAb than in day 28 post-vaccination sera, suggesting that vaccine-induced IgA is enriched in PPAb compared to sera. Functional activity was also detected in PPAb as determined by microneutralization and hemagglutination inhibition assays. In addition to bulk B cell cultures, we also cultured plasmablast subsets sorted by cell surface markers to generate PPAb. These results suggest that PPAb better reflects the mucosal IgA response than serum samples. Since PPAb are exclusively produced by recently activated B cells, it allows assessing vaccine-induced antibody response without interference from pre-existing cross-reactive serum antibodies and permits an assessment of antibody avidity based on antigen specific binding and antibody quantity. Therefore this assay is particularly useful for studying vaccine/infection-induced antibodies against antigens that might have previously circulated, such as antibody responses to rotavirus, dengue or influenza viruses in which cross-reactive antibodies against different virus serotypes/subtypes play a critical role in immunity and/or pathogenesis.
Keywords: Influenza virus, vaccines, antibodies, plasmablasts
1. Introduction
Influenza viruses are respiratory pathogens that cause annual epidemics and intermittent pandemics, resulting in significant disease burden in all age groups. Influenza vaccines, including live attenuated and inactivated vaccines, can effectively protect children and adults against influenza infection. Although the mechanisms for the protective efficacy of these vaccines are not completely understood, antibody response to natural influenza infection and vaccination is believed to be a critical part of the protective immunity (Gerhard 2001). However, the conventional evaluation of the antibody response to influenza vaccination by serum antibody titer, based on hemagglutination inhibition (HAI) assay or neutralization assay, does not always predict protection, especially in the situations such as live attenuated influenza vaccine (LAIV) (Treanor and Wright 2003). Therefore better correlates of the protective immune response induced by different vaccines are desirable.
After administration of a vaccine by different routes, naïve or memory B cells are activated at the site of immunization and draining lymph nodes. The activated B cells proliferate and differentiate into plasmablasts in the germinal centers of the local lymph nodes. At around day 6–8 after immunization, a large number of plasmablasts leave the germinal centers to transiently enter the circulation and form a sharp peak in the blood which is highly enriched for vaccine antigen-specific antibody secreting cells (ASC) (Cox et al. 1994; el-Madhun et al. 1998; Sasaki et al. 2008; Wrammert et al. 2008). Depending on local antigen-presenting environmental factors during the immune response (Sigmundsdottir and Butcher 2008), these plasmablasts express different trafficking receptors. These receptors direct the plasmablast migration to specific sites and tissues of the body (Kunkel and Butcher 2002; Kunkel and Butcher 2003). Those cells homing to the bone marrow become plasma cells, which are the primary source of serum antibodies (Benner et al. 1981). Other plasmablasts are targeted to different mucosal sites including the respiratory tract, and secrete musosal antibodies that are directly involved in protection against respiratory or other mucosal infections. Since the bone marrow plasma cells are only derived from a subset of the entire vaccine-activated B cell population, serum antibodies do not necessarily reflect the overall antibody response to the vaccine.
In addition to the conventional serological assays for measuring serum antibody titer before and after vaccination, different assays have been utilized to address the characteristics of the peripheral plasmablast response at day 7 after influenza vaccination. We and others have used ELISPOT assay to determine the number of total and influenza-specific IgG- and IgA-secreting plasmablasts in children and adults immunized with different types of influenza vaccines (Cox et al. 1994; el-Madhun et al. 1998; Sasaki et al. 2007; Sasaki et al. 2008). The plasmablasts can be identified and isolated by flow cytometry based on their surface markers, which allows analysis of the immunoglobulin gene repertoire of vaccine-activated B cell population (Wrammert et al. 2008). In addition, the mRNA encoding the heavy chain and light chain of immunoglobulin genes from individual plasmablasts can be cloned and co-expressed to generate recombinant monoclonal antibodies for functional analysis (Wrammert et al. 2008; Smith et al. 2009).
Instead of generating a panel of recombinant monoclonal antibodies from individual plasmablasts derived from each vaccinee to represent the overall antibody response to the vaccine, it is also possible to analyze polyclonal antibodies secreted ex vivo from the bulk plasmablast population (Ershler et al. 1982; Chang and Sack 2001). In this study we generated the plasmablast-derived polyclonal antibodies (PPAb) from a group of volunteers at day 7 after immunization with influenza vaccine and compared the PPAb with serum antibody response. Our results suggest that the PPAb analysis might provide new insights to the B cell and antibody responses to influenza vaccination, as well as other viral vaccines and infection.
2. Material and methods
2.1. Human participants, vaccination protocol and blood samples
Five healthy adults 18 – 30 years of age (donors No. 1 – No. 5) were enrolled during the 2008 – 2009 influenza season for the study. In addition, two healthy adults of the same age group (donors No. 6 and No. 7) were enrolled during the 2009 – 2010 influenza season for the flow cytometry sorting experiment (section 3.4). The study protocol was approved by the institutional review board at Stanford University. Informed consent was obtained from each participant. Participants were immunized with one dose of the trivalent inactivated influenza vaccine (TIV) of the current season: 2008 Afluria (CSL, Parkville, Victoria, Australia) for donors No. 1 – No. 5 or 2009 Fluzone (Safoni Pasteur, Swiftwater, PA, USA) for donors No. 6 and No. 7. Both the 2008 and 2009 TIV contained an A/Brisbane/59/2007 (H1N1)-like virus and an A/Brisbane/10/2007 (H3N2)-like virus. In addition, the 2008 TIV contained a B/Florida/4/2006-like virus, while the 2009 TIV contained a B/Brisbane/60/2008-like virus. Blood samples were collected on day 0 before vaccination, as well as day 7 and day 28 post vaccination. Sera were prepared with the day 0 and day 28 blood samples. PBMC were isolated using standard Ficoll gradient centrifugation from heparinized whole blood. B cells were isolated from heparinized whole blood by negative selection with the ResetteSep Human B cell Enrichment cocktail (Stemcell Technologies, Vancouver, BC, Canada), following the manufacturer’s instructions.
2.2. Generation of PPAb
For generation of PPAb from bulk plasmablasts, freshly isolated B cells were resuspended in complete medium (RPMI 1640 supplemented with 10% of heat inactivated fetal calf serum, 100 units/ml of penicillin G and 100 µg/ml of streptomycin) at 3 million cells per ml and cultured in 5% CO2 at 37°C for 4 – 7 days. The conditioned media with secreted PPAb were collected and kept at −20°C until being tested. For generation of PPAb from plasmablast subsets, PBMC and B cells were stained with a cocktail of antibodies against different cell surface markers: PE-Cy7-labeled anti-CD19, Pacific Blue-labeled anti-CD3, PE-labeled anti-CD27, PerCP-Cy5.5-labeled anti-CD38, APC-labeled anti-IgG (all from BD Biosciences, San Jose, CA, USA), and FITC-labeled anti-IgA (Invitorogen, Camarillo, CA, USA). The following cell populations were isolated on a FACS Aria flow cytometer: IgA+ plasmablasts (CD19+CD27highCD38highIgA+) and IgA− plasmablasts (CD19+CD27highCD38highIgA−) from B cells, and CD3+ T cells (CD19−CD3+) from PBMC. Variable numbers of the sorted plasmablast subsets were mixed with sorted T cells as feeder cells and cultured for 7 days in complete RPMI 1640 medium at 3 million total cells (plasmablasts plus T cells) per ml.
2.3. Determination of IgA and IgG concentration in PPAb
The concentration of IgA in the PPAb samples was determined with the IMMUNO-TEK Quantitative Human IgA ELISA kit (Zeptometrix, Buffalo, NY, USA), following the manufacturer’s instructions. The concentration of IgG was determined with a two-antibody sandwitch ELISA. In brief, Ninety-six-well Vinyl Microtiter Microplates (Thermo Fisher Scientific, Waltham, MA, USA) were first coated with goat anti-human IgG (Sigma, Saint Louis, USA) diluted 1:2000 with PBS. Plates were incubated overnight at 4°C, washed with wash buffer (0.1% of Tween 20 in PBS), and blocked with 3% of BSA in PBS for 1 hour at 37°C. PPAb were serially diluted with complete medium, then added to the wells of coated/blocked plates. Serially diluted purified human serum IgG (10 mg/ml, Innovative Research, Novi, MI, USA) was added to wells of the same plate to serve as the standard. The plates was incubated for 1 hour at 37°C, washed, and then incubated for 1 hour at 37°C with peroxidase-conjugated goat anti IgG γ (KPL, Gaithersburg, MD, USA) diluted 1:3000 with complete medium. After washing, the plates were developed with TMB substrate (KPL) and the OD450nm of each well recorded with an ELISA plate reader. The concentration of IgG in the PPAb samples were determined on the standard curve constructed with the OD values of serially diluted human serum IgG standard.
2.4. Enumeration of total and influenza-specific ASC
Total and influenza-specific IgA or IgG ASC were enumerated with ELISPOT. Ninety-six-well MultiScreen HTS plates (Millipore, Billerica, MA, USA) were coated with affinity purified goat antihuman IgA+IgG+IgM (H+L) (KPL) at a concentration of 4 µg/ml in PBS to detect total ASC, or coated with 2008 TIV (Fluzone) at a concentration of 4.5 µg/ml in PBS to detect influenza-specific ASC. Wells coated with PBS served as negative controls. Plates were incubated overnight at 4°C and then blocked for 2 hours at 37°C with complete medium. Freshly isolated B cells were resuspended in complete medium containing phosphatase conjugated goat anti-human IgA antibody (KPL) at 0.2 µg/ml or phosphatase conjugated goat anti-human IgG (H+L) antibody (KPL) at 0.2 µg/ml, dispensed into wells of the coated/blocked plates at 2 fold serial dilutions and incubated for 4 hours or longer at 37°C with 5% CO2. Plates were washed with PBS and developed with a Blue alkaline phosphatase substrate kit (Vector, Burlingame, CA, USA). The number of IgG and IgA ASC in each well was determined by counting the spots. Nonspecific spots detected in the negative control (PBS) wells were subtracted from the counts of influenza-specific and total ASC.
2.5. Influenza-specific binding activity of serum and PPAb samples
Influenza vaccine (TIV)- or individual vaccine virus strain-specific IgG or IgA binding activity was determined with ELISA. Ninety-six-well Vinyl Microtiter Microplates were coated with 2008 TIV at 2.7 µg/ml in PBS, or individual 2008 influenza vaccine strains (kindly provided by Dr. Kanta Subbarao of NIH) at 57 HA unit/ml in PBS (4 HA unit/well). Plates were incubated overnight at 4°C, washed with wash buffer, and blocked with complete medium for 1 hour at 37°C. PPAb or serum samples were serially diluted with complete medium, added to the wells of coated/blocked plates and incubated for 1 hour at 37°C. Wells incubated with complete medium served as negative control. The plates were then washed, incubated for 1 hour at 37°C with peroxidase-conjugated goat anti IgG γ (KPL) diluted 1:4000 with complete medium, or peroxidase-conjugated goat anti IgA α (KPL) diluted 1:2000 dilution with complete medium. After washing, the plates were developed with TMB substrate (KPL) and the OD450nm of each well determined with an ELISA plate reader. The cutoff OD450nm value was set at mean + 2 × SD (standard deviation) of OD450nm value of all negative control wells. Titer of each sample was determined as the highest dilution at which the mean OD450nm value of duplicate wells was greater than the cutoff.
2.6. Microneutralization and HAI assays
Serum samples were pretreated with receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) overnight at 37°C and subsequently heated at 56°C f or 45 minutes. Neutralization antibody titers were determined for 100% inhibition of cytopathic effect (CPE) in Madin-Darby canine kidney cells (European Collection of Animal Cell Cultures, Wiltshire, United Kingdom). Serially diluted serum or PPAb specimens were incubated with 50 TCID50 (50% tissue culture infective doses) of the indicated influenza virus for 1 hour at 33°C and transferred to the Madin-Darby canine kidney cell monolayers in 96-well culture plates. After 6 days of culture at 33°C in a CO2 incubator, CPE in the wells was examined under a light microscope. The neutralizing antibody titer of a given sample was defined as the reciprocal of the last serum or PPAb dilution with no observed CPE (Belshe et al. 2000). A titer of 10 was assigned to all serum samples in which the first dilution (1:20) was negative. A titer of 5 was assigned to all PPAb samples in which the first dilution (1:10) was negative.
The HAI assay was performed using a standard technique (Centers for Disease Control and Prevention 1998); serially diluted 25-µl aliquots of serum or PPAb samples in PBS were mixed with 25-µl aliquots of virus, corresponding to four HA units, in V-bottom 96-well plates (Nunc, Rochester, NY, USA) and incubated for 30 minutes at room temperature. At the end of the incubation, 50 µl of 0.5% chicken (for influenza A/H1N1 and B viruses) or turkey (for influenza A/H3N2 virus) red blood cells was added and incubated for a minimum of 45 minutes before reading for HAI activity. The HAI titer of a given sample was defined as the reciprocal of the last serum dilution with no HA activity. A titer of 2 was assigned to all samples in which the first dilution (1:4) was negative.
2.7. Statistical analysis
Paired t-tests were used for comparing different samples (PPAb versus serum). All titer data were logarithm transformed for analysis.
3. Results
3.1. Generation of PPAb with ex vivo culturing of B cells isolated before and after influenza vaccination
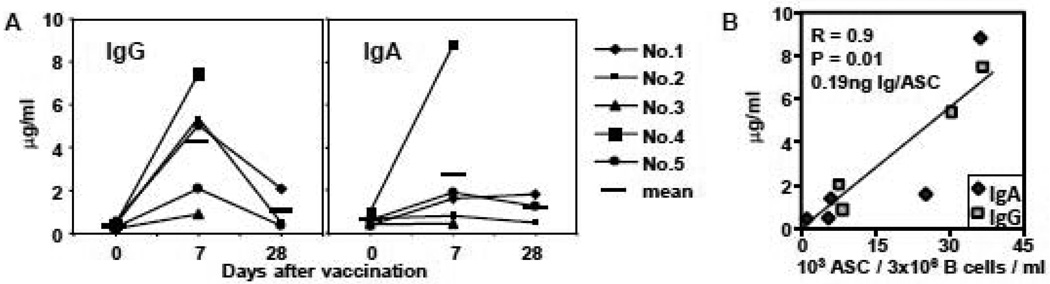
We immunized five adults with the 2008–09 inactivated influenza vaccine. B cells were isolated by negative selection from blood samples collected on day 0 (baseline), day 7, and day 28 after vaccination. The isolated B cells were cultured for one week. The conditioned media containing the secreted PPAb were recovered from the cultures and the concentrations of IgG and IgA determined. Both IgG and IgA were detected in the supernatant of B cell cultures (Figure 1A), indicating that the plasmablasts were capable of continued endogenous secretion of antibodies under the ex vivo culture conditions. The total IgG levels of all 5 donors and the IgA levels of 3 out of 5 donors are substantially higher in the day 7 samples than the day 0 samples, suggesting that the secreted antibodies, especially IgG, from the plasmablasts collected at day 7 after immunization with TIV are predominantly induced by vaccination, rather than reflecting background levels of antibodies produced by circulating plasmablasts specific for unrelated antigens (see Figures 2A and 2C in section 3.2 below). Therefore the levels of day 7 PPAb represent a reasonable estimate of the quantity of vaccine-induced antibodies. The concentration of antibodies in the day 7 PPAb samples correlated well with the frequency of total ASC determined by ELISPOT (Figure 1B). Based on the slope of the regression line, we estimate that 0.2 ng of IgG or IgA was secreted from each ASC under the ex vivo culturing condition (Figure 1B). Varying the culturing period from 4 – 7 days did not result in a significant increase in the amount of accumulated IgG or IgA in the PPAb samples (data not shown).
Figure 1.
Secretion of PPAb from cultured B cells. B cells collected from 5 subjects (No. 1 – No. 7) at different days after influenza vaccination were cultured at 3 million/ml for one week. A. Concentration of IgG and IgA in the conditioned media. The bars depict mean concentration. B. Linear regression between the concentration of PPAb and number of total ASC in culture of B cells collected on day 7 after vaccination. The number of IgA and IgG ASC in cultured B cells was determined with ELISPOT. IgG ELISPOT result is not available for subject No. 1.
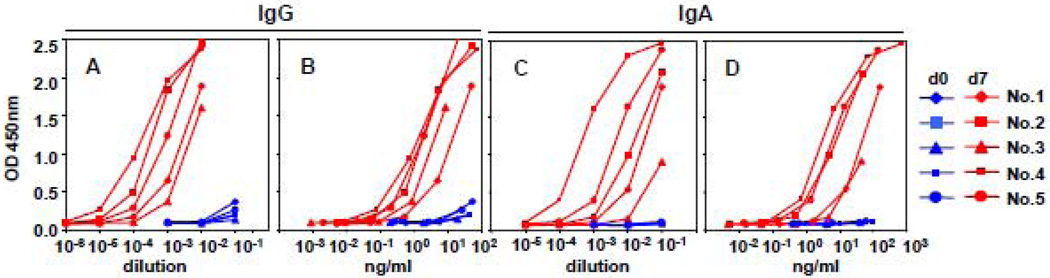
Figure 2.
Influenza-specific IgG and IgA binding reactivity of PPAb on day 0 (d0) and day 7 (d7) after vaccination. ELISA plates were coated with 2008 TIV. OD450nm values of 1:10 serially diluted PPAb samples were plotted against dilution factors (A, C) or PPAb concentration (B, D).
3.2. Influenza-specific binding activity of PPAb and serum before and after vaccination
We used ELISA to measure and compare the influenza-specific IgG and IgA binding activity in PPAb prepared from B cells collected before (day 0) and after (day 7) vaccination, as well as in serum samples collected before (day 0) and after (day 28) vaccination. As shown in Figures 2A and 2C, substantial levels of influenza-specific IgG and IgA binding reactivity were detected in PPAb at day 7 after vaccination, but not before vaccination. These results again indicate that the day 7 PPAb reflects antibodies specifically induced by the vaccine.
In Figures 2A and 2C the OD450nm values of serially diluted PPAb samples were plotted against the dilution factor to reflect the different titers of antibody binding activity present in the different samples, which are a function of both antibody concentration and antibody avidity. Since the day 7 PPAb are predominantly vaccine-induced and their immunoglobulin concentration has been determined, we were able to plot the OD450nm value against the actual concentration of PPAb, rather than the dilution factors, as seen in Figures 2B and 2D. Thus these different curves reflect the antibody concentration-normalized binding activity, or avidity of influenza-specific antibodies in PPAb samples, which represent the quality of the antibody response. Of note, the relative position of curves for individual vaccinees seen in figures 2A and 2C are not identical to those of the same donors plotted in figures 2B and 2D, suggesting both quantitative and qualitative differences among the five PPAb samples.
In Table 1 we summarize the influenza-specific ELISA binding titers of PPAb and serum samples collected before and after vaccination. The titers are defined as the highest dilution for each sample at which an influenza-specific binding signal was detectable by ELISA. In contrast to PPAb, which had negligible pre-vaccination titers compared to post-vaccination titers, substantial levels of baseline reactivity in the day 0 serum samples were detected in all donors, presumably reflecting prior exposures to influenza vaccine, infection or both. This difference is reflected in the fold-increase of titers after vaccination, which is on average greater than 483-fold in PPAb but smaller than 8-fold in serum sample pairs. These results suggest that a substantial portion of the influenza-specific reactivity in post-vaccination convalescent sera can be attributed to pre-existing antibodies resulting from prior exposure to influenza antigens that cross-react with the new vaccine antigens. In contrast, the post-vaccination PPAb reactivity represents the overall antibody response specifically induced by the current vaccination.
Table 1.
Influenza-specific reactivity of PPAb and serum samples before and after TIV immunization
| subject | PPAb |
serum |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG |
IgA |
IgG |
IgA |
|||||||||||
| titer (dilution) |
avidity (ng/ml) |
titer (dilution) |
avidity (ng/ml) |
titer (dilution) |
titer (dilution) |
|||||||||
| day 0 | day 7 | day 7/day 0 | day 7 | day 0 | day 7 | day 7/day 0 | day 7 | day 0 | day 28 | day 28/day 0 | day 0 | day 28 | day 28/day 0 | |
| No.1 | 10–100 | 32,000 | 320–3,200 | 0.16 | <10 | 3,200 | >320 | 0.50 | 2×106 | 1.6×107 | 8 | 8×104 | 1×105 | 1 |
| No.2 | 10–100 | 80,000 | 800–8,000 | 0.07 | <10 | 2,000 | >200 | 0.40 | 1×106 | 1×107 | 10 | 8×103 | 2×105 | 25 |
| No.3 | 10–100 | 8,000 | 80–800 | 0.11 | <10 | 400 | >40 | 1.13 | 4×106 | 1.6×107 | 4 | 8×104 | 1×10 | 1 |
| No.4 | 10–100 | 160,000 | 1,600–16,000 | 0.05 | <10 | 64,000 | >6,400 | 0.14 | 1×106 | 1.6×107 | 16 | 1.6×105 | 1×106 | 6 |
| No.5 | 10–100 | 80,000 | 800–8,000 | 0.03 | <10 | 16,000 | >1,600 | 0.12 | 2×106 | 1×107 | 5 | 8×104 | 1.6×106 | 20 |
| geo mean | 10–100 | 48,273 | 483–4,827 | <10 | 4,827 | >483 | 1.7×106 | 1.3×107 | 7.6 | 5.8×104 | 3.2×105 | 5 | ||
Serially (1:2) diluted PPAb and serum samples were tested in TIV-coated ELISA plates. Titer is defined as the highest dilution at which the mean OD value of duplicate wells is greater than mean+2×SD (standard deviation) of negative control wells. Avidity is defined as the lowest concentration of IgG or IgA at which the mean OD value of duplicate wells is greater than mean+2×SD (standard deviation) of negative control wells.
Table 1 also lists the influenza-specific binding avidity of day 7 PPAb samples, defined as the lowest antibody concentration for each sample at which an influenza-specific binding signal was detectable by ELISA. A similar assessment of antibody avidity is not feasible for serum samples due to the fact that the vast majority of antibodies in the serum are not specific for influenza antigens, and the proportion of vaccine-induced antibodies in serum cannot be easily determined.
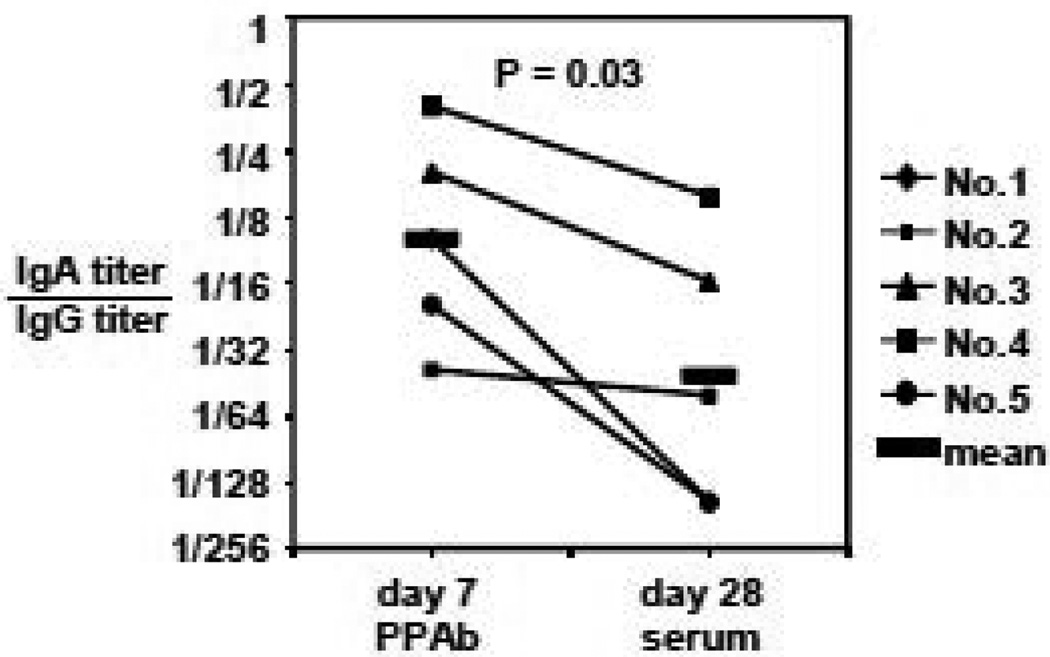
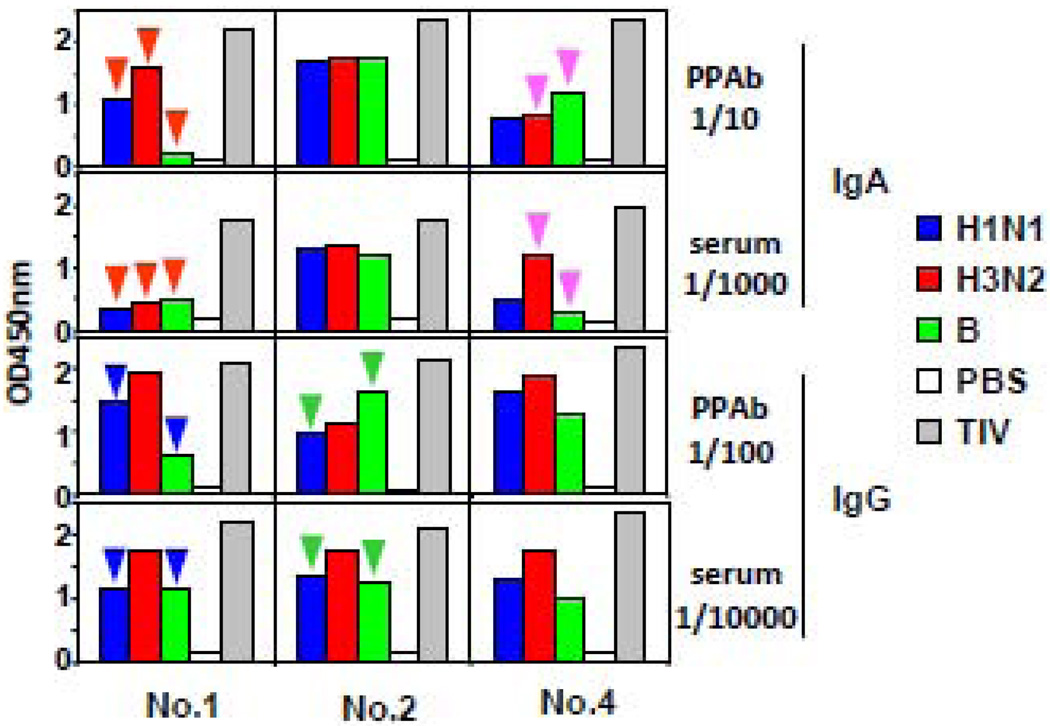
Next we compared the relative influenza-specific IgA and IgG reactivity in PPAb and sera (Figure 3). The ratio of influenza-specific IgA titer to IgG titer is on average 4-fold greater in the day 7 PPAb samples than the day 28 serum samples, indicating that vaccine-specific IgA is enriched in PPAb compared to serum. The different patterns of binding reactivity between PPAb and serum samples were further examined by measuring binding reactivity against individual vaccine component influenza strains (Figure 4). In donor No. 1, a hierarchy of IgA reactivity against the three vaccine component viruses is evident as H3N2>H1N1>B in the PPAb, but not in the serum (red arrows). Differences between PPAb and serum samples are also seen in the IgA reactivity of donor No. 4 (purple arrows), as well as IgG reactivity of donors No. 1 (blue arrows) and No. 2 (green arrows). This indicates that the relative antigenic specificities in PPAb and comparable convalescent serum specimens are not necessarily identical.
Figure 3.
Influenza-specific IgA reactivity is enriched in PPAb than sera. IgA and IgG binding titers against TIV were determined by ELISA.
Figure 4.
Patterns of influenza virus-specific binding reactivity in PPAb differ from those in serum. PPAb (day 7) and serum (day 28) samples from 3 subjects were tested for IgA and IgG reactivity in ELISA plates coated with the 2008 TIV or its individual component viruses. Distinct patterns of relative reactivity against the 3 different vaccine strains in PPAb versus sera are labeled with different color arrows.
3.3. Functional reactivity of PPAb
We next tested the day 7 PPAb and day 28 serum samples for functional activity against the three vaccine component viruses by microneutralization and HAI assays. As expected, most of the post-immunization serum samples tested positive in these assays. Out of the 15 tests conducted with the five PPAb samples against the three influenza viruses contained in the vaccine, positive results were obtained in six microneutralization tests and seven HAI tests (Table 2). Based on the neutralization titer and concentration of PPAb, we calculated the minimum concentration of each PPAb at which a neutralization effect was detected. This concentration-normalized neutralization reactivity is defined as the neutralization activity of the PPAb samples against each virus strain in the TIV, which ranged from 51 ng/ml to 309 ng/ml (Table 2). The six-fold variation in this parameter reflects the different efficiency of each PPAb in neutralizing specific viral strains, which depends on the intrinsic qualitative characteristic of the antibodies but not on their concentration. Similar to avidity (Table 1), this qualitative parameter can be directly evaluated with PPAb samples but not with serum samples, simply because the concentration of vaccine-induced antibodies in serum cannot be readily determined. Of note, in a recently published study, Jin et al. generated four recombinant human monoclonal antibodies with neutralization activity from influenza vaccine-induced plasmablasts (Jin et al. 2009). The neutralization activity of these monoclonal antibodies, determined with a similar assay, ranged from 250 ng/ml to 1,500 ng/ml, which is similar to or lower than the PPAb samples.
Table 2.
Functional reactivity of day 7 PPAb samples
| assay |
Microneutralization |
HAI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| sample | H1N1 |
H3N2 |
B |
H1N1 |
H3N2 |
B |
|||
| titer | neut. act. ng/ml |
titer | neut. act. ng/ml |
titer | neut. act. ng/ml |
titer | titer | titer | |
| No.1 | <10 | <10 | <10 | <4 | 4* | <4 | |||
| No.2 | 80** | 77 | 20* | 309 | 40** | 154 | 16** | 4* | 4* |
| No.3 | 10 | 10 | <10 | <4 | <4 | <4 | |||
| No.4 | 320** | 51 | 10 | 320** | 51 | 64** | <4 | 16** | |
| No.5 | 20* | 197 | 10 | <10 | 4* | <4 | <4 | ||
| medium# | 10 | 10 | <10 | <4 | <4 | <4 | |||
complete medium served as the negative control
2× increase than medium control
≥4× increase than medium control
Neutralizing activity (neut. Act.) = (IgG conc. + IgA conc.)/neutralization titer
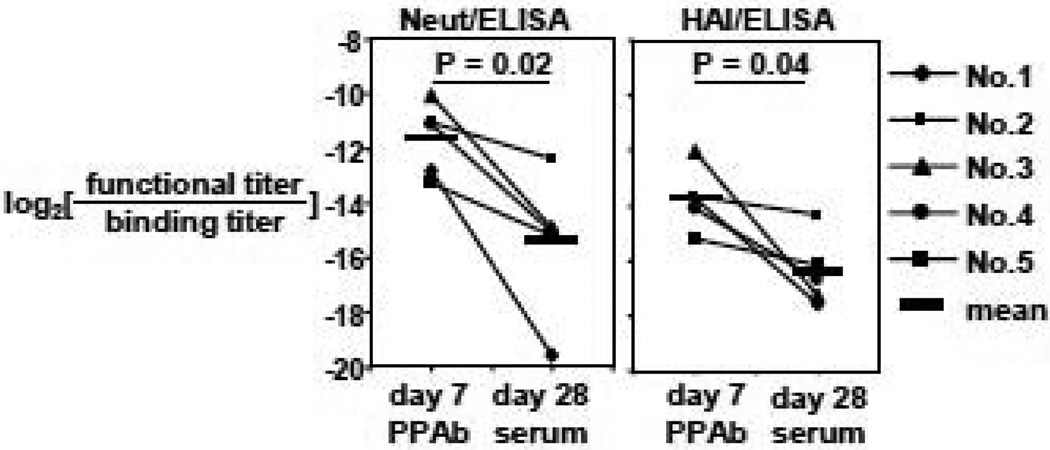
Finally, we sought to estimate what fraction of the total influenza-specific binding reactivity in the PPAb or serum samples could actually inhibit viral infection, which is measured by microneutralization assay, or block the binding of viral particles to red blood cells, which is measured by the HAI assay. The binding of influenza viral particles to red blood cells resembles their binding to the infection target cells. To this end we calculated the ratio of average functional activity titers (neutralization titer or HAI titer) to the total binding activity titers determined by ELISA for each PPAb and serum sample. As shown in Figure 5, this ratio is significantly greater in PPAb than in sera when either neutralization titer (16-fold difference) or HAI titer (8-fold difference) is used to represent functional reactivity. These data indicate that the functional reactivity, which varies between donors and, possibly, between immunoglobulin isotypes (see Figure 6 in section 3.4 below), is enriched in PPAb compared to sera.
Figure 5.
Influenza-specific functional reactivity against vaccine viruses are enriched in PPAb than serum. Functional titer is defined as the geometric mean of neutralization or HAI titer against each of the 3 vaccine component viruses. Neutralization titer of <10 (for PPAb) or <20 (for sera) is assigned a value of 5 or 10, respectively. HAI titer of <4 is assigned a value of 2. Binding titer is defined as the sum of ELISA IgA titer and IgG titer against TIV.
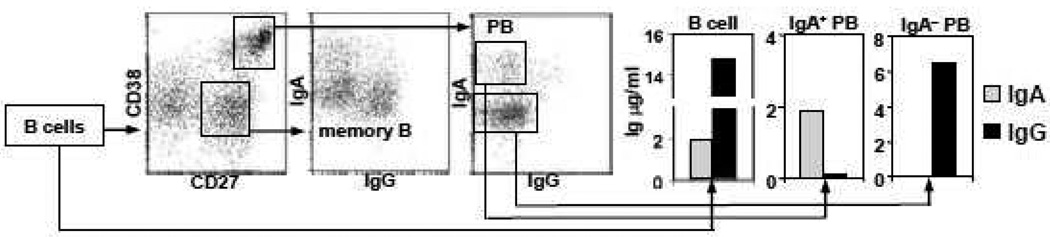
Figure 6.
Secretion of PPAb from total B cells and sorted plasmablast (PB) subsets. Total B cells, or sorted IgA+ subset (13,000 cells) and IgA− subset (80,000 cells) of CD27highCD38high PB mixed with homologous CD3+ cells (as feeder cells), were cultured for 7 days. The expression of surface IgG was down-modulated on IgG PB, compared to IgG memory B cells. Concentration of IgG and IgA in culture media was measured by ELISA. This is the representative data of two experiments conducted with day 7 post influenza vaccination blood samples from donors No. 6 and No. 7, respectively, with similar results.
3.4. Generation of PPAb from FACS-sorted plasmablast subsets
In addition to being generated from bulk B cell cultures, PPAb can also be generated from specific plasmablast subsets sorted by flow cytometer based on their surface phenotypes. In a representative experiment shown in Figure 6, surface IgA+ and IgA− subsets of plasmablasts from a blood sample collected on day 7 after immunization with the 2009–2010 TIV were sorted as indicated and then cultured. While both IgA and IgG were detected in the total B cell culture, the sorted IgA+ population and IgA− population produced only IgA or IgG, respectively. These results confirm that although IgG plasmablasts have down-modulated expression of cell surface B cell receptor in comparison to memory IgG cells (Figure 6), they can be identified in the IgA− plasmablast population. They also indicated that the FACS-sorted plasmablasts maintained the capability of secreting antibodies under ex vivo culturing condition.
4. Discussion
Almost three decades ago Ershler et al. developed a new assay for antibody response after tetanus toxoid immunization, which involved culturing lymphocytes in ELISA plates pre-coated with tetanus toxoid to capture and quantify antigen-specific IgG secreted from ASC (Ershler et al. 1982). When this assay, named microculture antibody synthesis enzyme linked assay (MASELA), was used to study antibody response after influenza vaccination, the authors reported highly correlated results with serum IgG antibody response (Ershler et al. 1988). Since then this assay has not been widely used to study antibody responses to influenza vaccines. In the current study we showed that after influenza vaccination, PPAb collected from cultured B cells demonstrate distinct patterns of influenza vaccine-specific and isotype-specific reactivity in comparison to serum antibodies, as well as a potential of providing new insights to the overall B cell response to viral vaccination or natural infection.
The primary function of the B cell response is production of antibodies. Influenza viruses are respiratory pathogens that infect the respiratory mucosal epithelial surface. Therefore mucosal antibodies, in particular secretory IgA, play a major role in protection, especially of the upper airway (Cox et al. 2004). However, collection of secreted mucosal antibodies from the respiratory tract is not practical in most clinical settings and standardization of such assays has proven difficult. Although conventional serological assays, including the serum neutralization assay and the HAI assay, provide quantitative measurements of the influenza-specific antibodies before or after vaccination, the direct role of serum antibodies in protective immunity is not known. In addition, serum antibody titers do not always correlate well with vaccine-induced protection, for example in adult recipients of LAIV (Treanor and Wright 2003), suggesting that serum antibody titers might not always correlate with the protective immune response. In this study we showed very distinct patterns of influenza specific binding and functional reactivity between PPAb and serum samples after vaccination, in particular the different relative reactivity of IgA versus IgG (Figures 3 and 5). These differences can likely be explained by the fact that serum antibodies are derived from a subset of vaccine-activated B cells homing to the bone marrow, while PPAb are derived from the overall vaccine-activated B cell population including those potentially migrating to the respiratory mucosa to become mucosal ASC. We propose that TIV-induced IgA and IgG plasmablasts have differential homing potential: the IgG plasmablasts preferentially migrate to bone marrow to become IgG plasma cells, while at least some of the IgA plasmablasts are less likely to migrate to bone marrow, resulting in a reduced presence of IgA plasma cells at this site, thus lower relative IgA level in the post-vaccination serum samples. Of note, a similar in vitro assay of secreted antibody in the lymphocyte supernatant (ALS assay) has been successfully used in the study of IgA responses after oral immunization and natural infection with bacterial pathogens of the gastrointestinal tract (Chang and Sack 2001; Qadri et al. 2003; Qadri et al. 2007; Sheikh et al. 2009). These studies support the proposition that PPAb provides an alternative and potentially more complete reflection of mucosal antibody responses following vaccination and infection. In particular, PPAb response after nasal immunization with the live attenuated influenza vaccine might reveal characteristics of the antibody response to the influenza vaccine delivered to the respiratory mucosal surface that are not available from the analysis of serum antibody.
An important issue in influenza vaccination is that most adults and older children have been previously exposed to influenza antigens via natural infection and/or previous vaccination and therefore have variable levels of baseline immunity against previously encountered influenza antigens, some of which might be similar to those present in the administered vaccine, including serum antibodies that cross-react with the new vaccine strains. Therefore the vaccine-specific antibody reactivity in the post-vaccination serum samples always represents the antibody response to the new vaccination plus pre-existing antibodies that cross-react with the new vaccine antigens, which can confound analysis of response to new vaccination or infection. In contrast, as shown here, PPAb are derived virtually exclusively from B cells recently activated by the new antigen exposure. This difference between serum antibody and PPAb also provides an explanation for the distinct patterns of influenza strain-specific binding activity in PPAb versus serum for the three TIV antigenic components shown in Figure 4. This feature of PPAb is particularly attractive for studying vaccine-induced heterosubtypic antibody reactivity against previously exposed viruses, without the interference from the cross-reacting serum antibodies resulting from prior exposure. It will also be useful in situations such as studying B cell response to dengue viruses in which cross-reactive antibodies against different virus serotypes can play a critical role in the pathogenesis of infection. Another important advantage of PPAb analysis, also based on the fact that PPAb is produced by recently activated B cells, is it allows assessment of vaccine-induced antibody avidity by measurement of antigen specific binding and antibody quantity. This type of analysis is not easily accomplished for serum antibodies because the amount of serum antibodies directly induced by the most recent vaccination cannot be readily determined.
An essential component of protective antibody response is targeting of the ASC to organs and tissues where the infecting pathogen enters and replicates (Kunkel and Butcher 2002; Kunkel and Butcher 2003). In the case of influenza, the targets are the mucosal surface of the upper and lower bronchial tree and the lung. Lymphocyte trafficking is determined by the patterns of trafficking receptors expressed on these cells, which depend on the site of antigen exposure and the local antigen-presenting environment (Sigmundsdottir and Butcher 2008). In this study we showed that in addition to being collected from bulk B cell cultures, PPAb can also be generated from FACS-sorted plasmablast subsets (Figure 6). This will be very useful for functional analysis of specific plasmablast subsets, for example those expressing specific homing receptors for certain tissues such as respiratory or intestinal mucosal sites, to determine their role in the protective antibody response against infection or immunization at the different mucosal sites in vivo.
In summary, the current work has extended previously published assays based on antibodies secreted from ex vivo cultured plasmablasts (Ershler et al. 1982; Chang and Sack 2001). Our results indicate that analysis of PPAb is likely to be a useful supplement to the conventional serological assays as well as other plasmablast-based assays for investigating the B cell and antibody responses to viral infection and vaccination.
Acknowledgements
We thank K. Subbarao for providing influenza virus vaccine strains, our study subjects for their participation, S. Mackey for coordinating the clinical study, and T. Quan, S. Swope, and C. Walsh for enrolling subjects and collecting blood samples.
Role of the funding source
The project described was supported by the NIH grants AI057229 and DK56339. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ASC
antibody secreting cells
- HAI
hemagglutination inhibition
- LAIV
live attenuated influenza vaccine
- PPAb
plasmablast-derived polyclonal antibodies
- TIV
trivalent inactivated trivalent influenza vaccine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, Treanor J, Zangwill K, Hayden FG, Bernstein DI, Kotloff K, King J, Piedra PA, Block SL, Yan L, Wolff M. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J. Infect. Dis. 2000;181:1133. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- Benner R, Hijmans W, Haaijman JJ. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin. Exp. Immunol. 1981;46:1. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Atlanta, Ga: Centers for Disease Control and Prevention; The 1998–99 WHO influenza reagent kit for the identification of influenza isolates. 1998
- Chang HS, Sack DA. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin. Diagn. Lab. Immunol. 2001;8:482. doi: 10.1128/CDLI.8.3.482-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 2004;59:1. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993. doi: 10.1016/0264-410x(94)90334-4. [DOI] [PubMed] [Google Scholar]
- el-Madhun AS, Cox RJ, Soreide A, Olofsson J, Haaheim LR. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J. Infect. Dis. 1998;178:933. doi: 10.1086/515656. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Coe CL, Gravenstein S, Schultz KT, Klopp RG. Specific antibody synthesis in vitro. IV. The correlation of in vitro and in vivo antibody response to influenza vaccine in rhesus monkeys. Clin. Exp. Immunol. 1988;73:355. [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Moore AL, Hacker MP. Specific in vivo and in vitro antibody response to tetanus toxoid immunization. Clin. Exp. Immunol. 1982;49:552. [PMC free article] [PubMed] [Google Scholar]
- Gerhard W. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 2001;260:171. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- Jin A, Ozawa T, Tajiri K, Obata T, Kondo S, Kinoshita K, Kadowaki S, Takahashi K, Sugiyama T, Kishi H, Muraguchi A. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody-secreting cells from human peripheral blood. Nat. Med. 2009;15:1088. doi: 10.1038/nm.1966. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Plasma-cell homing. Nat. Rev. Immunol. 2003;3:822. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- Qadri F, Ahmed T, Ahmed F, Bhuiyan MS, Mostofa MG, Cassels FJ, Helander A, Svennerholm AM. Mucosal and systemic immune responses in patients with diarrhea due to CS6-expressing enterotoxigenic Escherichia coli. Infect. Immun. 2007;75:2269. doi: 10.1128/IAI.01856-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F, Ryan ET, Faruque AS, Ahmed F, Khan AI, Islam MM, Akramuzzaman SM, Sack DA, Calderwood SB. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 2003;71:4808. doi: 10.1128/IAI.71.8.4808-4814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, Greenberg HB. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, Kemble GW, Arvin AM, Greenberg HB. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J. Virol. 2007;81:215. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh A, Bhuiyan MS, Khanam F, Chowdhury F, Saha A, Ahmed D, Jamil KM, LaRocque RC, Harris JB, Ahmad MM, Charles R, Brooks WA, Calderwood SB, Cravioto A, Ryan ET, Qadri F. Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin. Vaccine Immunol. 2009;16:1587. doi: 10.1128/CVI.00311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 2008;9:981. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 2009;4:372. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor J, Wright PF. Immune correlates of protection against influenza in the human challenge model. Dev. Biol. (Basel) 2003;115:97. [PubMed] [Google Scholar]
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]