Abstract
Mycobacterium tuberculosis is the causative agent of tuberculosis disease, which has developed a myriad of exceptional features contributing to its survival within the hostile environment of host cell. Unique cell wall structure with high lipid content plays an imperative role in the pathogenicity of mycobacteria. Cell wall components of MTB such as lipoarabinomannan and Trehalose dimycolate (cord factor) are virulent in nature apart from its virulence genes. Virulent effect of these factors on host cells reduces host cell immunity. LAM has been known to inhibit phagosome maturation by inhibiting the Ca2+/calmodulin phosphatidyl inositol-3-kinase hvps34 pathways. Moreover, TDM (Trehalose dimycolate) also inhibits fusion between phospholipid vesicles and migration of polymorphonuclear neutrophils. The objective of this paper is to understand the virulence of LAM and cord factor on host cell which might be helpful to design an effective drug against tuberculosis.
1. Introduction
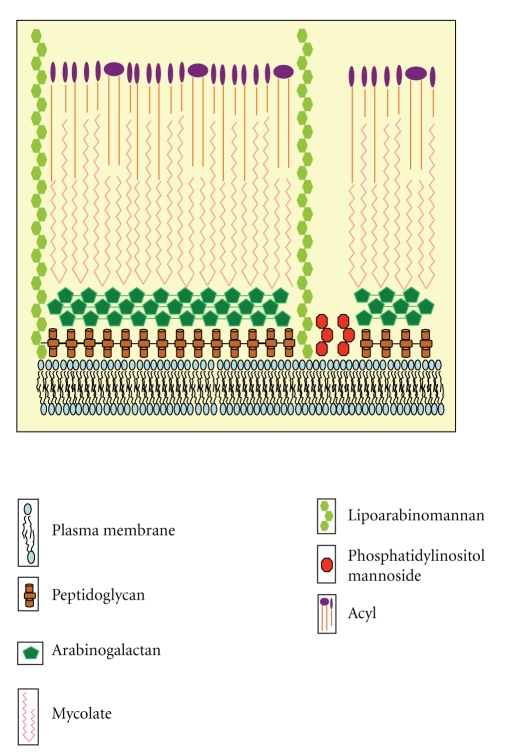
Mycobacterium tuberculosis (MTB) is exceptionally successful pathogen with unique characteristic features which make it highly pathogenic [1]. Cell wall of MTB is composed of 60% of lipids. Major fraction of its cell wall is mycolic acid, Cord factor, and Wax-D [2, 3]. The cell wall of MTB is composed of two segments: outer part and core of cell wall (Figure 1). Core of cell wall is made up of peptidoglycan (PG), covalently attached with arabinogalactan (AG) and mycolic acids subsequently, forming the mycolyl arabinogalactan-peptidoglycan (mAGP) complex. Upper part is composed of free lipids which are linked with fatty acids. Mostly this part is made up of different cell wall proteins, the phosphatidylinositol mannosides (PIMs), Lipomannan (LM), and Lipoarabinomannan (LAM). These proteins along with lipids and glycoconjugate lipids act as effector molecules of signaling process, and the insoluble core is essential for the viability of the cell [3]. LAM blocks phagosomal maturation in host cell either by blocking the trafficking pathway from trans-Golgi network (TGN) to phagosomes which itself depends on early endosomal autoantigen 1 (EEA1), an essential Rab5 factor recruitment to early phagosomes, or by inhibiting the Ca2+ concentration in macrophages, as Ca2+ is an essential factor for phagosomal maturation [4]. Another virulence factor, produced by MTB, is Cord factor. Cord factor behaves differentially according to its localization. It is nontoxic, when present on organisms and protects them from macrophage destruction, but it becomes toxic on lipid surfaces. TDM inhibits the phagosome-lysosome fusion and contributes to the maintenance of granulomatous response. Removal of surface lipids enhances trafficking of organisms to acidic compartments [5]. Accumulation of TDM causes weight loss in organisms, resulting in the condition known as Cachexia. In this condition, animals exhibit hypertriglyceridemia, hypoglycemia, and Tumor- Necrosis factor (TNF) in plasma [6]. MTB produces diversity of lipids which are responsible for its pathogenicity.
Figure 1.
Structure of mycobacterial cell wall.
2. Lipoarabinomannan (LAM)
Lipoarabinomannan commonly known as LAM, is a glycoconjugate and one of the virulence factor associated with MTB. It is a major cell wall component and allows the MTB to survive in host cell environment by affecting host resistance and immune responses [7]. LAM inhibits T-cell proliferation and bactericidal activities of macrophages [8]. In addition, LAM eliminates cytotoxic oxygen-free radicals produced by macrophages and inhibits the activity of protein kinase C and also blocks the activation of gamma-interferon at transcriptional level [8]. LAM is capped with short mannose containing oligosaccharides which allow the bacteria to bind with the mannose receptors present on the macrophages. LAM also has ability to bind with the toll receptors and thus can insert itself into biological membranes affecting signaling events [3]. LAM causes the release of TNF in vitro in human blood monocytes and in vivo in mice. TNF release may be responsible for the characteristics of tuberculosis, such as, loss in weight, fever, and cytokine-mediated necrosis [9]. It was also observed that LAM binds to the DC-SIGN molecule which is expressed on the surface of dendritic cells.
DC-SIGN is indispensable for the maturation of dendritic cells, but binding of LAM inhibits the process. This inhibition also results in decreased IL-12 production and induction of dendritic cells to secrete IL-10, which in turn inhibits antigen presentation, expression of MHC molecules, and costimulatory receptors. In view of these observations recent studies also found that TB patients exhibit considerably elevated levels of IL-10 [10]. This can be demonstrated in vitro by the inhibition of polyethylene glycol- (PEG-) induced lipid vesicle fusion with Fluorescence resonance energy transfer (FRET). PEG absorbs water molecules around the lipid vesicles and promotes fusion of these vesicles. Lower FRET signals are obtained with LAM, which shows that LAM inhibits the association of adjacent vesicles. Instead of Polyethylene glycol (PEG), SNARE proteins act as fusion attachment receptors in vivo [11].
LAM is virulent in nature and causes phagosome maturation arrest by blocking Ca2+/Calmodulin phosphatidyl-inositol-3-kinase hvps34 pathways resulting in the long-term survival of MTB in host cell environment [12].
2.1. Structure
LAM is mainly made-up of three components: membrane anchor, mannosyl-phosphatidyl-myo-inositol, backbone, mannopyranose, and arabinofuranose which are homopolysaccharides and the capping motif. Membrane anchor attaches the molecule to cell wall at the time of infection, and homopolysaccharides serve as carbohydrate skeleton [13, 14].
2.2. Classification of LAM
Classification of LAM is based on the presence and structure of capping and classified LAMs into three major classes.
2.2.1. Mannosylated LAMs (Man LAM)
In Man LAMs, mannosyl groups are present on the D-arabinan group. After the mannosyl capping, Man LAM acts as anti-inflammatory molecule and inhibits the production of TNF-α and IL-12. These properties of Man LAM allow the bacteria to survive in the host cell for long time. Man LAMs are mainly found in pathogenic mycobacterial species such as MTB, Mycobacterium leprae (M. leprae), and Mycobacterium bovis (M. bovis) [15].
2.2.2. Phosphoinositol-Capped LAM (PILAM)
LAMs capped with phosphoinositol groups are called as PILAMs, mainly found in nonpathogenic species such as Mycobacterium smegmatis (M. smegmatis). PILAMs can bind with CD14 receptor, present on macrophages. CD14 receptor is associated with toll like receptor 2 (TLR 2) which acts as recognition receptor for PILAMs. PILAMs induce the production of cytokines such as TNF-alpha, IL-8, and IL-12 [16].
2.2.3. Arabinofuranosyl-Terminated LAM (Ara LAM)
Ara LAM 1, 3-mannosyl side chains are present instead of 1, 2-mannosyl side chain, commonly found in many mycobacterial species. Ara LAM also induces the production of many cytokines such as TNF-alpha, IL 1-alpha, IL 1-β, IL-6, IL-8, and IL-10. Ara LAM is generally found in Mycobacterium chelonae (M. chelonae) and laboratory strains, M. tuberculosis H37Ra [15].
2.3. Biosynthesis of LAM
Many other lipid components of bacterial cell wall like LM and PIMs are involved in the synthesis of LAM. LAM is synthesized by the addition of mannopyranosyl to a phosphoinositol. PIMs are considered as precursors of LAMs in the biosynthesis pathway [9]. PIMs and LM are synthesized by the addition of mannopyranosyl to a phosphoinositol. Glycosylation of PIMs and LM with arabinan forms LAM. Mannosyltransferases are involved in the synthesis of PIMs encoded by the different genes as shown in Table 1. PIMs have been recognized as the major nonpeptidic Antigens of the host immune responses. PIMs are also TLR-2 agonists and are involved in the stimulation of unconventional αβ T lymphocytes in the perspective of CD-1 proteins. PIMs contribute in the opsonic and nonopsonic binding of MTB to phagocytic and nonphagocytic cells, since it is recognized by the C-type lectins, mannose receptor (MR), mannose binding protein (MBP), and DC-SIGN. Degree of mannosylation of PIMs and their fatty acyl appendages are crucial to their interactions with host cells [17].
Table 1.
Different genes involved in the biosynthesis of LAM.
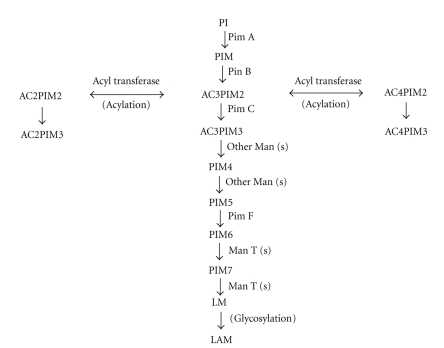
Mannosyl transferase, Pim A transfers one ManP group to the 2-position of the myo-inositol of PI, which is now converted in PIM1. Pim B transfers one ManP group to the 6 position of the myoinositol of PIM1 to form PIM2. PimA and Pim B both use GDP-Mannose as the sugar donor. Pim C transfers another group of ManP to PIM2 to form PIM3, using GDP-mannose as the sugar donor. Higher PIMs are formed by the further mannosylation of PIM3 with different mannosyl transferases. These PIMs are precursors for the synthesis of LM and LAM as shown in Figure 2. LM is glycosylated with arabinan to form LAM [18].
Figure 2.
Biosynthesis of LAM in mycobacteria. PI: Phosphatidyl-myo-inositol, PIM: Phosphatidylinositol mannoside, AC2PIM2: diacylphosphatidylinositol dimannoside, AC2PIM3: diacylphosphatidylinositol trimannoside, AC3PIM2: triacylphosphatidylinositol dimannosides, AC3PIM3: triacylphosphatidylinositol trimannoside, AC4PIM2: tetra-acylated Phosphatidyl-myo-inositol dimannoside, AC4PIM3: tetra-acylated Phosphatidyl-myo-inositol trimannoside, PIM4: Phosphatidylinositol tetramannoside, PIM5: Phosphatidylinositol pentamannoside, PIM6: Phosphatidylinositol hexamannoside, PIM7: Phosphatidylinositol heptamannoside, LM: lipomannan, LAM: Lipoarabinomannan.
PimF is involved in the synthesis of higher PIMs and involved in a later step of PIM synthesis. PimF transfers two sequential mannose group to AC4PIM5, resulting in the synthesis of AC4PIM7, an intermediate in the biosynthetic pathway of LAM. Mutation in PimF gene causes accumulation of AC4PIM5 but the synthesis of Pim6 species is not affected. Acylation of Pim1 and Pim2 is mediated by Rv2611c, an acyltransferase [20].
2.4. Maturation of Phagosomes into Phagolysosomes
Phagosomal maturation is a biological process in which phagosomes interact with endosomes and lysosomes. The membrane removed at the time of maturation of phagosomes into phagolysosomes. Maturing phagosomes form phagolysosomes which are essential for the destruction of foreign particles. Late endosomal and lysosomal constituents delivered to phagosomes via various sorting pathways [21, 22]. Two specific rab5 factors are essential for the maturation of phagosomes; (a) the phosphatidyl-inositol (PI) 3 kinase hvps34 and (b) early endosome autoantigen (EEA) [23], which interacts with endosome via its phosphatidyl-inositol-3-phosphate binding domain. These rabs are active when present in GTP-bound state and inactive when present in GDP-bound state. Replacement of rab5 by rab7 is essential for the maturation of phagosomes. The contents of early stage of phagosomes delivered to the late phagosomes via vesicular and intracellular membrane trafficking [24]. EEA1 binds with phagosomes by interacting with PI3P which is generated on early stage of phagosomes by the rab5 effector hvps34 [25]. Additional elements such as Rabaptin-5 [26], tuberin (a Rab5-GTPase activating protein) [27], Rabex 5 (a Rab 5 nucleotide exchange factor) [28] also participate in membrane tethering and fusion. EEA1 causes membrane fusion via interacting with the SNARE syntaxin 13, SNARE priming factor NSF, and alpha-SNAP, essential for the membrane-tethering and fusion [29].
2.5. LAM Causes Phagosome Maturation Arrest
LAM contributes to MTB's pathogenicity by blocking the phosphatidyl-inositol-3-kinase- and phosphatidylinositol-3-phosphate-dependent pathways, resulting in blocking of phagosome maturation [30]. LAM inhibits the recruitment of EEA1 to the early phagosome which is an essential factor for the maturation of phagosomes. LAM also inhibits Ca2+ increase which is required for the Ca2+/Calmodulin-PI3K hvps34 pathways, essential for the PI3P production. PI3P acts as a mediator for the association of phagosome and lysosome via EEA1 rab5 factor. An increase in the concentration of Ca2+ prolongs the binding of Ca2+/Calmodulin to CAMK II, a Serine/Threonine Kinase essential for the EEA1 recruitment to phagosome [5].
Apart from this, a coat protein, TACO, also inhibits the delivery of phagosomal contents to lysosomes, thus prevents the lysosomal delivery of mycobacteria and allow mycobacteria to survive in host macrophages for long time. TACO is formed around the phagosomes after infection [31].
3. Cord Factor (Trehalose 6, 6′-di-mycolate; TDM)
Cord factor is the most abundant glycolipid in the mycobacterial cell wall [32], one of the major constituent of MTB cell wall, is toxic to mammalian cells, and affects the host immune system by inhibiting the migration of polymorphonuclear neutrophils [33]. Cord factor has long chain lipids as structural component of the hydrophobic cell wall which is found to be crucial for the survival of mycobacteria within phagosomes of host [34]. Cord factor is responsible for the specific microscopic morphology called serpentine cords [35].
3.1. Structure
Cord factor molecules are made up of trehalose sugar which is esterified to two mycolic acid residues. Length of the residues can vary from species to species but generally these are present in the range of 20–80 carbons [36]. Two molecules of mycolic acids and one molecule of the disaccharide trehalose are obtained from alkaline hydrolysis of cord factor [37].
3.2. TDM Inhibits Fusion between Phospholipid Vesicles: Like Phagosomes and Lysosomes
TDM consists of trehalose which is attached to hydrophobic components. So, TDM inhibits fusion between two phospholipids bilayers by immobilizing the trehalose at the aqueous interphase. It acts as a barrier to fusion by increasing hydration force and creating a steric hindrance to fusion [38–41]. It has been proved that intact cord factor is required for the inhibition of fusion of phospholipid vesicles, as the free mycolic acid molecules and free trehalose are not able to inhibit fusion of vesicles [36].
In addition, cord factor also stimulates NADase activity in host cell, thus lowering the level of NAD, especially in liver, lung, and spleen. As a result, it reduces the activity of NAD-dependent enzymes. Oxidative phosphorylation and electron transport chain in mitochondria are also affected by the cord factor-mediated disruption of mitochondrial membranes [42].
3.3. Unique Biological Behavior of Cord Factor
Biological activities of Cord factor are dependent on its physical conformation. Cord factor is amphiphilic by nature and it forms micelles in aqueous medium and monolayer on hydrophobic surfaces. In monolayer formation, trehalose and mycolate domains are arranged in two dimensions and form crystal structure [43–45]. The outer surface of micelle is completely made-up of trehalose molecules which make it hydrophilic, and mycolate groups are covered in inner part of micelle [46]. Both micellar and monolayer forms have different biological properties. In micellar form, TDM is nontoxic but in monolayer configuration, it becomes highly toxic. As soon as macrophages come in contact with them, they are killed by mycobacterium [47]. TDM also acquire distinctive immunostimulatory activity, such as humoral responses, granulomagenesis, adjuvant activity for cell-mediated immune responses, and tumor regression [32].
3.4. Host Immune System versus TDM
TDM induces host immune system to secrete cytokines as immune response. Initially macrophages are present in their resting stage. After phagocytosis of bacilli, they gain activity. CD4 and CD8 T-cells are responsible for the immune response of host cells against MTB infection. Macrophages act as antigen presenting cells and interact with CD4 T-cells. CD4 T-cells release IFN-γ after this interaction, which stimulates macrophages for the release of cytokines such as TNF-α, IL-1, IL -1β, IL-12, and IL-6. Host macrophages produce higher amount of proinflammatory cytokines, when exposed to TDM. These cytokines are essential for the formation of granulomas. It also induces chemokine production like as MCP-1 and MIP-1α [48]. In a study it was also proposed that TDM can also be converted into glucose mono mycolate (GMM) inside host cell [49]. In a biochemical study, it has been postulated that TDM is a direct Mincle ligand. Based on this observation, a study was carried out to show that mycobacteria causes conversion of TDM into GMM upon infection into host in order to escape from Mincle- mediated host immunity [50].
3.5. Viability of MTB in Host Macrophages
Petroleum ether methods are used to remove TDM from virulent M. tuberculosis and MTB becomes delipidated [34]. Delipidated MTB is less viable than native MTB. Survival of MTB in macrophages is reduced after delipidation. Cells which are infected with delipidated MTB produce less amount of IL-1β, IL-6, TNF-alpha, and IL-12 but higher amount of IL-10. MCP-1 and MIP-1 alpha production is also delayed after delipidation. Viability of MTB can be restored after addition of pure TDM [56]. Role of TDM in different forms of TB is described in Table 2.
Table 2.
Role of TDM in different forms of TB.
| Type of tuberculosis | Role of TDM | References |
|---|---|---|
| Primary tuberculosis | (1) For survival of MTB in host cell environment mainly in macrophages by inhibiting phagosome-lysosome fusion. | [50] |
| (2) At the time of caseating granulomas formation, a sufficient dose of TDM is required. | [5] | |
| Secondary tuberculosis | (1) Preliminary stage of secondary tuberculosis is called as lipid pneumonia, which is caused by the infected material spilled from granuloma cavities. | [51] |
| (2) Due to the presence of TDM, MTB is able to survive in foamy alveolar macrophages. | ||
| (3) Bronchial obstruction takes place either because of granuloma or lipid content present on MTB cell wall. | [52–54] | |
| Caseation necrosis | (1) TDM monolayer triggers caseation necrosis in MTB. | [55] |
| (2) Toxic effects of TDM contribute to the maintenance of the lesions. | ||
3.6. Cachexia
Cachexia is caused by accumulation of TDM in body in which body weight is reduced even on consuming a proper diet by the animal. Cachectin (TNF) mediates this condition [5]. In this condition, animals lost ability to produce granulomas and die of hemorrhagic pneumonia [57, 58].
4. Conclusion
Mycobacterial lipids have a major role in pathogenicity of MTB. This paper presents evidence that LAM inhibits phagosome maturation and TDM inhibits fusion between phospholipid vesicles (phagosomes and lysosomes). Therefore, it is responsible for the long time survival of MTB in host body. Because of the presence of these unique characteristics features, MTB is highly pathogenic. As we all know that these are not the only factors which provide virulence to MTB. MTB has a plethora of defense mechanisms against host immune system and virulence so targeting a single drug target cannot be a good strategy against MTB infection. Drug resistance is causing another problem in the way of effective treatment of TB, so we should always look for newer drug targets and we can take advantage of the virulence mechanism of LAM and cord factor in the development of new drugs. Further research and investigations may lead to a better understanding for tuberculosis and be helpful in controling it effectively.
Acknowledgments
The authors thank Dr. Rajesh S. Gokhale for making this work possible. The authors acknowledge financial support from GAP0050 of the DST (Department of Science and Technology) and CSIR (Council of Scientific & Industrial Research).
Abbreviations
- MTB:
Mycobacterium tuberculosis
- LAM:
Lipoarabinomannan
- TDM:
Trehalose di-mycolate
- TACO:
Tryptophane aspartate-containing coat protein
- FRET:
Fluorescence resonance energy transfer
- PI:
Phosphatidyl-myo-inositol
- PIM:
Phosphatidylinositol mannoside
- LM:
Lipomannan
- TGN:
Trans-Golgi network
- EEA1:
Early endosomal autoantigen 1
- MHC:
Major histocompatibility complex.
References
- 1.Brennan PJ, Nikaido H. The envelope of mycobacteria. Annual Review of Biochemistry. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 2.Alderwick LJ, Birch HL, Mishra AK, Eggeling L, Besra GS. Structure, function and biosynthesis of the Mycobacterium tuberculosis cell wall: arabinogalactan and lipoarabinomannan assembly with a view to discovering new drug targets. Biochemical Society Transactions. 2007;35(5):1325–1328. doi: 10.1042/BST0351325. [DOI] [PubMed] [Google Scholar]
- 3.Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis. 2003;83(1–3):91–97. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- 4.Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca /calmodulin-PI3K hVPS34 cascade. Journal of Experimental Medicine. 2003;198(4):653–659. doi: 10.1084/jem.20030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva CL, Faccioli LH. Tumor necrosis factor (cachectin) mediates induction of cachexia by cord factor from mycobacteria. Infection and Immunity. 1988;56(12):3067–3071. doi: 10.1128/iai.56.12.3067-3071.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Annals of Clinical and Laboratory Science. 2006;36(4):371–386. [PubMed] [Google Scholar]
- 7.Guérardel Y, Maes E, Briken V, et al. Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: novel structural features and apoptosis-inducing properties. Journal of Biological Chemistry. 2003;278(38):36637–36651. doi: 10.1074/jbc.M305427200. [DOI] [PubMed] [Google Scholar]
- 8.Knutson KL, Hmama Z, Herrera-Velit P, Rochford R, Reiner NE. Lipoarabinomannan of Mycobacterium tuberculosis promotes protein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes: role of the Src homology 2 containing tyrosine phosphatase 1. Journal of Biological Chemistry. 1998;273(1):645–652. doi: 10.1074/jbc.273.1.645. [DOI] [PubMed] [Google Scholar]
- 9.Cotran RS, Kumar V, Robbins SL. Pathologic Basis of Disease. Vol. 4. Philadelphia, Pa, USA: W. B. Saunders; 1989. [Google Scholar]
- 10.Dietrich J, Doherty TM. Interaction of Mycobacterium tuberculosis with the host: consequences for vaccine development. APMIS. 2009;117(5-6):440–457. doi: 10.1111/j.1600-0463.2009.02458.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa E, Tokumasu F, Nardone GA, Jin AJ, Hackley VA, Dvorak JA. A Mycobacterium tuberculosis-derived lipid inhibits membrane fusion by modulating lipid membrane domains. Biophysical Journal. 2007;93(11):4018–4030. doi: 10.1529/biophysj.107.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik ZA, Denning GM, Kusner DJ. Inhibition of Ca signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. Journal of Experimental Medicine. 2000;191(2):287–302. doi: 10.1084/jem.191.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee D, Khoo KH. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8(2):113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 14.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie. 2003;85(1-2):153–166. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 15.Gilleron M, Himoudi N, Adam O, et al. Mycobacterium smegmatis phosphoinositols-glyceroarabinomannans: structure and localization of alkali-labile and alkali-stable phosphoinositides. Journal of Biological Chemistry. 1997;272(1):117–124. doi: 10.1074/jbc.272.1.117. [DOI] [PubMed] [Google Scholar]
- 16.Vignal C, Guérardel Y, Kremer L, et al. Lipomannans, but not lipoarabinomannans, purified from Mycobacterium chelonae and Mycobacterium kansasii induce TNF-α and IL-8 secretion by a CD14-Toll-like receptor 2-dependent mechanism. Journal of Immunology. 2003;171(4):2014–2023. doi: 10.4049/jimmunol.171.4.2014. [DOI] [PubMed] [Google Scholar]
- 17.Guerin ME, Kordulakova J, Alzari PM, Brennan PJ, Jackson M. Molecular basis of phosphatidylinositol mannoside biosynthesis and regulation in mycobacteria. Journal of Biological Chemistry. 2010;285:33577–33583. doi: 10.1074/jbc.R110.168328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer L, Gurcha SS, Bifani P, et al. Characterization of a putative α-mannosyltransferase involved in phosphatidylinositol trimannoside biosynthesis in Mycobacterium tuberculosis. Biochemical Journal. 2002;363(3):437–447. doi: 10.1042/0264-6021:3630437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korduláková J, Gilleron M, Mikuová K, et al. Definition of the first mannosylation step in phosphatidylinositol mannoside synthesis: PimA is essential for growth of mycobacteria. Journal of Biological Chemistry. 2002;277(35):31335–31344. doi: 10.1074/jbc.M204060200. [DOI] [PubMed] [Google Scholar]
- 20.Alexander DC, Jones JRW, Tan T, Chen JM, Liu J. Mannosyltransferase of mycobacteria, is involved in the biosynthesis of phosphatidylinositol mannosides and lipoarabinomannan. Journal of Biological Chemistry. 2004;279(18):18824–18833. doi: 10.1074/jbc.M400791200. [DOI] [PubMed] [Google Scholar]
- 21.Méresse S, Steele-Mortimer O, Moreno E, Desjardins M, Finlay B, Gorvel JP. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nature Cell Biology. 1999;1(7):E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- 22.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochemical Journal. 2002;366(3):689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volinia S, Dhand R, Vanhaesebroeck B, et al. A human phosphatidylinolsitol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO Journal. 1995;14(14):3339–3348. doi: 10.1002/j.1460-2075.1995.tb07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122(5):735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 25.Christoforidis S, Miaczynska M, Ashman K, et al. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature Cell Biology. 1999;1(4):249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- 26.Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83(3):423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 27.Xiao GH, Shoarinejad F, Jin F, Golemis EA, Yeung RS. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. Journal of Biological Chemistry. 1997;272(10):6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]
- 28.Horiuchi H, Lippé R, McBride HM, et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90(6):1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 29.McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98(3):377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 30.Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. Journal of Cell Biology. 2001;154(3):631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97(4):435–447. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 32.Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Annals of Clinical and Laboratory Science. 2006;36(4):371–386. [PubMed] [Google Scholar]
- 33.Asano M, Nakane A, Minagawa T. Endogenous gamma interferon is essential in granuloma formation induced by glycolipid-containing mycolic acid in mice. Infection and Immunity. 1993;61(7):2872–2878. doi: 10.1128/iai.61.7.2872-2878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Indrigo J, Hunter RL, Actor JK. Influence of trehalose 6,6′-dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiology. 2002;148(7):1991–1998. doi: 10.1099/00221287-148-7-1991. [DOI] [PubMed] [Google Scholar]
- 35.Middlebrook G, Dubos RG, Pierce C. Virulence and morphological characteristics of mammalian tubercle bacilli. Journal of Experimental Medicine. 1947;86:175–184. doi: 10.1084/jem.86.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spargo BJ, Crowe LM, Ioneda T, Beaman BL, Crowe JH. Cord factor (α,α-trehalose 6,6'-dimycolate) inhibits fusion between phospholipid vesicles. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(3):737–740. doi: 10.1073/pnas.88.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noll H, Bloch H, Asselineau J, Lederer E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochimica et Biophysica Acta. 1956;20:299–318. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- 38.Parsegian VA, Fuller N, Rand RP. Measured work of deformation and repulsion of lecithin bilayers. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rand RP, Parsegian VA. Physical force considerations in model and biological membranes. Canadian Journal of Biochemistry and Cell Biology. 1984;62(8):752–759. doi: 10.1139/o84-097. [DOI] [PubMed] [Google Scholar]
- 40.Cowley AC. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- 41.Marsh D. Water adsorption isotherms and hydration forces for lysolipids and diacyl phospholipids. Biophysical Journal. 1989;55(6):1093–1100. doi: 10.1016/S0006-3495(89)82906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goren MB, Brennan PJ. Mycobacterial lipids: chemistry and biological activities. In: Youmans GP, editor. Tuberculosis. Philadelphia, Pa, USA: W. B. Saunders; 1980. pp. 63–193. [Google Scholar]
- 43.Retzinger GS, Meredith SC, Takayama K. The role of surface in the biological activities of trehalose 6,6’-dimycolate. Surface properties and development of a model system. Journal of Biological Chemistry. 1981;256(15):8208–8216. [PubMed] [Google Scholar]
- 44.Schabbing RW, Garcia A, Hunter RL. Characterization of the trehalose 6,6’-dimycolate surface monolayer by scanning tunneling microscopy. Infection and Immunity. 1994;62(2):754–756. doi: 10.1128/iai.62.2.754-756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Behling CA, Bennett B, Takayama K, Hunter RL. Development of a trehalose 6,6’-dimycolate model which explains cord formation by Mycobacterium tuberculosis. Infection and Immunity. 1993;61(6):2296–2303. doi: 10.1128/iai.61.6.2296-2303.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Retzinger GS, Meredith SC, Hunter RL. Identification of the physiologically active state of the mycobacterial glycolipid trehalose 6,6'-dimycolate and the role of fibrinogen in the biologic activities of trehalose 6,6'-dimycolate monolayers. Journal of Immunology. 1982;129(2):735–744. [PubMed] [Google Scholar]
- 47.Youmans GP. Mycobacterial lipids: chemistry and biologic activities. In: Youmans GP, editor. Tuberculosis. Philadelphia, Pa, USA: W. B. Saunders; 1979. pp. 63–193. [Google Scholar]
- 48.Actor JK, Leonard CD, Watson VE, et al. Cytokine mRNA expression and serum cortisol evaluation during murine lung inflammation induced by Mycobacterium tuberculosis. Combinatorial Chemistry and High Throughput Screening. 2000;3(4):343–351. [PubMed] [Google Scholar]
- 49.Matsunaga I, Naka T, Talekar RS, et al. Mycolyltransferase-mediated glycolipid exchange in mycobacteria. Journal of Biological Chemistry. 2008;283(43):28835–28841. doi: 10.1074/jbc.M805776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishikawa E, Ishikawa T, Morita YS, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. Journal of Experimental Medicine. 2009;206(13):2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagel W, Pagel W. Fat and lipoid content of tuberculous tissue-histochemical investigation. Virchow's Archiv für pathologische Anatomie. 1925;256:629–640. [Google Scholar]
- 52.Hatipoğlu ON, Osma E, Manisali M, et al. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax. 1996;51(4):397–402. doi: 10.1136/thx.51.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurasawa T, Ikeda N, Yamadori H, et al. Two cases of elderly people diagnosed with acute tuberculous pneumonia possibly succeeded by the perforation of lymph nodes in the bronchus. Kekkaku. 1994;69(2):83–88. [PubMed] [Google Scholar]
- 54.Soni R, Barnes D, Torzillo P. Post obstructive pneumonia secondary to endobronchial tuberculosis—an institutional review. Australian and New Zealand Journal of Medicine. 1999;29(6):841–842. doi: 10.1111/j.1445-5994.1999.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 55.Hunter RL, Olsen M, Jagannath C, Actor JK. Trehalose 6,6′-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. American Journal of Pathology. 2006;168(4):1249–1261. doi: 10.2353/ajpath.2006.050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Indrigo J, Hunter RL, Actor JK. Cord factor trehalose 6,6′-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology. 2003;149(8):2049–2059. doi: 10.1099/mic.0.26226-0. [DOI] [PubMed] [Google Scholar]
- 57.Retzinger GS. Dissemination of beads coated with trehalose 6,6’-dimycolate: a possible role for coagulation in the dissemination process. Experimental and Molecular Pathology. 1987;46(2):190–198. doi: 10.1016/0014-4800(87)90065-7. [DOI] [PubMed] [Google Scholar]
- 58.Seggev JS, Goren MB, Kirkpatrick CH. The pathogenesis of trehalose dimycolate-induced interstitial pneumonitis. III. Evidence for a role for T-lymphocytes. Cellular Immunology. 1984;85(2):428–435. doi: 10.1016/0008-8749(84)90256-9. [DOI] [PubMed] [Google Scholar]