Abstract
Group A Streptococcus (GAS) can be internalized by epithelial cells, including keratinocytes from human skin or pharyngeal epithelium. Internalization of GAS by epithelial cells has been postulated both to play a role in host defense and to provide a sanctuary site for GAS survival. The cholesterol-binding cytolysin streptolysin O (SLO) appears to enhance virulence in part by inhibiting GAS internalization by human keratinocytes and by disrupting the lysosomal degradation of internalized GAS. We now report that low-level production of SLO by an inducible expression system reduced GAS internalization by keratinocytes. Induced SLO expression also prevented lysosomal colocalization with intracellular bacteria and acidification of GAS-containing vacuoles. Exogenous recombinant SLO mimicked the inhibitory effect of SLO secretion on GAS entry but not that on colocalization with the lysosomal marker LAMP-1, implying that disruption of lysosomal degradation requires intracellular secretion of SLO. The internalization of SLO-negative GAS was blocked by the depletion of host cell cholesterol and by the inhibition or knocking down of the expression of clathrin or dynamin. SLO also inhibited the cellular uptake of other cargos that are internalized by clathrin-mediated uptake or by macropinocytosis. We conclude that SLO interferes with the internalization of GAS through local perturbation of the keratinocyte cell membrane and disruption of a clathrin-dependent uptake pathway.
IMPORTANCE
Streptolysin O (SLO) is a member of a family of pore-forming toxins, the cholesterol-dependent cytolysins, that are produced by many Gram-positive bacterial pathogens. While SLO can lyse host cells at high doses, much smaller amounts appear to contribute to pathogenesis by inhibiting the internalization of group A Streptococcus (GAS) by pharyngeal keratinocytes and by preventing efficient intracellular killing by lysosomal fusion. This study provides evidence that SLO blocks a clathrin-dependent pathway for the internalization of GAS through effects on the cell surface, whereas inhibition of lysosomal fusion depends on the intracellular production of SLO. These observations may have broader implications for understanding the pathogenesis of multiple bacterial species that produce cholesterol-dependent cytolysins.
INTRODUCTION
Streptococcus pyogenes or group A Streptococcus (GAS) is an exclusively human pathogen that causes pharyngitis, infections of the skin and soft tissues, invasive syndromes such as necrotizing fasciitis, bloodstream infection, and streptococcal toxic shock, as well as the postinfectious syndromes of acute rheumatic fever and poststreptococcal glomerulonephritis (1–3). In most cases, the pharyngeal mucosa is thought to be the initial site of GAS acquisition, and pharyngeal carriage may persist after clinical infection has resolved or even in the absence of symptomatic infection. The central role of pharyngeal colonization in GAS infection has focused attention on interactions of the bacteria with pharyngeal epithelial cells as a potentially critical aspect of streptococcal disease pathogenesis.
While GAS is adapted to live primarily at extracellular sites on mucosal surfaces and in tonsillar crypts, multiple studies have demonstrated the internalization of GAS by human epithelial cells in vitro (4–8). Internalization efficiency appears to be higher for isolates associated with pharyngitis or asymptomatic carriage than for isolates associated with bloodstream infection, a pattern that has suggested that internalization of GAS by pharyngeal epithelial cells may contribute to carriage by providing an intracellular sanctuary from host immune effectors and antibiotics (9). On the other hand, GAS fails to proliferate within human keratinocytes in vitro, and intracellular organisms are killed by endolysosomal and/or autophagosomal degradation (8, 10, 11). The net effect of GAS internalization by pharyngeal keratinocytes on bacterial persistence in the human host likely depends on multiple factors that influence the efficiency of internalization and intracellular killing.
The process of GAS internalization requires energy-dependent rearrangement of cellular actin and, in general, appears to involve direct or indirect binding of GAS to cell surface integrins (7, 12–14). Fibronectin and other plasma proteins have been implicated as bridging molecules that bind to several bacterial surface proteins and to host cell integrins. Bacterial uptake is stimulated through interaction with integrins and/or other signaling molecules on the host cell.
The GAS cholesterol-dependent cytolysin streptolysin O (SLO) appears to play a central role in modulating the internalization and intracellular killing of GAS by epithelial cells. Nakagawa et al. found that GAS intracellular killing by epithelial cells involved the formation of bacterium-containing autophagosome-like vacuoles, a process triggered by SLO (11). However, Hakansson et al. reported that SLO markedly reduced the efficiency of GAS internalization by human keratinocytes and interfered with the trafficking of internalized GAS to lysosomes for efficient killing (10). The goals of the present study were to better define how GAS is internalized by human pharyngeal keratinocytes and to determine how internalization is modulated by SLO. The results support a clathrin-dependent model of GAS internalization, a process that is sensitive to disruption by SLO-mediated damage to the cell membrane.
RESULTS
Inducible expression of SLO inhibits GAS internalization by keratinocytes and intracellular trafficking to lysosomes.
The inhibitory effects of SLO on the internalization of GAS and on intracellular trafficking to lysosomes for efficient intracellular killing in human keratinocytes might be a result of a general cytotoxic effect of SLO and/or its associated cotoxin, NAD-glycohydrolase (NADase), although inhibitory effects on internalization and intracellular killing are observed at a multiplicity of infection (MOI) that does not produce substantial changes in cell membrane integrity (10). Alternatively, SLO might act locally to disrupt cell membrane microdomains to prevent bacterial internalization and/or to dysregulate intracellular trafficking. To better distinguish between these possibilities, we constructed a strain of GAS in which slo is expressed under the control of an inducible promoter (see Materials and Methods) (Fig. 1A). Under inducing conditions, 188SLO−(piSLO) produced approximately 10% of the amount of SLO produced by parent strain 188, as assessed by Western blotting and hemolytic activity (Fig. 1B). In assays measuring internalization by OKP7/bmi1/TERT keratinocytes, we found that induction of SLO production by isopropyl-β-d-thiogalactopyranoside (IPTG) reduced the internalization of 188SLO−(piSLO) by 65% compared to that seen under noninducing conditions (Fig. 1C). Induction of SLO expression was not associated with any significant increase in keratinocyte cytotoxicity, as reflected by lactate dehydrogenase (LDH) release (data not shown). Thus, the production of even a relatively small amount of SLO had a substantial inhibitory effect on GAS internalization, although internalization was still somewhat more efficient than that of strain 188, which produces 10-fold more SLO [3.5% internalization of 188SLO−(piSLO) under inducing conditions compared to 0.5% internalization of strain 188 containing the empty pSIV vector]. Parallel experiments with 188SLO−/NADase−(piSLO) showed a degree of inhibition of internalization upon the induction of SLO expression similar to that observed in 188SLO−(piSLO), a result that implies that SLO is sufficient to inhibit GAS uptake in the absence of NADase. These results supported the hypothesis that SLO prevents GAS entry into keratinocytes and are consistent with earlier findings that the internalization of parent strain 188 was reduced compared to that of isogenic SLO mutant strain 188SLO− (10).
FIG 1 .
Induction of low levels of SLO reduces GAS internalization by keratinocytes. (A) The streptococcal inducible vector (pSIV) is an E. coli–Gram-positive shuttle vector that includes an IPTG-inducible promoter, lacO-guaB-lacO, upstream of unique restriction endonuclease sites for the cloning of a gene of interest. The slo gene from GAS was cloned into the pSIV vector to produce plasmid iSLO. (B) GAS parent strain 188, 188SLO−, and 188SLO−/NADase− containing the empty pSIV vector or the inducible SLO expression vector piSLO were grown in the absence (−) or presence (+) of 2 mM IPTG to assess the inducible expression of SLO. SLO in supernatants of exponential-phase cultures was detected by Western blotting, and activity was quantified in HU. (C) Keratinocytes were infected with GAS strain 188, 188SLO−, or 188SLO−/NADase− containing either the empty vector pSIV or piSLO in the absence or presence of 2 mM IPTG. Internalization in the induced condition is expressed as a percentage of that observed with the uninduced control. Mean values and standard errors of the means (SEM) from four independent experiments are graphed. *, P < 0.05.
We used confocal microscopy to study the intracellular trafficking of internalized GAS. As expected, under noninducing conditions, 188SLO−(piSLO) was efficiently trafficked to an intracellular compartment that contained LAMP-1, characteristic of late endosomes and lysosomes (Fig. 2A). In contrast, under inducing conditions, i.e., when SLO was expressed by 188SLO−(piSLO), we observed only very incomplete colocalization of intracellular bacteria with LAMP-1: some bacteria appeared to colocalize with punctate accumulations of LAMP-1, but almost none exhibited the circumferential pattern of LAMP-1 staining routinely seen in cells infected with 188SLO−(piSLO) under noninducing conditions (Fig. 2A).
FIG 2 .
Induction of low levels of SLO partially inhibits LAMP-1 colocalization and bacterial acidification following uptake by keratinocytes. (A) Confocal microscopy was utilized to determine if GAS expressing low levels of SLO colocalized with LAMP-1. Extracellular GAS was labeled in blue, all GAS in green, and LAMP-1 in red. Keratinocytes infected with GAS strains containing either the pSIV empty vector or piSLO were analyzed at 3 h postinfection. Arrows indicate GAS colocalization with LAMP-1 as follows: short white arrows, circumferential colocalization with LAMP-1; long gray arrows, partial colocalization; long white arrows, no colocalization. Scale bars represent 5 µm. (B, C) To measure the pH of internalized GAS, the bacteria were labeled with a pH-independent fluorophore (Alexa Fluor 405) and a pH-dependent fluorophore (FITC). The pH was determined by comparing the ratio of FITC/Alexa Fluor 405 fluorescence in each sample to a standard fluorescence curve constructed by exposing infected cells to buffer at a range of pH values in the presence of nigericin and valinomycin to equalize the intracellular pH with that of the buffer. As shown in panel B, the fluorescence of FITC is strongly quenched at pH 4.5 (gray histogram) compared to the fluorescence emitted by the same cells at pH 7.2 (open histogram). In contrast, when the same cells are analyzed for Alexa Fluor 405 fluorescence (C), the two histograms at pH 4.5 and 7.2 appear superimposed, showing that the pH-dependent difference in FITC emission is due to pH sensitivity rather than to partial degradation of the dye or the bacteria. (D) The intracellular pH of GAS harboring pSIV or piSLO was measured 3 h after infection of keratinocytes by ratiometric analysis of FITC/Alexa Fluor 405 fluorescence compared to a pH standard curve. GAS strains were grown in the absence (−) or presence (+) of 2 mM IPTG. Mean values and SEM are graphed from four independent experiments. *, P < 0.05; **, P < 0.01.
For a more quantitative assessment of the functional consequences of the production of small amounts of SLO on the intracellular fate of GAS, we also tested whether induction of SLO expression in 188SLO−(piSLO) inhibited the acidification of the bacterium-containing vacuole, as observed for SLO-producing strain 188. For this purpose, GAS was covalently labeled with both fluorescein isothiocyanate (FITC), a pH-sensitive fluorophore, and Alexa Fluor 405 SE, a relatively pH-insensitive fluorophore. After infection of keratinocytes, the intracellular pH environment of the labeled bacteria was assessed by flow cytometry with ratiometric analysis of the relative fluorescence intensities of the two fluorophores in infected cells (Fig. 2B to D). Consistent with the incomplete colocalization of 188SLO−(piSLO) with LAMP-1, under inducing conditions, the intracellular environment of 188SLO−(piSLO) was only partially acidified to pH 5.7, a value intermediate between that for the same strain in the absence of IPTG induction (pH 5.0) and that of SLO-producing parent strain 188 (pH 6.8 to 7.1) (Fig. 2D). Thus, the small amount of SLO produced under inducing conditions by 188SLO−(piSLO) both inhibited GAS internalization and partially prevented lysosomal acidification of the internalized bacteria.
In order to discriminate further between the effects of SLO on bacterial internalization versus those on intracellular trafficking, we performed internalization assays and assessed the intracellular localization of SLO-negative strain 188SLO− in keratinocytes treated with purified recombinant SLO (rSLO). For these experiments, rSLO was added to infections at a concentration that produces a low level of cytotoxic injury similar to that caused by infection at an MOI of 1 with SLO-producing strain 188. Exogenous SLO reduced the internalization of strains 188SLO− and 188SLO−/NADase− by 86% and 65%, respectively (Fig. 3A). In contrast, treatment of the cells with exogenous SLO had no significant effect on the colocalization of strain 188SLO− with LAMP-1 or on acidification of the bacterial vacuole (Fig. 3B and C). The fact that treatment with exogenous SLO reduced the internalization of 188SLO− to an extent similar to that achieved by the induced expression of SLO in strain 188SLO−(piSLO) but failed to inhibit colocalization with LAMP-1 or acidification of intracellular bacteria implies that the latter effects are dependent on the intracellular secretion of SLO by internalized bacteria. The dissociation of SLO effects on internalization and intracellular trafficking suggests that these effects are not simply consequences of a global cytotoxic effect of SLO on cellular functions but rather that they reflect more specific and localized modulation of two relatively distinct cellular processes. Together with the results obtained using the inducible expression system, these experiments suggest that inhibition of internalization results from the action of SLO on the eukaryotic cell surface, whereas effects of SLO on intracellular trafficking depend on secretion of the toxin after bacterial internalization.
FIG 3 .
Addition of rSLO reduces internalization but has no effect on intracellular pH or LAMP-1 colocalization. (A) Keratinocytes were infected with GAS 188SLO− or 188 SLO−/NADase− in the absence or presence of 10 HU of rSLO. Total association and intracellular bacteria were quantified in antibiotic protection assays. Internalization of GAS in the presence of rSLO is expressed as a percentage of the values for control samples not treated with SLO. (B) The pH of intracellular GAS following infection in the presence or absence of 10 HU of rSLO was measured as described in the legend to Fig. 2. Mean values and SEM for four individual experiments are shown. *, P < 0.05; **, P < 0.01. (C) Keratinocytes infected with GAS 188SLO− in the absence or presence of 10 HU of rSLO were fixed and stained with antibodies to extracellular GAS (blue), all GAS (green), and LAMP-1 (red). Scale bars represent 5 µm.
Inhibitory effects of SLO on GAS internalization are mimicked by depletion of cell membrane cholesterol and by inhibition of clathrin-mediated uptake.
The pore-forming activity of SLO, like that of other cholesterol-dependent cytolysins, requires the presence of cholesterol in the target cell membrane, although cholesterol itself may not be the membrane receptor for SLO binding (15). Integrin molecules that mediate the attachment and internalization of GAS are associated with cholesterol-rich lipid raft microdomains of the cell membrane (16–18). Insertion of SLO complexes into these cholesterol-containing membrane domains could disrupt integrin signaling, thereby inhibiting GAS internalization. We tested whether depletion of membrane cholesterol mimicked SLO’s effect on GAS internalization. Treatment of keratinocytes with methyl-β-cyclodextrin or with nystatin to deplete or sequester plasma membrane cholesterol reduced the internalization of SLO-negative GAS strain 188SLO− by >90%, to a level similar to that of SLO-producing strain 188 (Fig. 4A). Thus, cholesterol depletion of target cells had an inhibitory effect on GAS internalization similar to that of SLO.
FIG 4 .
SLO prevents GAS uptake via a clathrin- and dynamin-dependent process. (A to D) Keratinocytes were pretreated for 30 min with chemical inhibitors or for 36 h with siRNA prior to infection with GAS 188 or 188SLO−. Association and internalization were determined in an antibiotic protection assay. Mean values and SEM for four independent experiments are shown as percentages of the untreated control. Chemical inhibitor name abbreviations: MeCD, methyl-β-cyclodextrin; Nyst, nystatin; MDC, monodansylcadaverine; CPZ, chlorpromazine. **, P < 0.01. (E) Knockdown of targeted protein was confirmed by Western blotting and compared to a control protein (tubulin).
Integrin-mediated internalization of some bacterial species has been shown to involve clathrin (19, 20). Therefore, we tested whether treatment with inhibitors that interfere with clathrin-mediated uptake also block the internalization of GAS. Treatment of keratinocytes with either monodansylcadaverine or chlorpromazine prevented the internalization of both SLO-producing strain 188 and 188SLO− (Fig. 4B). Because the cellular effects of these agents are not entirely specific for the clathrin pathway, we used small interfering RNA (siRNA) to knock down clathrin expression in keratinocytes (Fig. 4E). We also tested the effect of interfering with dynamin, the large GTPase that plays an essential role in clathrin-mediated uptake. Knockdown of clathrin or dynamin expression with specific siRNA inhibited the uptake of 188SLO− by about 50% but did not affect the internalization of SLO-producing strain 188 (Fig. 4D). Similarly, treatment of cells with the dynamin inhibitor dynasore reduced the uptake of 188SLO− but not that of 188 (Fig. 4C). Together, these results suggest that, in the absence of SLO, GAS is efficiently internalized by a clathrin- and dynamin-dependent process and that this entry pathway is inhibited by the action of SLO.
SLO inhibits the uptake of other cargo, but inhibition is most efficient for bacterial cells secreting the toxin.
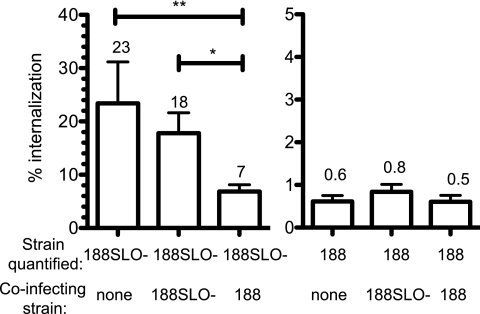
As discussed above, we found that the addition of exogenous rSLO to keratinocytes reduced the internalization of GAS strain 188SLO−. We performed additional experiments to investigate further the inhibitory effect of SLO not only on the uptake of bacterial cells secreting the toxin but also on that of other cargo. Coinfection of keratinocytes with SLO-producing strain 188 together with strain 188SLO− reduced the internalization of 188SLO− by 60% (Fig. 5), to a level intermediate between the high level of internalization observed in single-strain infection with 188SLO− and the low rate of internalization seen in single-strain infection with 188. Thus, secreted SLO can inhibit the uptake not only of GAS cells actually secreting the toxin but also, to a lesser extent, of neighboring bacterial cells. Consistent with this idea, we found that treatment of keratinocytes with rSLO at a dose with cytolytic activity equivalent to that associated with infection of the cells with the standard inoculum of GAS strain 188 reduced the internalization not only of GAS strain 188SLO−, as discussed above, but also of a Yersinia pseudotuberculosis strain that enters cells by an integrin- and Rac1-dependent pathway (21) (Fig. 6). In a similar fashion, SLO also inhibited the uptake of Salmonella enterica serovar Typhimurium, which enters by a Rac1/Cdc42-dependent “trigger” mechanism similar to macropinocytosis (22, 23) (Fig. 6).
FIG 5 .
Coinfection with SLO-producing GAS reduces the internalization of SLO-negative GAS. (A) Keratinocytes were infected with GAS strain 188 (spectinomycin resistant) or 188SLO− (spectinomycin sensitive) singly or mixed together. The number of associated and internalized bacteria of each strain was measured in antibiotic protection assays by quantitative cultures on blood agar plates and replica plating onto medium containing spectinomycin. Percent internalization (intracellular CFU/total associated CFU) of each strain in single-strain infections and coinfections is graphed. For coinfections, the quantified strain is listed above the coinfecting strain. Results are the mean and SEM of four experiments. *, P < 0.05; **, P < 0.01.
FIG 6 .
rSLO inhibits the entry of other bacterial pathogens. The internalization of Yersinia and Salmonella by keratinocytes was measured in the absence or presence of 10 HU of rSLO in antibiotic protection assays. The mean percent internalization in four independent experiments is expressed as a percentage of the untreated control (no rSLO). **, P < 0.01.
To investigate the generality of SLO’s inhibitory effect on the internalization of other cargo, we used flow cytometry to analyze the uptake by keratinocytes of fluorescent dextran with a molecular weight (MW) of approximately 10,000 or 70,000, a molecule expected to be internalized by endocytosis or macropinocytosis, respectively (24, 25). In cells exposed to SLO-producing GAS strain 188, the internalization of 70,000-MW dextran was reduced by 34% compared to that of cells exposed to 188SLO− (Fig. 7A). Simultaneous staining for GAS revealed that dextran uptake was reduced in those cells associated with cell-bound bacteria, whereas there was no significant change in the overall dextran uptake by cells without associated bacteria (not shown). These results support the idea of a gradient effect of SLO such that its inhibitory effect on internalization is most evident near the site of SLO secretion by bacteria bound to the host cell. In contrast, exposure to strain 188 had no inhibitory effect on the uptake of 10,000-MW dextran or on the uptake of transferrin, which is internalized by receptor-mediated, clathrin-dependent endocytosis (25). Treatment of keratinocytes with rSLO had little or no effect on the internalization of 10,000-MW dextran or transferrin but reduced the internalization of 70,000-MW dextran by 50% (Fig. 7B). Thus, SLO appeared to preferentially inhibit macropinocytosis, with little or no effect on endocytosis of smaller molecules or on receptor-mediated endocytosis.
FIG 7 .
SLO reduces the uptake of other large cargo. The effect of infection with SLO+ or SLO− GAS strains (A) or rSLO (B) on the uptake of various endocytic cargos was measured by flow cytometry. Keratinocytes were infected with GAS for 1.5 h or pretreated with rSLO for 30 min, and then fluorescently labeled 10,000-MW dextran (Dex10), 70,000-MW dextran (Dex 70), or transferrin was added. Uptake by infected or treated cells is compared to that by uninfected controls. Mean values and SEM of four experiments are shown. *, P < 0.05; **, P < 0.01.
DISCUSSION
GAS may transiently colonize human skin and can survive in food or on environmental surfaces, but its preferred ecological niche is the human oropharynx. Persistent colonization of the pharynx requires firm attachment to the epithelium and resistance to mechanical and immunological clearance mechanisms of the human host. The internalization of GAS by pharyngeal keratinocytes has been suggested to represent a local defense against GAS infection, as internalized organisms fail to proliferate intracellularly and lose viability over time (8, 10, 11). On the other hand, the persistence of a small population of intracellular organisms could provide a sanctuary site for chronic colonization or recrudescent infection that is relatively resistant to the action of immune effectors and antibiotics (9, 26).
We have shown previously that production of SLO and the associated toxin NADase reduces the internalization of GAS by human oropharyngeal keratinocytes and interferes with trafficking of internalized GAS to lysosomes for efficient intracellular killing (10). In the present study, we have analyzed in greater depth the cellular mechanisms involved in internalization and those that are targeted by the actions of SLO on the host cell. By engineering a GAS strain that produces SLO under the control of an inducible promoter, we found that the process of internalization could be inhibited by relatively small amounts of SLO. The internalization of SLO-negative GAS could be inhibited to a similar extent by the addition of a sublytic amount of exogenous SLO. Intracellular trafficking of the bacteria to lysosomes and acidification of the bacterium-containing vacuole were also partially inhibited by the inducible expression of SLO, but in contrast to internalization, these processes were not inhibited by the addition of exogenous SLO. This discrepancy provides strong evidence that SLO prevents the internalization of GAS through the action of extracellular toxin on the host cell membrane, whereas inhibition of lysosomal colocalization and the acidification of internalized GAS depends on the secretion of SLO by intracellular bacteria. In studies of GAS interactions with HeLa cells, Sakurai et al. also found that SLO production was associated with a failure of GAS-containing early endosomes to colocalize with LAMP-1 and suggested that SLO-producing GAS was released into the cytoplasm soon after internalization and later incorporated into autophagosomes (27).
The sensitivity of GAS internalization to low levels of SLO suggests that, rather than exerting a nonspecific cytotoxic effect on the cell, relatively low concentrations of SLO preferentially inhibit one or more cellular pathways for the uptake of particulate cargo. The most extensively characterized pathway for GAS internalization by epithelial cells involves the binding of host fibronectin (7, 14, 28). Fibronectin is thought to act as a molecular bridge between fibronectin-binding protein(s) or M protein on the GAS surface and integrins (primarily α5β1) on the epithelial cell surface, an interaction that stimulates zipper-like internalization of the bound bacteria in a manner similar to that described for Yersinia and Listeria (21, 29).
Results of the present study implicate plasma membrane cholesterol, clathrin, and dynamin in GAS internalization. Sequestration or depletion of membrane cholesterol with nystatin or methyl-β-cyclodextrin reduced the internalization of SLO-negative GAS but had little effect in further reducing the relatively inefficient internalization of SLO-positive GAS. These results are compatible with a model in which binding of GAS (through fibronectin) to integrins on the cell surface acts to cluster integrin-containing lipid rafts, thereby enhancing integrin-mediated cell signaling to stimulate the process of bacterial internalization. By forming pores in cholesterol-rich membrane domains, SLO may mimic cholesterol depletion and inhibit the clustering of lipid rafts, thereby interfering with integrin signaling and bacterial internalization.
Inhibition of clathrin or dynamin function by chemical inhibitors or by siRNA also inhibited GAS internalization. These findings suggest that GAS, in the absence of SLO, is internalized in a clathrin- and dynamin-dependent manner, consistent with the general model proposed by Veiga et al. for clathrin-dependent internalization of bacteria that enter cells through a “zipper” mechanism (20). The facts that SLO-producing GAS bacteria are internalized poorly and that the internalization of these organisms is minimally affected by the blocking of clathrin or dynamin suggest that SLO targets the clathrin-dependent internalization pathway. However, SLO did not block the endocytosis of transferrin, a result that suggests that its site of action must be downstream or relatively independent of that of clathrin-dependent, receptor-mediated endocytosis.
Exposure of keratinocytes to SLO-producing GAS or to purified rSLO also inhibited the uptake of high-molecular-weight dextran, which is thought to be internalized by macropinocytosis. Endocytic and receptor-mediated uptake remained intact, however, highlighting the relatively selective effect of SLO on the uptake of large molecules and particles, including bacterial cells. Consistent with this model, SLO inhibited the internalization of Y. pseudotuberculosis and S. enterica serovar Typhimurium, organisms that enter cells via an integrin-mediated zipper mechanism (Yersinia) and by a macropinocytosis-like process stimulated by type III effector proteins (Salmonella). Although SLO is a secreted toxin that is expected to diffuse freely in extracellular fluids, its action on the uptake of bacteria and other cargo was relatively localized. In flow cytometry experiments, SLO-producing GAS inhibited the uptake of fluorescent 70,000-MW dextran, but effective inhibition was only observed in keratinocytes that had bound GAS. Thus, the inhibitory effect of SLO on the cellular uptake of bacteria and other cargo is relatively localized, preferentially affecting the infected cell. This pattern of cellular response implies that SLO secreted by GAS bound to an epithelial cell has its maximal effect locally, acting to block the internalization and intracellular killing of the bound bacterium.
Results of the present study provide evidence that GAS is internalized by human oropharyngeal keratinocytes through a clathrin-dependent uptake mechanism. SLO blocks bacterial internalization and the uptake of unrelated cargo by the infected cell probably by disrupting integrin clustering or other signaling events by inserting itself into cholesterol-rich lipid rafts. While SLO appears to preferentially affect the internalization of large molecules and particles such as bacteria, it inhibits the uptake of cargo entering through either clathrin-mediated uptake or macropinocytosis. The exquisite sensitivity of pharyngeal epithelial cells to inhibition of bacterial uptake by SLO represents a critical biological response that may serve to enhance GAS survival in the human pharynx.
MATERIALS AND METHODS
Bacterial strains.
GAS strain 188 is an isogenic unencapsulated mutant of M type 3 necrotizing fasciitis isolate 950771 (30). Because the hyaluronic acid capsule inhibits GAS internalization, the use of an unencapsulated mutant permits clearer discrimination of the role of SLO in preventing internalization by epithelial cells. GAS strain 188SLO− is a slo deletion mutant of strain 188, and 188SLO−/NADase− is an nga deletion mutant of strain 188SLO− (10, 31). GAS strains 188 and 188SLO− were transformed with phasp-gfp, which encodes green fluorescent protein and spectinomycin resistance, for use in coinfection experiments (32). S. enterica serovar Typhimurium SL1344 was a gift of Denise Monack (33). The Y. pseudotuberculosis YPIII plasmid minus strain was a gift of Joan Mecsas (34). Escherichia coli, Salmonella, and Yersinia were grown in Luria-Bertani broth (LB) or on LB agar. GAS strains were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract or on sheep blood agar plates (VWR). For the inducible-expression experiments, GAS strains were grown in L3 medium (35). Antibiotics were used when appropriate at the following concentrations: ampicillin at 100 µg/ml, erythromycin at 1 µg/ml for GAS and 250 µg/ml for E. coli, spectinomycin at 50 µg/ml, and kanamycin at 50 µg/ml for E. coli, penicillin at 20 µg/ml, and gentamicin at 200 µg/ml.
Plasmids and molecular biology.
E. coli XL1Blue was used for cloning. PCR products were ligated into the pGEM-T Easy vector (Promega, Madison, WI) before restriction enzyme digestion, purification, and ligation into the appropriate vector. The inducible vector pSIV (streptococcal inducible vector) (Fig. 1) was constructed from two separate plasmids, the inducible expression vector pLIV1 (courtesy of Darren Higgins) (36) and the E. coli-GAS shuttle vector pOri23 (courtesy of June Scott) (37). The p23 promoter was removed from pOri23 by digestion with PstI and EcoRI; this region was replaced with the portion of the pUC19 multiple cloning site flanked by PstI and EcoRI sites to create pOri-MCS. The inducible expression region of pLIV1 was modified by removing the erythromycin cassette and the P60 promoter by digestion with XbaI/SnaBI and ligation of the P23 promoter between the XbaI and SnaBI sites, upstream of the lacI gene to ensure constitutive expression of LacI in GAS. The inducible promoter (SPAC-lacOid) was replaced with the GAS guaB promoter flanked by lacOid sequences (lacOid-guaB-lacOid) using the EcoRI and XbaI sites. This new promoter improves expression in GAS and reduces background expression in the absence of IPTG induction. The modified inducible region was ligated to pOri-MCS after both plasmids had been digested with PstI and BamHI. The pSIV plasmid allows the introduction of a gene of interest downstream of the lacOid-guaB-lacOid promoter by using any of four unique restriction sites (AatII, PvuI, NcoI, and SphI). This promoter is repressed by the lac repressor, LacI, and repression can be alleviated by addition of IPTG. Since IPTG diffuses across eukaryotic cell membranes, intracellular induction is possible with this system (36). The slo gene from GAS 188 was cloned into the SphI site of the pSIV vector to create the piSLO plasmid. All cloning steps were confirmed by restriction digestion and sequence analysis.
The oligonucleotide primers used in cloning were from Eurofins MWG Operon (Huntsville, AL) and are, in 5′–3′ orientation, CGATCTAGAGCATGCTCGAAAAGCCCTGACAACCC (F) and TACGTAAACATCATTGTCATTCATATTTTT (R) for the P23 promoter and TCCGCATGCGAATGATAAAAAGGTATGAAGGAC (F) and AAGGCATGCACAGTCACAAAATAGTGAGACAACAG (R) for the slo gene.
The lacOid-guaB-lacOid sequence was synthesized by Integrated DNA Technologies, Inc. (Coralville, IA) as follows: GCATGCTCTAGACCATGGCGATCGGACGTCAATTGTGAGCGCTCACAATTCTCGAGGGGAATATTACCCTGGTCTATTTTTTATTGTTACTATCATATCACGCTAAAATCATTTGTCAATAAAATATTCCTACAGCTGAAGTCACTATCAGCATAATATATCAGTATTATCCGTTTTTTATTAATCAAAAAATTCATTTGGGTCTTATTTTTAATCAAAAATAAGACTATATAAAACATAATCTCTACTATCCTGTAAAGGCGGCCGCAATTGTGAGCGCTCACAATTCCGCGGGAATTC.
Reagents.
Reagents were purchased from Sigma unless otherwise noted. Restriction enzymes were purchased from New England Biolabs (Ipswich, MA). The rSLO and anti-SLO antibodies were previously described (38). Antibodies were purchased from BD Biosciences (San Jose, CA), with the exception of anti-dynamin from Affinity BioReagents (Rockford, IL) and anti-tubulin from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antiserum to GAS group A carbohydrate was from Immucell (Portland, ME); the IgG fraction of this antiserum was conjugated to Alexa Fluor 488 or Alexa Fluor 660 using an IgG conjugation kit from Molecular Probes/Invitrogen (Carlsbad, CA). Dynasore was a gift of Tomas Kirchhausen (39). The siRNA reagents clathrin ON-TARGET plus SMART pool L-004001-0020, dynamin ON-TARGET plus SMART pool L-004007-00-0020, and nontargeting pool D-001810-10-20 were purchased from Dharmacon (Lafayette, CO). Fluorescent dyes (FITC and Alexa Fluor 405), dextrans, and transferrin were purchased from Molecular Probes/Invitrogen (Carlsbad, CA).
Keratinocytes and cell culture.
The OKP7/bmi1/TERT (OKP7) keratinocytes used in this study are normal human soft palate keratinocytes that have been immortalized by the expression of bmi1 (which inhibits the growth arrest mechanisms of p16INK4A and p14ARF) and the catalytic subunit of human telomerase (tert) (40, 41). These cells were a gift of James Rheinwald and were provided through the Harvard Skin Disease Research Center. Keratinocytes were cultured in keratinocyte serum-free medium (KSFM) from Gibco/Invitrogen as previously described (10).
Cytotoxicity assays.
Keratinocyte cytotoxicity was assessed by LDH release assays as described previously (38).
Antibiotic protection assays.
Total bacterial association and internalization levels were determined in antibiotic protection assays at 3 h postinfection with GAS at an MOI of 1 as previously described (10). Briefly, keratinocytes were cultured for 3 to 4 days to confluence at 37°C in 5% CO2 prior to infection. Bacteria were grown for 2 h to mid-exponential phase, washed, diluted, and added to washed cells. Bacteria were allowed to adhere for 2 min, followed by the addition of KSFM. Infections were allowed to proceed for 2 h 15 min at 37°C in 5% CO2, at which point penicillin and gentamicin were added to half of the wells to kill extracellular bacteria. At 3 h postinfection, cells were washed with phosphate-buffered saline (PBS), trypsinized, and lysed in water for 15 min. Serial dilutions were cultured on blood agar plates to determine the total numbers of cell-associated bacteria in wells without antibiotics and intracellular bacteria in wells with antibiotics.
Chemical inhibitors of uptake pathways were added 30 min prior to infection at the following concentrations: methyl-β-cyclodextrin, 10 mM preincubation and 2.5 mM during infection; nystatin, 7.5 µg/ml; monodansylcadaverine, 30 µg/ml; chlorpromazine, 12 µg/ml; dynasore, 20 µM. At these concentrations, the inhibitors did not affect bacterial association. Knockdowns of proteins involved in internalization were performed by siRNA treatment of cells with Lipofectamine (Invitrogen) prior to infection. Keratinocytes were treated with 100 pmol siRNA 12 and 36 h after seeding, according to the manufacturer’s instructions. Knockdowns were confirmed by Western blotting 12 h after the second siRNA treatment, the same time point at which infections were performed. For coinfections, cells were infected with a total MOI of 4, i.e., with each strain at an MOI of 2.
For infections with Yersinia or Salmonella, the bacteria were grown in broth with aeration overnight at room temperature or 37°C, respectively. On the morning of infection, cultures were diluted 1:40 into fresh LB and grown for 2 h to mid-exponential phase at room temperature with aeration for Yersinia or at 37°C with no aeration for Salmonella to ensure the expression of appropriate adherence factors as previously described (33, 34). Bacteria were washed and diluted for infection at an MOI of 1. Following infection, antibiotic protection assays were performed as described above.
Inducible expression of SLO.
GAS strains carrying the pSIV or piSLO plasmid were grown in L3 medium with erythromycin and with or without 2 mM IPTG. For hemolysis assays, bacteria were grown to late exponential/early stationary phase, at which point supernatants were collected and filtered to remove bacteria. SLO protein was detected by Western blotting with rabbit antiserum to SLO, and SLO activity was measured in hemolysis assays as previously described (42). Briefly, hemolytic activity was determined as the reciprocal of the dilution of culture supernatant that resulted in 50% erythrocyte lysis and was expressed in hemolytic units (HU). For infections, bacteria were grown to mid-exponential phase in L3 medium, washed and diluted, and then used to infect keratinocytes at an MOI of 1 with or without 2 mM IPTG to ensure induction during infection.
Measurement of vacuolar pH.
Prior to infection, live GAS was covalently labeled with FITC (pH sensitive) and Alexa Fluor 405 SE (pH insensitive) in the presence of sodium hydrogen carbonate buffer at pH 8.3 for 30 min at room temperature. Labeled GAS was extensively washed with sodium hydrogen carbonate buffer, resuspended in KSFM, and added to keratinocyte cultures at an MOI of 2. At 2 h postinfection, bacterial internalization was arrested by addition of the actin polymerization inhibitor cytochalasin D at 5 µg/ml and extracellular bacteria were killed by treatment with penicillin and gentamicin for 1 h. Synchronized infected cultures were washed, trypsinized, and then briefly incubated with Alexa Fluor 660-conjugated anti-GAS IgG to stain the remaining extracellular bacteria. Infected keratinocytes were immediately analyzed by flow cytometry, gating for cells that had internalized GAS but did not display extracellular bacteria on their surface. The ratio of the mean fluorescence emission intensity of FITC to that of Alexa Fluor 405 was determined for each sample. Experimental pH values were extrapolated from standard curves obtained by resuspending infected cells in K+-rich buffers of known pH supplemented with 10 µg/ml nigericin and 10 µg/ml valinomycin, which equalized the intracellular pH with the pH of the extracellular environment (43). The calibration buffer contained 140 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 5 mM glucose and was titrated to pH 4 or 5 with 25 mM acetic acid, to pH 6 with 25 mM morpholineethanesulfonic acid (MES), or to pH 7 with 25 mM morpholinepropanesulfonic acid (MOPS).
Microscopy.
Keratinocytes were infected as described above at an MOI of 2 for 3 h and then washed with PBS to remove unattached bacteria. Extracellular GAS was stained with Alexa Fluor 660-conjugated anti-GAS IgG at 4°C for 20 min. Infected cells were fixed and permeabilized with ice-cold methanol at −20°C for 5 to 10 min. Cells were blocked in PBS with 1% bovine serum albumin and then stained with anti-LAMP-1 antibody, Alexa Fluor 568-conjugated anti-mouse secondary antibody, and Alexa Fluor 488-conjugated anti-GAS IgG. Each staining step was performed at room temperature for 1 h, and cells were washed three times with PBS after each step. Confocal microscopy was performed at the Harvard Digestive Diseases Center core facility on a Nikon Eclipse TE2000-E inverted microscope interfaced with a Yokogawa CSU-10 spinning-disc scan head, an Orca ER AG cooled charge-coupled device camera, a 100× Plano apo DICH oil objective, and a Kr/Ar laser producing excitation wavelengths of 488, 568, and 647 nm. Image acquisition and normalization were performed with Slidebook 4.2 (Intelligent Imaging Innovations, Denver, CO).
Uptake of other cargo.
Effects of SLO on the internalization of other cargo were investigated by measuring the uptake of three fluorescent compounds, 10,000-MW dextran, 70,000-MW dextran, and transferrin. The uptake of dextrans was assayed 2 h after addition to keratinocytes, and transferrin uptake was assayed 30 min after addition. To measure uptake, keratinocytes were gently trypsinized to detach them, treated with 10% fetal bovine serum, fixed with 1% paraformaldehyde, and analyzed using a MoFlo high-speed flow cytometer. Data were analyzed with Summit v 4.3 software (Cytomation, Inc.).
Statistical analysis.
The statistical significance of differences between experimental groups was evaluated by Mann-Whitney U or one-way analysis-of-variance tests using Prism 5.0 (GraphPad, San Diego, CA).
ACKNOWLEDGMENTS
We thank James Rheinwald and Tomas Kirchhausen for providing materials and helpful advice and Elizabeth Boush, Jessica Wagner, and Santana Chavez for excellent technical assistance.
This work was supported in part by grants AI 070926 and AI 029952 from the National Institutes of Health. L.K.L. was supported in part by training grant T32 AI007061 and individual NRSA F32 AI 074229, both from the National Institutes of Health.
Footnotes
Citation Logsdon, L. K., A. P. Håkansson, G. Cortés, and M. R. Wessels. 2011. Streptolysin O inhibits clathrin-dependent internalization of group A Streptococcus. mBio 2(1):e00332-10. doi:10.1128/mBio.00332-10.
REFERENCES
- 1. Bisno A. L., Stevens D. L. 1996. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 334:240–245 [DOI] [PubMed] [Google Scholar]
- 2. Cunningham M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stevens D. L. 2000. Group A beta-hemolytic streptococci: virulence factors, pathogenesis, and spectrum of clinical infections, p. 19–36 In Stevens D. L., Kaplan E. L., Streptococcal infections. Clinical aspects, microbiology, and molecular pathogenesis. Oxford University Press, New York, NY. [Google Scholar]
- 4. Greco R., De Martino L., Donnarumma G., Conte M. P., Seganti L., Valenti P. 1995. Invasion of cultured human cells by Streptococcus pyogenes. Res. Microbiol. 146:551–560 [DOI] [PubMed] [Google Scholar]
- 5. LaPenta D., Rubens C., Chi E., Cleary P. P. 1994. Group A streptococci efficiently invade human respiratory epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 91:12115–12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molinari G., Talay S. R., Valentin-Weigand P., Rohde M., Chhatwal G. S. 1997. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect. Immun. 65:1357–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ozeri V., Rosenshine I., Mosher D. F., Fassler R., Hanski E. 1998. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol. Microbiol. 30:625–637 [DOI] [PubMed] [Google Scholar]
- 8. Schrager H. M., Rheinwald J. G., Wessels M. R. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Invest. 98:1954–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Molinari G., Chhatwal G. S. 1998. Invasion and survival of Streptococcus pyogenes in eukaryotic cells correlates with the source of the clinical isolates. J. Infect. Dis. 177:1600–1607 [DOI] [PubMed] [Google Scholar]
- 10. Hakansson A., Bentley C. C., Shakhnovic E. A., Wessels M. R. 2005. Cytolysin-dependent evasion of lysosomal killing. Proc. Natl. Acad. Sci. U. S. A. 102:5192–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakagawa I., Amano A., Mizushima N., Yamamoto A., Yamaguchi H., Kamimoto T., Nara A., Funao J., Nakata M., Tsuda K., Hamada S., Yoshimori T. 2004. Autophagy defends cells against invading group A Streptococcus. Science 306:1037–1040 [DOI] [PubMed] [Google Scholar]
- 12. Cue D., Dombek P. E., Lam H., Cleary P. P. 1998. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 66:4593–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dombek P. E., Cue D., Sedgewick J., Lam H., Ruschkowski S., Finlay B. B., Cleary P. P. 1999. High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol. Microbiol. 31:859–870 [DOI] [PubMed] [Google Scholar]
- 14. Molinari G., Rohde M., Guzman C. A., Chhatwal G. S. 2000. Two distinct pathways for the invasion of Streptococcus pyogenes in non-phagocytic cells. Cell. Microbiol. 2:145–154 [DOI] [PubMed] [Google Scholar]
- 15. Giddings K. S., Johnson A. E., Tweten R. K. 2003. Redefining cholesterol’s role in the mechanism of the cholesterol-dependent cytolysins. Proc. Natl. Acad. Sci. U. S. A. 100:11315–11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krauss K., Altevogt P. 1999. Integrin leukocyte function-associated antigen-1-mediated cell binding can be activated by clustering of membrane rafts. J. Biol. Chem. 274:36921–36927 [DOI] [PubMed] [Google Scholar]
- 17. Miyamoto S., Akiyama S. K., Yamada K. M. 1995. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 267:883–885 [DOI] [PubMed] [Google Scholar]
- 18. Simons K., Toomre D. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39 [DOI] [PubMed] [Google Scholar]
- 19. Veiga E., Cossart P. 2005. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 7:894–900 [DOI] [PubMed] [Google Scholar]
- 20. Veiga E., Guttman J. A., Bonazzi M., Boucrot E., Toledo-Arana A., Lin A. E., Enninga J., Pizarro-Cerda J., Finlay B. B., Kirchhausen T., Cossart P. 2007. Invasive and adherent bacterial pathogens co-opt host clathrin for infection. Cell Host Microbe 2:340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Isberg R. R., Barnes P. 2001. Subversion of integrins by enteropathogenic Yersinia. J. Cell Sci. 114:21–28 [DOI] [PubMed] [Google Scholar]
- 22. Chen L. M., Hobbie S., Galan J. E. 1996. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 274:2115–2118 [DOI] [PubMed] [Google Scholar]
- 23. Garcia-del Portillo F., Finlay B. B. 1994. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect. Immun. 62:4641–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conner S. D., Schmid S. L. 2003. Regulated portals of entry into the cell. Nature 422:37–44 [DOI] [PubMed] [Google Scholar]
- 25. Doherty G. J., McMahon H. T. 2009. Mechanisms of endocytosis. Annu. Rev. Biochem. 78:857–902 [DOI] [PubMed] [Google Scholar]
- 26. Osterlund A., Popa R., Nikkila T., Scheynius A., Engstrand L. 1997. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107:640–647 [DOI] [PubMed] [Google Scholar]
- 27. Sakurai A., Maruyama F., Funao J., Nozawa T., Aikawa C., Okahashi N., Shintani S., Hamada S., Ooshima T., Nakagawa I. 2010. Specific behavior of intracellular Streptococcus pyogenes that has undergone autophagic degradation is associated with bacterial streptolysin O and host small G proteins Rab5 and Rab7. J. Biol. Chem. 285:22666–22675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cue D., Lam H., Cleary P. P. 2001. Genetic dissection of the Streptococcus pyogenes M1 protein: regions involved in fibronectin binding and intracellular invasion. Microb. Pathog. 31:231–242 [DOI] [PubMed] [Google Scholar]
- 29. Cossart P., Pizarro-Cerda J., Lecuit M. 2003. Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell Biol. 13:23–31 [DOI] [PubMed] [Google Scholar]
- 30. Ashbaugh C. D., Warren H. B., Carey V. J., Wessels M. R. 1998. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J. Clin. Invest. 102:550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sierig G., Cywes C., Wessels M. R., Ashbaugh C. D. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group A streptococci. Infect. Immun. 71:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gryllos I., Cywes C., Shearer M. H., Cary M., Kennedy R. C., Wessels M. R. 2001. Regulation of capsule gene expression by group A Streptococcus during pharyngeal colonization and invasive infection. Mol. Microbiol. 42:61–74 [DOI] [PubMed] [Google Scholar]
- 33. Monack D. M., Raupach B., Hromockyj A. E., Falkow S. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. U. S. A. 93:9833–9838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mecsas J., Bilis I., Falkow S. 2001. Identification of attenuated Yersinia pseudotuberculosis strains and characterization of an orogastric infection in BALB/c mice on day 5 postinfection by signature-tagged mutagenesis. Infect. Immun. 69:2779–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eichenbaum Z., Federle M. J., Marra D., de Vos W. M., Kuipers O. P., Kleerebezem M., Scott J. R. 1998. Use of the lactococcal nisA promoter to regulate gene expression in gram-positive bacteria: comparison of induction level and promoter strength. Appl. Environ. Microbiol. 64:2763–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dancz C. E., Haraga A., Portnoy D. A., Higgins D. E. 2002. Inducible control of virulence gene expression in Listeria monocytogenes: temporal requirement of listeriolysin O during intracellular infection. J. Bacteriol. 184:5935–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Que Y. A., Haefliger J. A., Francioli P., Moreillon P. 2000. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 68:3516–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michos A., Gryllos I., Hakansson A., Srivastava A., Kokkotou E., Wessels M. R. 2006. Enhancement of streptolysin O activity and intrinsic cytotoxic effects of the group A streptococcal toxin, NAD-glycohydrolase. J. Biol. Chem. 281:8216–8223 [DOI] [PubMed] [Google Scholar]
- 39. Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10:839–850 [DOI] [PubMed] [Google Scholar]
- 40. Crowe D. L., Hu L., Gudas L. J., Rheinwald J. G. 1991. Variable expression of retinoic acid receptor (RAR beta) mRNA in human oral and epidermal keratinocytes; relation to keratin 19 expression and keratinization potential. Differentiation 48:199–208 [DOI] [PubMed] [Google Scholar]
- 41. Dickson M. A., Hahn W. C., Ino Y., Ronfard V., Wu J. Y., Weinberg R. A., Louis D. B., Li F. P., Rheinwald J. G. 2000. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruiz N., Wang B., Pentland A., Caparon M. 1998. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol. Microbiol. 27:337–346 [DOI] [PubMed] [Google Scholar]
- 43. Savina A., Jancic C., Hugues S., Guermonprez P., Vargas P., Moura I. C., Lennon-Dumenil A. M., Seabra M. C., Raposo G., Amigorena S. 2006. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126:205–218 [DOI] [PubMed] [Google Scholar]