Abstract
Two new eunicellin-type diterpenoids, cladielloides A (1) and B (2), which were found to possess a 2-hydroxybutyroxy group in their structures, were isolated from an Indonesian octocoral identified as Cladiella sp. The structures of eunicellins 1 and 2 were elucidated by spectroscopic methods. Cladielloide B (2) exhibited moderate cytotoxicity toward CCRF-CEM tumor cells and this compound displayed significant inhibitory effects on superoxide anion generation and elastase release by human neutrophils.
Keywords: cladielloide, eunicellin, octocoral, cytotoxicity, superoxide anion, elastase
1. Introduction
Previous chemical investigations on the octocorals belonging to the genus Cladiella have resulted in a series of interesting eunicellin-based (2,11-cyclized cembranoid) diterpenoids [1–6], and the compounds of this type have been found to possess complex structures and various bioactivities [1–3,5–11]. In continuation of our search for bioactive substances from the marine invertebrates distributed in the tropical West Pacific Ocean, an Indonesian octocoral identified as Cladiella sp. was studied, and its organic extract exhibited cytotoxicity toward DLD-1 (human colorectal adenocarcinoma), HL-60 (human promyelocytic leukemia cells), and P388D1 (macrophage-like murine tumor cells), with IC50 = 2.7, 8.9, 7.2 μg/mL, respectively. Two new eunicellins, cladielloides A (1) and B (2), were isolated from this marine organism. In this paper, we report the isolation, structure determination, and bioactivity of the above new diterpenoids 1 and 2 (Scheme 1).
Scheme 1.
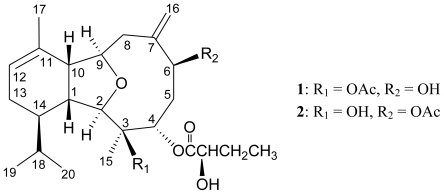
The structures of cladielloides A (1) and B (2).
2. Results and Discussion
Cladielloide A (1) was isolated as a colorless oil and the molecular formula for this compound was determined to be C26H40O7 (seven degrees of unsaturation) by HRESIMS (C26H40O7 + Na, m/z 487.2674, calculated 487.2672). The IR spectrum of 1 showed bands at 3460 and 1734 cm−1, consistent with the presence of hydroxy and ester groups. From the 1H and 13C NMR spectra (Table 1), 1 was found to possess a trisubstituted olefin (δH 5.43, 1H, m, H-12; δC 132.1, s, C-11; 122.2, d, C-12), an exocyclic carbon-carbon double bond (δH 5.21, 1H, s, H-16a; 5.58, 1H, s, H-16b; δC 147.6, s, C-7; 115.2, t, C-16), an acetoxy group (δH 2.14, 3H, s; δC 20.6, q; 171.1, s), and a 2-hydroxybutyrate (δH 1.03, 3H, t, J = 7.2 Hz; 1.91, 2H, m; 4.86, 1H, dd, J = 6.8, 6.0 Hz; δC 9.3, q; 24.3, t; 74.1, d; 171.4, s) moiety. Thus, from the above data, four degrees of unsaturation were accounted for and compound 1 must be a tricyclic compound.
Table 1.
1H and 13C NMR data, 1H–1H COSY, and HMBC correlations for diterpenoid 1.
| C/H | 1H a | 13C b | 1H–1H COSY | HMBC (H→C) | |
|---|---|---|---|---|---|
| 1 | 2.74 m | 39.7 | (d) d | H-2, H-10, H-14 | C-2, −3, −10, −11, −14 |
| 2 | 3.86 d (8.0) c | 87.1 | (d) | H-1 | C-3, −4, −9, −14, −15 |
| 3 | 74.6 | (s) | |||
| 4 | 5.14 dd (4.4, 3.6) | 74.4 | (d) | H2-5 | C-3, −6, −15, −23 |
| 5α | 2.97 ddd (16.0, 4.4, 2.8) | 37.2 | (t) | H-4, H-5β, H-6 | C−3, −4 |
| β | 1.75 ddd (16.0, 5.6, 3.6) | H-4, H-5α, H-6 | C-4, −6, −7 | ||
| 6 | 4.21 br s | 72.6 | (d) | H2-5, OH-6 | n.o. e |
| 7 | 147.6 | (s) | |||
| 8 | 2.35 br d (2.4) | 40.0 | (t) | H-9 | C-6, −7, −9, −10, −16 |
| 9 | 4.16 dt (3.6, 3.2) | 81.3 | (d) | H2-8, H-10 | n.o. |
| 10 | 2.63 br s | 44.6 | (d) | H-1, H-9 | C-11 |
| 11 | 132.1 | (s) | |||
| 12 | 5.43 m | 122.2 | (d) | H2-13, H3-17 | n.o. |
| 13α | 2.10 m | 22.8 | (t) | H-12, H-13β, H-14 | n.o. |
| β | 1.97 m | H-12, H-13α, H-14 | n.o. | ||
| 14 | 1.58 m | 39.0 | (d) | H-1, H2-13, H-18 | n.o. |
| 15 | 1.37 s | 22.4 | (q) | C-2, −3, −4 | |
| 16a | 5.21 s | 115.2 | (t) | H-16b | C-6, −8 |
| b | 5.58 s | H-16a | C-6, −7, −8 | ||
| 17 | 1.68 d (0.8) | 22.0 | (q) | H-12 | C-10, −11, −12 |
| 18 | 1.62 m | 28.8 | (d) | H-14, H3-19, H3-20 | C-1, −14 |
| 19 | 0.92 d (6.4) | 21.3 | (q) | H-18 | C-14, −18, −20 |
| 20 | 0.83 d (6.4) | 20.5 | (q) | H-18 | C-14, −18, −19 |
| OH-6 | 2.84 d (7.2) | H-6 | n.o. | ||
| 3-OC(O)CH3 | 171.1 | (s) | |||
| 21 22 | 2.14 s | 20.6 | (q) | C-21 | |
 |
171.4 | (s) | |||
| 4.86 dd (6.8, 6.0) | 74.1 | (d) | H2-25 | C-23, −25, −26 | |
| 1.91 m | 24.3 | (t) | H-24, H3-26 | C-23, −24, −26 | |
| 1.03 t (7.2) | 9.3 | (q) | H2-25 | C-24, −25 | |
Spectra measured at 400 MHz in CDCl3 at 25 °C;
Spectra measured at 100 MHz in CDCl3 at 25 °C;
J values (in hertz) in parentheses;
Attached protons were deduced by DEPT and HMQC experiments;
n.o. = not observed.
In the 1H NMR spectrum of 1, two doublets at δH 0.92 and 0.83 (each 3H, d, J = 6.4 Hz, H3-19 and H3-20) were deduced from two methyls of an isopropyl group. A singlet of the tertiary methyl bonded to an oxygenated carbon was due to the resonance of signal at δH 1.37 (3H, s, H3-15). In addition, a suite of resonances of proton signals at δH 2.74 (1H, m, H-1), 2.63 (1H, br s, H-10), 3.86 (1H, d, J = 8.0 Hz, H-2), 4.16 (1H, dt, J = 3.6, 3.2 Hz, H-9), and carbon signals at δC 39.7 (d, C-1), 44.6 (d, C-10), 87.1 (d, C-2), and 81.3 (d, C-9), indicated the presence of a tetrahydrofuran structural unit. Based on the above data, the proposed skeleton of 1 was suggested to be a eunicellin-based metabolite.
From the 1H–1H COSY spectrum of 1, it was possible to identify the separate spin systems among H-1/H-2; H-4/H2-5/H-6; H2-8/H-9/H-10; and H-10/H-1 (Table 1). These data, together with the HMBC correlations between H-1/C-2, −3, −10; H-2/C-3, −4, −9; H-4/C-3, −6; H2-5/C-3, −4, −6, −7; and H2-8/C-6, −7, −9, −10, established the connectivity from C-1 to C-10 within the ten-membered ring (Table 1). An exocyclic carbon-carbon double bond at C-7 was confirmed by the HMBC correlations between H2-16/C-6, −7, −8 and H2-8/C-16. The hydroxy proton signal at δH 2.84 was revealed by its 1H–1H COSY correlations to H-6 (δH 4.21), indicating its attachment to C-6. The location of 2-hydroxybutyrate group in 1 was confirmed by an HMBC correlation between H-4 (δH 5.14) and the 2-hydroxybutyrate carbonyl (δC 171.4, s). Thus, the remaining acetate ester was at C-3, an oxygenated quaternary carbon which bonded to the C-15 tertiary methyl and is confirmed by the HMBC correlations between H-2/C-15; H-4/C-15; and H3-15/C-2, −3, −4. The ether bridge between C-2 and C-9 was supported by an HMBC correlation between H-2/C-9. The 1-isopropyl-4-methylcyclohexene ring, which is fused to the ten-membered ring at C-1 and C-10, was elucidated by the 1H–1H COSY correlations between H-12/H2-13/H-14/H-1; H-14/H-18; and H-18/H3-19(H3-20) and further supported by the HMBC correlations between H-1/C-11, −14; H-2/C-14; H-10/C-11; H3-17/C-10; and H-18/C-1. The vinyl methyl at C-11 was confirmed by the HMBC correlations between H3-17/C-10, −11, −12 and further supported by the allylic coupling between the olefin proton H-12 and H3-17 (J = 0.8 Hz). Therefore, the planar structure of 1 was established.
The relative configuration of 1 was elucidated from the interactions observed in a NOESY experiment. In the NOESY experiment of 1 (Table 2), the correlations between H-1 with H-4 and H-10, indicated that these protons are situated on the same face and assigned as β protons. H-2 exhibited interactions with H-14 and H3-15 and no correlation was found between H-1 and H-2, indicating that H-2, H-14, and Me-15 should be α-oriented. H-6 correlated with one proton of C-5 methylene (δH 2.97), but not with H-4, reflecting the α-orientation of H-6. Furthermore, H-9 correlated with H2-8 and H3-17. From consideration of molecular models, H-9 was found to be reasonably close to H2-8 and H3-17, when H-9 was α-oriented in 1.
Table 2.
The stereoview of 1 (generated from computer modeling) and the calculated distances (Å) between selected protons having key NOESY correlations.
| Cladielloide A (1) | H/H | (Å) |
|---|---|---|
 |
H-1/H-4 | 2.59 |
| H-1/H-10 | 2.33 | |
| H-2/H-14 | 2.45 | |
| H-2/H3-15 | 2.32 | |
| H-6/H-5α | 2.44 | |
| H-8α/H-9 | 2.44 | |
| H-8β/H-9 | 2.50 | |
| H-9/H3-17 | 2.61 |
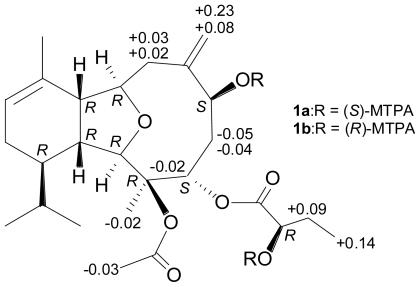
In order to determine the absolute configuration, the eunicellin 1 was treated with (−) or (+)-MTPA chloride to yield the (S)- and (R)-MTPA esters 1a and 1b, respectively [12–14]. Comparison of the 1H NMR chemical shifts for 1a and 1b (Δ values shown in Figure 1) led to the assignment of the S-configuration at C-6. The C-24 hydroxy group in the 2-hydroxybutyrate moiety was also assigned as R-configuration. Therefore, the absolute configurations of all chiral centers of 1 were assigned as 1R, 2R, 3R, 4S, 6S, 9R, 10R, 14R, 24R.
Figure 1.
The key 1H NMR chemical shift differences Δδ (δS–δR) in ppm for the MTPA esters of 1.
Cladielloide B (2) had the same molecular formula as that of 1, C26H40O7, as determined by HRESIMS (C26H40O7 + Na, m/z 487.2675, calculated 487.2672). The spectral data (1D, 2D NMR (Table 3), IR, and MS) were similar to those of 1. However, the polarity of 2, which was checked by TLC, was substantially different from that of 1, indicating that these two compounds are isomers. In the 1H NMR spectrum of 2, an acetate methyl was observed at δH 2.14 (3H, s). The additional acyl group was found to be a 2-hydroxybutyrate group, which showed six contiguous protons (δH 1.02, 3H, t, J = 7.2 Hz; 1.91, 2H, m; 4.87, 1H, dd, J = 6.8, 6.0 Hz). The 13C NMR signal at δC 170.2 (s) correlated with the signal of an oxymethine proton at δH 4.87 in the HMBC spectrum and was consequently assigned as the carbon atom of the 2-hydroxybutyrate carbonyl. A correlation observed in the HMBC experiment of 2 further revealed the connectivity between H-4 (δH 5.21) and the carbonyl carbon (δC 170.2) of 2-hydroxybutyrate unit and demonstrated the location of the 2-hydroxybutyrate to be at C-4. The position of acetoxy group at C-6 was also confirmed by the connectivity between the oxymethine proton at δH 4.66 (H-6) and the ester carbonyl at δC 171.6 (s) in the HMBC spectrum of 2. Thus, the remaining hydroxy group should be positioned at C-3. In addition, by comparison of the NOESY correlations of 2 with those of 1, the chiral centers of 2 were confirmed to be the same as those of 1.
Table 3.
1H and 13C NMR data, 1H–1H COSY, and HMBC correlations for diterpenoid 2.
| Position | 1H a | 13Cb | 1H 1H COSY | HMBC (H→C) | |
|---|---|---|---|---|---|
| 1 | 2.51 m | 40.6 | (d) d | H-2, H-10, H-14 | C-10 |
| 2 | 3.90 d (3.6) c | 88.1 | (d) | H-1 | C-1, −3, −4 |
| 3 | 74.8 | (s) | |||
| 4 | 5.21 dd (8.0, 4.0) | 73.8 | (d) | H2-5 | C-6, −21 |
| 5α | 2.48 m | 34.2 | (t) | H-4, H-5β, H-6 | C-6, −7 |
| β | 1.97 m | H-4, H-5α, H-6 | n.o. e | ||
| 6 | 4.66 dd (8.8, 3.2) | 83.8 | (d) | H2-5 | C-4, −7, −16, −25 |
| 7 | 144.2 | (s) | |||
| 8α | 2.65 dd (14.0, 4.8) | 41.4 | (t) | H-8β, H-9 | C-6, −7, −9, −10, −16 |
| β | 2.46 dd (14.0, 2.0) | H-8α, H-9 | C-6, −7, −9, −10, −16 | ||
| 9 | 4.06 br s | 82.4 | (d) | H2-8, H-10 | n.o. |
| 10 | 2.58 br s | 44.7 | (d) | H-1, H-9 | C-8, −9, −11 |
| 11 | 131.1 | (s) | |||
| 12 | 5.49 m | 123.1 | (d) | H2-13, H3-17 | n.o. |
| 13α | 2.01 m | 22.9 | (t) | H-12, H-13β, H-14 | n.o. |
| β | 1.80 m | H-12, H-13α, H-14 | n.o. | ||
| 14 | 1.39 m | 39.8 | (d) | H-1, H2-13, H-18 | C-2 |
| 15 | 1.33 s | 22.8 | (q) | C-2, −3, −4 | |
| 16a | 5.26 s | 117.7 | (t) | H-16b | C-6, −8 |
| b | 5.47 s | H-16a | C-6, −7, −8 | ||
| 17 | 1.69 d (1.2) | 22.8 | (q) | H-12 | C-10, −11, −12 |
| 18 | 1.80 m | 27.8 | (d) | H-14, H3-19, H3-20 | C-14, −19, −20 |
| 19 | 0.94 d (6.8) | 21.7 | (q) | H-18 | C-14, −18, −20 |
| 20 | 0.77 d (6.8) | 17.5 | (q) | H-18 | C-14, −19, −20 |
 |
170.2 | (s) | |||
| 4.87 dd (6.8, 6.0) | 74.3 | (d) | H2-23 | C-21, −23, −24 | |
| 1.91 m | 24.5 | (t) | H-22, H3-24 | C-21, −22, −24 | |
| 1.02 t (7.2) | 9.3 | (q) | H2-23 | C-22, −23 | |
| 6-OC(O)CH3 | 171.6 | (s) | |||
| 25 26 | 2.14 s | 20.6 | (q) | C-25 | |
Spectra measured at 400 MHz in CDCl3 at 25 °C;
Spectra measured at 100 MHz in CDCl3 at 25 °C;
J values (in hertz) in parentheses;
Attached protons were deduced by DEPT and HMQC experiments;
n.o. = not observed.
The cytotoxicity of metabolites 1 and 2 toward various tumor cell lines, including DLD-1, HL-60, CCRF-CEM (human T-cell acute lymphoblastic leukemia), and P388D1 cells was evaluated. The results, in Table 4, show that eunicellin 2 exhibited moderate cytotoxicity toward CCRF-CEM cells.
Table 4.
Cytotoxic data of diterpenoids 1 and 2.
| Compound | Cell lines IC50 (μg/mL) |
|||
|---|---|---|---|---|
| DLD-1 | HL-60 | CCRF-CEM | P388D1 | |
| 1 | >40 | >40 | >40 | >40 |
| 2 | 10.2 | >40 | 4.7 | >40 |
| Doxorubicin a | 0.09 | 0.03 | 0.18 | 0.11 |
Doxorubicin was used as a reference compound.
The in vitro anti-inflammatory effects of metabolites 1 and 2 were tested. Metabolite 2 displayed significant inhibitory effects on superoxide anion generation and elastase release by human neutrophils at 10 μg/mL (Table 5).
Table 5.
Inhibitory effects of diterpenoids 1 and 2 on superoxide anion generation and elastase release by human neutrophils in response to FMLP/CB.
| Compound | Superoxide anion | Elastase release |
|---|---|---|
| IC50 (μg/mL) a or (Inh %) b | IC50 (μg/mL) or (Inh %) | |
| 1 | (20.5 ± 5.0) | (27.1 ± 4.8) |
| 2 | 5.9 ± 0.7 | 6.5 ± 1.9 |
| DPI c | 0.8 ± 0.2 | |
| Elastatinal c | 30.8 ± 5.7 | |
Concentration necessary for 50% inhibition (IC50);
Percentage of inhibition (Inh %) at 10 μg/mL;
DPI (diphenylene indonium) and elastatinal were used as reference compounds.
3. Experimental
3.1. General Experimental Procedures
Optical rotation values were measured with a JASCO P-1010 digital polarimeter at 25 °C. Infrared spectra were obtained on a VARIAN DIGLAB FTS 1000 FT-IR spectrometer. The NMR spectra were recorded on a VARIAN MERCURY PLUS 400 FT-NMR at 400 MHz for 1H and 100 MHz for 13C, in CDCl3 at 25 °C. Proton chemical shifts were referenced to the residual CHCl3 signal (δH 7.26 ppm). 13C NMR spectra were referenced to the center peak of CDCl3 at δC 77.1 ppm. ESIMS and HRESIMS data were recorded on a BRUKER APEX II mass spectrometer. Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck) and spots were visualized by spraying with 10% H2SO4 solution followed by heating. HPLC was performed using a system comprised of a HITACHI L-7100 pump, a HITACHI photodiode array detector L-7455, and a RHEODYNE 7725 injection port. A normal phase column (Hibar 250 × 10 mm, Merck, silica gel 60, 5 μm,) was used for HPLC.
3.2. Animal Material
The octocoral Cladiella sp. were collected from Indonesia in 2004 and stored in a freezer until extraction. A voucher specimen was deposited in the National Museum of Marine Biology and Aquarium, Taiwan (NMMBA). This organism was identified by comparison with previous descriptions [15,16].
3.3. Extraction and Isolation
Slices of Cladiella sp. (wet weight 924 g) were extracted with a mixture of MeOH and CH2Cl2 (1:1) and the residue was partitioned between EtOAc and H2O. The EtOAc layer was subjected to silica gel column chromatography and eluted using a mixture of n-hexane and EtOAc (stepwise, 100:1 to pure EtOAc) to obtain 19 fractions A–S. Fractions K and N were repurified by normal phase HPLC, using the mixture of n-hexane/ethyl acetate to afford 2 (2.4 mg, 5.5:1) and 1 (7.9 mg, 3:1), respectively.
Cladielloide A (1). Colorless oil; [α]D23 −24° (c 0.4, CHCl3); IR (neat) νmax 3460, 1734 cm−1; 1H (CDCl3, 400 MHz) and 13C (CDCl3, 100 MHz) NMR data, see Table 1; ESIMS m/z 487 (M + Na)+; HRESIMS m/z 487.2674 (calculated for C26H40O7 + Na, 487.2672).
Cladielloide B (2). Colorless oil; [α] D23 −10° (c 0.1, CHCl3); IR (neat) νmax 3446, 1738 cm−1; 1H (CDCl3, 400 MHz) and 13C (CDCl3, 100 MHz) NMR data, see Table 3; ESIMS m/z 487 (M + Na)+; HRESIMS m/z 487.2675 (calculated for C26H40O7 + Na, 487.2672).
3.4. Preparation of (S)- and (R)-MTPA Esters of Cladielloide A (1)
To a solution of 1 (1 mg) in pyridine (0.4 mL), R-(−)-α-methoxy-α-(trifluoromethyl) phenylacetyl (MPTA) chloride (25 μL) was added, and the mixture was allowed to stand for 24 h at room temperature. The reaction was quenched by addition of 1.0 mL of water, and the mixture was subsequently extracted with EtOAc (3 × 1.0 mL). The EtOAc-soluble layers were combined, dried over anhydrous MgSO4 and evaporated. The residue was subjected to column chromatography over silica gel using n-hexane–EtOAc (13:2) to yield the (S)-MTPA ester, 1a (0.7 mg, 44%). The same procedure was used to prepare the (R)-MTPA ester, 1b (1.4 mg, 89%), from the reaction of (S)-MPTA chloride with 1 in pyridine. The key 1H NMR chemical shift differences Δδ (δS − δR) in ppm for the MTPA esters of 1 are shown in Figure 1.
3.5. Cytotoxicity Testing
The cytotoxicity of compounds 1 and 2 was assayed with a modification of the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric method. Cytotoxicity assays was carried out according to the procedures described previously [17,18].
3.6. Human Neutrophil Superoxide Anion Generation and Elastase Release
Human neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Superoxide generation and elastase release were carried out according to the procedures described previously [19,20]. Briefly, superoxide anion production was assayed by monitoring the superoxide dismutase-inhibitable reduction of ferricytochrome c. Elastase release experiments were performed using MeO-Suc-Ala-Ala-Pro-Valp-nitroanilide as the elastase substrate.
Acknowledgements
This study was supported by grants from the National Museum of Marine Biology and Aquarium (Grant No. 99200321 and 99200322); National Dong Hwa University; Asia-Pacific Ocean Research Center, National Sun Yat-sen University (Grant No. 98C031702); and the National Science and Technology Program for Biotechnology and Pharmaceuticals, National Science Council (Grant No. NSC 99-2323-B-291-001 and 98-2320-B-291-001-MY3), Taiwan, awarded to P.-J.S.
Footnotes
Samples Availability: Not available.
References
- 1.Radhika P. Chemical constituents and biological activities of the soft corals of genus Cladiella: A review. Biochem Syst Ecol. 2006;34:781–789. [Google Scholar]
- 2.Yamada K, Ogata N, Ryu K, Miyamoto T, Komori T, Higuchi R. Bioactive terpenoids from octocorallia. 3. A new eunicellin-based diterpenoid from the soft coral Cladiella sphaeroides. J Nat Prod. 1997;60:393–396. [Google Scholar]
- 3.Gray CA, Davies-Coleman MT, Schleyer MH. Cembrane diterpenes from the Southern African soft coral Cladiella kashmani. J Nat Prod. 2000;63:1551–1553. doi: 10.1021/np000179r. [DOI] [PubMed] [Google Scholar]
- 4.Chill L, Berrer N, Benayahu Y, Kashman Y. Eunicellin diterpenes from two Kenyan soft corals. J Nat Prod. 2005;68:19–25. doi: 10.1021/np049772p. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed AF, Wu M-H, Wang G-H, Wu Y-C, Sheu J-H. Eunicellin-based diterpenoids, australins A–D, isolated from the soft coral Cladiella australis. J Nat Prod. 2005;68:1051–1055. doi: 10.1021/np0500732. [DOI] [PubMed] [Google Scholar]
- 6.Hassan HM, Khanfar MA, Elnagar AY, Mohammed R, Shaala LA, Youssef DTA, Hifnawy MS, El Sayed KA. Pachycladins A–E, prostate cancer invasion and migration inhibitory eunicellin-based diterpenoids from the Red Sea soft coral Cladiella pachyclados. J Nat Prod. 2010;73:848–853. doi: 10.1021/np900787p. [DOI] [PubMed] [Google Scholar]
- 7.Chen B-W, Wu Y-C, Chiang MY, Su J-H, Wang W-H, Fan T-Y, Sheu J-H. Eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Tetrahedron. 2009;65:7016–7022. [Google Scholar]
- 8.Chen B-W, Chao C-H, Su J-H, Wen Z-H, Sung P-J, Sheu J-H. Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org Biomol Chem. 2010;8:2363–2366. doi: 10.1039/b926353e. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto T, Yamada K, Ikeda N, Komori T, Higuchi R. Bioactive terpenoids from octocorallia, I. Bioactive diterpenoids: litophynols A and B from the mucus of the soft coral Litophyton sp. J Nat Prod. 1994;57:1212–1219. doi: 10.1021/np50111a004. [DOI] [PubMed] [Google Scholar]
- 10.Ortega MJ, Zubía E, Salva J. Structure and absolute configuration of palmonine F, a new eunicellin-based diterpene from the gorgonian Eunicella verrucosa. J Nat Prod. 1994;57:1584–1586. doi: 10.1021/np50113a021. [DOI] [PubMed] [Google Scholar]
- 11.Cóbar OM. Survey of 2,11-cyclized cembranoids from Caribbean sources. Nat Prod Res. 2009;23:26–43. doi: 10.1080/14786410701760797. [DOI] [PubMed] [Google Scholar]
- 12.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. High-Field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J Am Chem Soc. 1991;113:4092–4096. [Google Scholar]
- 13.Seco JM, Quinoá E, Riguera R. The assignment of absolute configuration by NMR. Chem Rev. 2004;104:17–117. doi: 10.1021/cr2003344. [DOI] [PubMed] [Google Scholar]
- 14.Hoye TR, Jeffrey CS, Shao F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat Protoc. 2007;2:2451–2458. doi: 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
- 15.Bayer FM. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: anthozoa), with diagnoses of new taxa. Proc Biol Soc Wash. 1981;94:902–947. [Google Scholar]
- 16.Fabricius K, Alderslade P. Soft Corals and Sea Fans–A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Vol. 49. Australian Institute of Marine Science; Queensland, Australia: 2001. pp. 84–85. [Google Scholar]
- 17.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 18.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 19.Hwang T-L, Li G-L, Lan Y-H, Chia Y-C, Hsieh P-W, Wu Y-H, Wu Y-C. Potent inhibitors of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic Biol Med. 2009;46:520–528. doi: 10.1016/j.freeradbiomed.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Hwang T-L, Su Y-C, Chang H-L, Leu Y-L, Chung P-J, Kuo L-M, Chang Y-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J Lipid Res. 2009;50:1395–1408. doi: 10.1194/jlr.M800574-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]