Abstract
Cetuximab is an epidermal growth factor receptor (EGFR)-blocking antibody that is approved to treat several types of solid cancers in patients. We recently showed that cetuximab can induce autophagy in cancer cells by both inhibiting the class I phosphatidylinositol 3-kinase (PtdIns3K)/Akt/mammalian target of rapamycin (mTOR) pathway and activating the class III PtdIns3K (hVps34)/beclin 1 pathway. In the current study, we investigated the relationship between cetuximab-induced autophagy and apoptosis and the biological roles of autophagy in cetuximab-mediated cancer therapy. We found that cetuximab induced autophagy in cancer cells that show strong or weak induction of apoptosis after cetuximab treatment but not in those that show only cytostatic growth inhibition. Inhibition of cetuximab-induced apoptosis by a caspase inhibitor prevented the induction of autophagy. Conversely, inhibition of cetuximab-induced autophagy by silencing the expression of autophagy-related genes (Atg) or treating the cancer cells with lysosomal inhibitors enhanced the cetuximab-induced apoptosis, suggesting that autophagy was a protective cellular response to cetuximab treatment. On the other hand, cotreatment of cancer cells with cetuximab and the mTOR inhibitor rapamycin resulted in an Atg-dependent and lysosomal inhibition-sensitive death of cancer cells that show only growth inhibition or weak apoptosis after cetuximab treatment, indicating that cell death may be achieved by activating the autophagy pathway in these cells. Together, our findings may guide the development of novel clinical strategies for sensitizing cancer cells to EGFR-targeted therapy.
Key words: EGFR, cetuximab, autophagy, apoptosis, cancer therapy
Introduction
The epidermal growth factor receptor (EGFR), a 170 kD glycosylated transmembrane receptor with intrinsic tyrosine kinase activity, is commonly expressed in a variety of normal and malignant epithelial cells.1,2 EGFR-mediated cell signaling is aberrantly regulated in cancer cells.1,3 Cetuximab, a monoclonal antibody that effectively inhibits EGFR by blocking its ligand-induced activation, was the first EGFR antibody approved by the US Food and Drug Administration to be used in combination with either chemotherapy or radiotherapy to treat colorectal and head and neck cancers.4 We and others have shown that the therapeutic effects of cetuximab on cancer cells include cell cycle arrest and/or the induction of apoptotic cell death.5–13 More recently, we found that treating cancer cells with cetuximab can also induce autophagy through inhibition of the class I phosphatidylinositol 3-kinase (PtdIns3K)/Akt/mammalian target of rapamycin (mTOR) pathway and activation of the class III PtdIns3K(hVps34)/Beclin 1 complex;14 the induction of autophagy and apoptosis by cetuximab appears to share at least one common pathway involving the downregulation of the hypoxia-inducible factor-1alpha after cetuximab treatment.13,14 However, whether there is a relationship between cetuximab-induced apoptosis and autophagy and how the autophagy induced by cetuximab or by other biological and pharmacological agents may affect the response of cancer cells to cetuximab is largely unknown. Our understanding of the roles of autophagy in cetuximab-mediated cancer therapy against EGFR is just beginning.
Apoptosis and autophagy, both genetically regulated and evolutionarily conserved, are two important cellular processes that regulate cell survival and death. Morphologically featured by highly condensed membrane blebbing, cell shrinkage, the formation of apoptotic bodies and nuclear condensation, apoptosis is triggered by the activation of a group of cysteine-aspartic proteases (caspases), including the initiator and effector caspases that together initiate the apoptotic cascade leading to the cleavage of hundreds of targeted proteins, which eventually disassembles the cells.15–17 In contrast, autophagy, which is morphologically characterized by the appearance of double-membrane vacuoles (autophagosomes) in the cytoplasm, is regulated by a group of autophagy-related (Atg) genes. The Atgs control a coordinated process leading to the induction and nucleation of autophagic vesicles that eventually fuse with lysosomes where the macromolecules engulfed within the autophagosomes are degraded and recycled.18–20 By selectively recycling macromolecules and organelles, autophagy is an integral part of normal cellular function, helping cells survive under starvation conditions and maintaining cell growth and development and the homeostasis of the organism.21 When cells lack nutrients or are deprived of growth factors, which govern the uptake of nutrients, autophagy is rapidly induced to fuel the cells' bioenergetics and to prevent cell death. In such circumstances, inhibiting autophagy results in accelerated cell death through apoptosis.22,23 Autophagy can also protect cells from various other apoptotic stimuli.24 mTOR is an important anti-autophagy protein functioning upstream of the Atgs and is centrally regulated by multiple upstream signaling pathways involving PtdIns3K/Akt, AMP-activated protein kinase and several other proteins. Inhibition of mTOR by rapamycin, a lipophilic macrolide antibiotic once used as an immunosuppressant, can induce autophagy.25
On the other hand, autophagy can also lead to autophagic cell death, which is also known as type II programmed cell death to distinguish it from apoptosis or type I programmed cell death.26–28 One of the best examples of autophagic cell death is the death of cells that have defective apoptosis machinery, such as the etoposide-induced death of embryonic fibroblasts from Bax/Bak double knockout mice,29 or the cell death induced by caspase inhibitors.30 Thus, autophagy can have both positive and negative effects on cell survival.
To understand the relationship between apoptosis and autophagy in cetuximab-mediated cancer therapy, in this study, we investigated the ability of cetuximab to induce autophagy in several types of cancer cells that respond to cetuximab treatment with strong or weak induction of apoptosis or with only cytostatic growth inhibition. We used a combination of several techniques to detect autophagy and apoptosis, including transmission electron microscopy, fluorescent microscopy, enzyme-linked immunosorbent assay (ELISA), western blot analysis and cell viability assays. We explored novel approaches for enhancing the therapeutic effect of cetuximab through the regulation of autophagy. The findings from our study provide important insights that may aid in the development of novel strategies to improve the response of cancer cells to cetuximab by exploiting the role of autophagy in EGFR-targeted therapy.
Results
Autophagy induced by cetuximab is a resistance mechanism of cancer cells to cetuximab-induced apoptotic cell death.
Depending on the cancer cells' dependence on EGFR-mediated cell signaling, which is an intrinsic property of the cells, cetuximab can induce cell death through apoptosis, partially or completely arrest the cell cycle, or have no effect on cell survival and proliferation.5–13 DiFi colorectal carcinoma cells are highly dependent on EGFR-mediated cell signaling; treatment of the cells with cetuximab leads to cell death through apoptosis.12,31 Following transfection of these cells with a cDNA construct containing green fluorescent protein (GFP)-tagged microtubule-associated light chain 3 (LC3, mammalian Atg8), we detected an abundance of punctate fluorescent dots in the cells 24 h after cetuximab treatment (Fig. 1A), which reflects the conversion of the cytoplasmic LC3-I to the membrane-associated autophagosome form, LC3-II, a marker of autophagy.32 Transmission electron microscopy performed 48 h after cetuximab treatment showed an abundance of autophagosomes that are characteristic of autophagy, which further confirmed the induction of autophagy in these cells (Fig. 1B). To further demonstrate the induction of autophagy by cetuximab, we examined the effects of autophagy-lysosomal inhibition on the autophagic flux in DiFi cells after cetuximab treatment. Western blot analysis showed the appearance of LC3-II in DiFi cells 24 h after cetuximab treatment (Fig. 1C), which was accumulated when the flux of autophagosomes, from their formation to fusion with the lysosomes, was inhibited by several inhibitors, including bafilomycin A1, a vacuolar ATPase inhibitor and chloroquine and NH4Cl, both of which are autophagy-lysosomal inhibitors. Collectively, these results strongly show that autophagy was induced in the DiFi cells after cetuximab treatment.
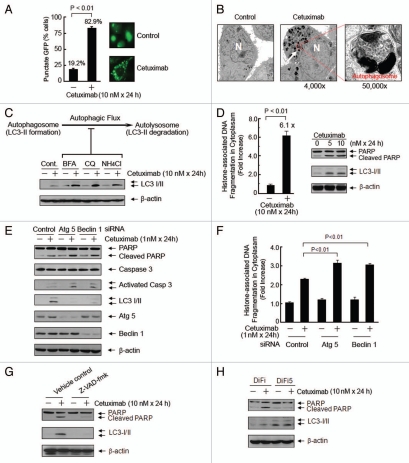
Figure 1.
Cetuximab induces autophagy that is related to the strong induction of apoptosis by cetuximab in DiFi cells. (A) Cetuximab induced appearance of the membrane-associated LC3 (punctate fluorescence). DiFi cells were transiently transfected with a GFP-LC3 construct for 24 h and then treated with or without cetuximab as indicated. The number of cells with punctate fluorescence was counted in 10 different fields under a fluorescent microscope and is shown as a percentage of the total number of cells counted. (B) Cetuximab induced formation of autophagosomes. DiFi cells were either untreated or treated with cetuximab for 48 h. Cell samples were prepared for transmission electron microscopy analysis as described in the Materials and Methods. A magnified view of the electron photomicrograph shows a characteristic autophagosome. N = nucleus. (C) Inhibition of the autophagic flux led to accumulation of cetuximab-induced LC3-II. DiFi cells were either untreated or treated with 10 nM cetuximab for 24 h in the absence or presence of bafilomycin A1 (BFA, 200 nM), chloroquine (CQ, 100 µM) or NH4C1 (50 mM). Cell lysates were analyzed by western blot. β-actin was used as protein-loading control. (D) Cetuximab induced both apoptosis and autophagy. Left, DiFi cells were either untreated or treated with cetuximab, as indicated. Cell lysates were analyzed for the levels of histone-associated DNA fragmentation in the cytosol by an ELISA. Right, DiFi cells were either untreated or treated with cetuximab, as indicated. Cell lysates were analyzed by western blot for the level of PARP cleavage and the appearance of LC3-II . (E and F) Silencing Atg5 or beclin 1 enhanced cetuximab-induced apoptosis. DiFi cells were transiently transfected with siRNA directed against Atg5 or beclin 1 or control siRNA for 72 h. Cells were then either untreated or treated, as indicated. Cell lysates were analyzed by (E) western blot and (F) an apoptosis ELISA. (G) Inhibition of caspase abolished cetuximab-induced autophagy. DiFi cells were first treated with 100 µM Z-VAD-fmk or vehicle control (DMSO) for 6 h and then treated for another 24 h in the presence or absence of 10 nM cetuximab. Cell lysates were analyzed by western blot. (H) Cetuximab failed to induce autophagy in a cetuximab-resistance subline. DiFi and DiFi5 cells were either untreated or treated with 10 nM cetuximab for 24 h. Cell lysates were analyzed by western blot. Values shown in (A, D and F) are means ± SD. p values for the comparisons were determined by Student's t-test.
We found that after treating DiFi cells with cetuximab, the induction of autophagy coincides with the induction of apoptosis. An apoptosis ELISA detected a marked increase (6.1X) in histone-associated DNA fragmentation in the cytoplasm of DiFi cells after cetuximab treatment (Fig. 1D). Western blot analysis showed both cleavage of poly (ADP-ribose) polymerase (PARP), which is a marker of apoptosis and the appearance of LC-3II. To understand the relationship between cetuximab-induced apoptosis and autophagy, we asked whether inhibition of autophagy by silencing Atg5 or beclin 1 (Atg6) has any effect on cetuximab-induced apoptosis. Because DiFi cells are highly sensitive to cetuximab, in these studies, we used a suboptimal dose (1 nM), which only induces a modest level of apoptosis, allowing us to detect increases in apoptosis after inhibition of autophagy. Figure 1E shows that knockdown of Atg5 or beclin 1 by small-interfering RNA (siRNA) successfully inhibited the LC3-I to LC3-II conversion after cetuximab treatment. We found that knockdown of Atg5 or beclin 1 led to an increase in cetuximab-induced apoptosis, as shown by an increase in the level of PARP cleavage and the level of activated caspase 3 (Fig. 1E). We further confirmed this finding with an apoptosis ELISA showing that after cetuximab treatment more DNA fragmentation was observed in the cells with knockdown of Atg5 or beclin 1 than in the control cells (Fig. 1F). Together, these data indicate that the induction of autophagy protects the cells from cetuximab-induced apoptosis, as inhibition of autophagy enhanced the cetuximab-induced apoptosis in these cells.
To further confirm this cause-and-effect relationship between autophagy and apoptosis after cetuximab treatment, we cotreated DiFi cells with cetuximab and benzyloxycarbonyl Val-Ala-Asp (O-methyl)-fluoro-methylketone (Z-VAD-fmk), a broad-spectrum caspase inhibitor.33 Z-VAD-fmk inhibited cetuximab-induced apoptosis, as shown by the inhibition of cetuximab-induced PARP cleavage in the presence of the caspase inhibitor (Fig. 1G). We found that this inhibition of cetuximab-induced apoptosis also abolished the appearance of LC3-II, which was seen in the cells cotreated with cetuximab and vehicle control. This result strongly suggests that the autophagy induced after cetuximab treatment occurs as a response to cetuximab-induced apoptosis.
We further analyzed the induction of autophagy by cetuximab in a cetuximab-resistant DiFi subline, DiFi5,31 to confirm our findings. The baseline level of LC3-II was higher in DiFi5 cells than in DiFi cells. Compared with the response of DiFi cells to cetuximab treatment, cetuximab failed to induce apoptosis in DiFi5 cells, as shown by the lack of PARP cleavage after cetuximab treatment and the LC3-II level was not further increased after the treatment (Fig. 1H). This finding provides further evidence suggesting that cetuximab-induced autophagy occurs as a mechanism of resistance to cetuximab-induced apoptosis.
We next explored whether pharmacologically inhibiting autophagy would enhance the cellular response to cetuximab. We found that treatment of DiFi cells with chloroquine markedly enhanced the cleavage of PARP induced by a suboptimal dose of cetuximab (1 nM), whereas treatment of the cells with either the suboptimal dose of cetuximab or chloroquine alone only weakly induced apoptosis (Fig. 2A). It is noteworthy that both cetuximab and chloroquine individually led to an increase in LC3-II and the combination treatment resulted in a further increase; however, the chloroquine-induced increase in LC3-II is caused by the inhibition of the autophagic flux (Fig. 1C), which is mechanistically different from the cetuximab-induced increase in LC3-II. Figure 2B further shows that changes in the relative number of surviving cells measured with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Chloroquine sensitized DiFi cells to cetuximab, as the combination of chloroquine and a suboptimal dose of cetuximab led to more cell death than either treatment alone; the relative number of surviving cells after treatment with 1 nM cetuximab plus chloroquine was similar to the number of surviving cells after treatment with 10 nM cetuximab, indicating that treatment with chloroquine sensitized the cells to cetuximab-induced apoptosis.
Figure 2.
Chloroquine, but not rapamycin, enhances cetuximab-induced apoptosis and restores the sensitivity of cetuximab-resistant cells to cetuximab-induced apoptosis. (A and B) Chloroquine enhanced cetuximab-induced apoptosis. (A) DiFi cells were untreated or treated with cetuximab and chloroquine, either alone or in combination, for 24 h, as indicated. Cell lysates were analyzed by western blot. (B) DiFi cells were untreated or treated with cetuximab and chloroquine, either alone or in combination, for 72 h, as indicated. The relative number of surviving cells was determined with an MTT assay. The optical density (O.D.) values of the treated cells at wavelength 570 nM were normalized to the O.D. values of the untreated cell lysates (control) and expressed as a percentage of the O.D. value of the control. (C and D) Chloroquine restored cetuximab sensitivity. (C) The cetuximab-resistant DiFi5 cells were untreated or treated with cetuximab and chloroquine, either alone or in combination, for 24 h, as indicated. Cell lysates were analyzed by western blot. (D) DiFi5 cells were either untreated or treated with cetuximab and chloroquine, either alone or in combination, for 72 h, as indicated. The relative number of surviving cells was determined with an MTT assay as described in (B). (E and F) Rapamycin had no effect on cetuximab-induced apoptosis. (E) DiFi cells were untreated or treated with cetuximab and rapamycin, either alone or in combination, for 24 h, as indicated. Cell lysates were analyzed by western blot. (F) DiFi cells were untreated or treated with cetuximab and rapamycin, either alone or in combination, for 72 h, as indicated. The relative number of surviving cells was determined by an MTT assay, as described in (B). Values in (B, D and F) are means ± SD. p values for the comparisons were determined by Student's t-test.
The effect of chloroquine on enhancing cetuximab-induced apoptosis was even more biologically significant in the cetuximab-resistant DiFi5 cells (Fig. 2C and D). Consistent with the finding shown in Figure 1H, DiFi5 cells showed a relatively higher basal level of LC3-II and were resistant to both cetuximab-induced apoptosis and autophagy. The sensitivity of DiFi5 cells to cetuximab-induced apoptosis was restored markedly after cotreatment of the cells with chloroquine and cetuximab, as shown by a marked increase in the amount of PARP cleavage (Fig. 2C) and a decrease in the relative number of surviving cells (Fig. 2D).
Using the same experimental approach to answer the opposite question, we further investigated whether inducing autophagy by cotreatment of cells with cetuximab and rapamycin, which is well known to induce autophagy through inhibition of mTOR, would have any effect on cetuximab-induced apoptosis. We found that the cetuximab-rapamycin combination had no appreciable effect on the cetuximab-induced PARP cleavage or inhibition of cell survival compared with either treatment alone (Fig. 2E and F).
We also found a similar result in another cell line, HCC827 non-small cell lung cancer cells, in which cetuximab strongly induced apoptosis (data not shown). Taken together, these data indicate that autophagy is induced by cetuximab and functions as a cetuximab resistance mechanism in cancer cells with cetuximab- induced apoptotic cell death. Inhibition of autophagy may, therefore, be a novel strategy for circumventing the resistance of cancer cells to cetuximab-induced apoptosis.
Both inhibition of cetuximab-induced autophagy and additional induction of autophagy by rapamycin may enhance cell death.
The A431 vulvar squamous carcinoma cells respond to cetuximab mainly through induction of cell cycle arrest; cetuximab only induces a weak apoptosis in these cells.13 Compared with DiFi cells (Fig. 1D), A431 cells showed only a modest (1.8x) increase in the level of histone-associated DNA fragmentation in the cytoplasm after 24 h of treatment with 10 nM cetuximab (Fig. 3A), which is far great than the Kd (1–2 nM) of cetuximab for binding to the EGFR.34 Using the same methods as shown in Figure 1A, we also detected cetuximab-induced punctate fluorescent dots in A431 cells transiently transfected with the same GFP-tagged LC3 construct; however, the percentage of punctate GFP-positive cells after cetuximab treatment was less in A431 cells (12.6% of untreated cells and 41.9% of cetuximab-treated cells; Fig. 3B) than in DiFi cells (19.2% of untreated cells and 82.9% of cetuximab-treated cells; Fig. 1A). The induction of autophagy by cetuximab in A431 cells was also confirmed by transmission electron microscopy, which showed the appearance of the characteristic autophagosomes (Fig. 3C). Similar to the results shown in Figure 1C, bafilomycin A1, chloroquine and NH4Cl inhibited the autophagic flux and resulted in the accumulation of LC3 after cetuximab treatment (Fig. 3D).
Figure 3.
Cetuximab induces autophagy that is related to the weak induction of apoptosis by cetuximab in A431 cells. (A) Cetuximab induced a weak apoptosis in A431 cells. A431 cells were either untreated or treated with cetuximab, as indicated. Cell lysates were analyzed for the levels of histone-associated DNA fragmentation in the cytosol by an ELISA. (B) Cetuximab induced appearance of membrane-associated LC3 (punctate fluorescence). A431 cells were transiently transfected with a GFP-LC3 construct for 24 h and then treated with cetuximab or untreated as indicated. The number of cells with punctate fluorescence was counted in 10 different fields under a fluorescent microscope and is shown as a percentage of the total number of cells counted. (C) Cetuximab induced formation of autophagosomes. A431 cells were either untreated or treated with cetuximab for 48 h. Cell samples were prepared for transmission electron microscopy analysis, as described in Materials and Methods. A magnified view of the electron photomicrograph shows a characteristic autophagosome. N = nucleus. (D) Inhibition of the autophagic flux led to accumulation of cetuximab-induced LC3-II. A431 cells were either untreated or treated with 10 nM cetuximab for 24 h, in the absence or presence of bafilomycin A1 (BFA, 200 nM), chloroquine (CQ, 100 µM) or NH4Cl (50 mM). Cell lysates were analyzed by western blot. β-actin was used as protein-loading control. (E) Inhibition of caspase abolished cetuximab-induced autophagy. A431 cells were first treated with 100 µM Z-VAD-fmk or vehicle control (DMSO) for 6 h and then treated for another 24 h in the presence or absence of 10 nM cetuximab. Cell lysates were analyzed by western blot. (F–H) Lysosomal inhibition enhanced cetuximab-induced apoptosis. (F and G), A431 cells were either untreated or treated with cetuximab in presence or absence of chloroquine or NH4Cl for 24 h, as indicated. The cell lysates were analyzed by (F) western blot and (G) an apoptosis ELISA. (H), A431 cells were either untreated or treated with cetuximab in the presence or absence of chloroquine, for 72 h, as indicated. The relative number of surviving cells was determined with an MTT assay. The optical density (O.D.) values of the treated cells at wavelength 570 nM were normalized to the O.D. value of the untreated cells (control) and expressed as a percentage of the O.D. value of the control. Values in (A, B, G and H) are means ± SD. p values for the comparisons were determined by Student's t-test.
To determine whether inhibition of the weak apoptosis induced by cetuximab in A431 cells inhibits cetuximab-induced autophagy, as it did in DiFi cells, we cotreated A431 cells with cetuximab and the caspase inhibitor Z-VAD-fmk. Cetuximab induced a weak cleavage of PARP, along with an appearance of LC3-II and treatment of A431 cells with Z-VAD-fmk inhibited the PARP cleavage and prevented the appearance of LC3-II after cetuximab treatment (Fig. 3E). This result further supports our finding from DiFi cells that the autophagy induced after cetuximab treatment occurs as a response to cetuximab-induced apoptosis.
Because cetuximab only induced a weak apoptosis in A431 cells, we next evaluated whether pharmacological inhibition of autophagy in A431 cells may enhance cetuximab-induced apoptosis, similar to the result we found in the cetuximab-resistant DiFi5 cells (Fig. 2C and D). Figure 3F shows that the amount of PARP cleavage was clearly enhanced after cotreatment of A431 cells with either NH4Cl or chloroquine and cetuximab (Fig. 3F), compared with the cleavage after treatment with cetuximab, NH4Cl or chloroquine alone. This result was further confirmed by a quantitative apoptosis ELISA (Fig. 3G). Compared with either treatment alone, cotreatment of the cells with cetuximab and chloroquine significantly reduced the relative number of surviving cells (Fig. 3H). These findings, showing that inhibiting autophagy enhanced the induction of apoptosis in A431 cells after cetuximab treatment, are consistent with our findings from DiFi cells, which showed a much stronger induction of apoptosis.
In contrast to our results from the DiFi cells (Fig. 2E and F), cotreatment of A431 cells with the autophagy inducer rapamycin and cetuximab substantially reduced the relative number of surviving cells compared with either treatment alone (Fig. 4A, solid columns). Knockdown of Atg5 expression largely negated the effect of rapamycin on the cetuximab-treated cells (Fig. 4A, open columns and inset), which strongly indicates that the enhanced cell death induced by the cetuximab-rapamycin combination was mainly caused by autophagic cell death. A trypan blue exclusion assay showed that there was a statistically significant difference in cell viability between the groups of cells treated with cetuximab or rapamycin alone and those treated with both agents (Fig. 4B). To acquire further evidence of cell death, we used a quantitative fluorescent cell live/dead assay kit to measure the percentage of live (green) and dead (red) cells after treatment with cetuximab, rapamycin or both (Fig. 4C). We also found a statistically significantly higher percentage of dead cells after treating cells with the cetuximab-rapamycin combination than after treating cells with either agent alone.
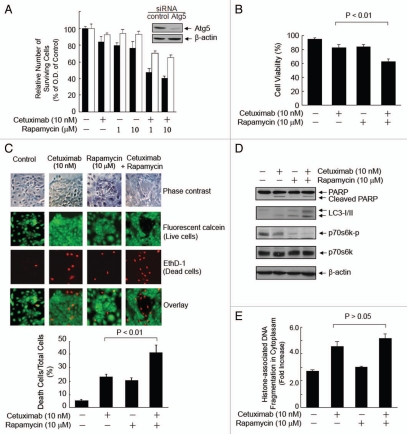
Figure 4.
Rapamycin induces autophagic cell death, leading to an enhanced cell death in A431 cells where cetuximab induces only weak apoptosis. (A) Rapamycin-induced autophagy enhanced the effect of cetuximab on inhibiting A431 cell growth and survival. A431 cells were transiently transfected with control or Atg 5 siRNA for 48 h and then were untreated or treated with cetuximab and rapamycin, either alone or in combination, for 72 h, as indicated prior to an MTT assay. The inset shows a western blot showing knockdown of Atg5 48 h after transfection. ■: control siRNA, □: Atg5-siRNA. (B) The cetuximab-rapamycin combination reduced cell viability. A431 cells were untreated or treated with cetuximab and rapamycin, either alone or in combination, for 72 h, as indicated. Cell viability after the treatments was determined by a trypan blue exclusion assay. (C) The cetuximab-rapamycin combination increased cell death. A431 cells were either untreated or treated, as described in (B). A fluorescence-based cell viability assay was performed, as described in Materials and Methods. The results shown are the average from three independent experiments. The values are expressed as means ± SD. p values were determined by student's t-test. (D) The cetuximab-rapamycin combination increased LC3-II but not PARP cleavage. A431 cells were either untreated or treated for 24 h, as indicated. Cell lysates were analyzed by western blot. (E) The cetuximab-rapamycin combination did not increase apoptosis. The cell lysates from (D) were analyzed for the levels of histone-associated DNA fragmentation by ELISA. The statistical significance of the differences between the cetuximab-treated groups (treated alone and in combination with rapamycin) was determined with Student's t-test.
In addition, there was no evidence that the enhanced cell death induced by the combination treatment was caused by an increase in apoptosis, because the combination treatment did not enhance the cleavage of PARP (Fig. 4D) or the level of histoneassociated DNA fragmentation (Fig. 4E) more than either treatment alone. In contrast, the LC3-II level was increased by the combination treatment and the level of phosphorylated p70s6k, which reflected the activity of mTOR, was significantly lower in the cells treated with rapamycin than the cells treated with cetuximab and the combination of rapamycin and cetuximab further lowered the p70s6k levels (Fig. 4D).
Together, these data suggest that, when cell death is not maximally induced through the apoptosis owing to suboptimal or limited inhibition of the EGFR pathway or other resistance mechanisms, cell death can be achieved through either inhibition of cetuximab-induced autophagy or induction of autophagic cell death by rapamycin, which utilizes a different mechanism than cetuximab to induce autophagy.
Induction of autophagy is a novel approach for killing cancer cells that are resistant to cetuximab-induced apoptosis.
We further tested the modality of cotreatment with cetuximab and either rapamycin or chloroquine in cancer cells that show growth inhibition when treated with cetuximab but are resistant to cetuximab-induced apoptosis. We found that treatment of HN5 and FaDu head and neck cancer cells, particularly HN5 cells, with cetuximab resulted in a remarkable reduction in the relative cell numbers after a five-day culture, similar to the results seen for the DiFi and A431 cells (Fig. 5A). However, in contrast to the findings from DiFi and A431 cells, neither apoptosis nor autophagy was detected in FaDu or HN5 cells after cetuximab treatment, by either quantitative apoptosis ELISA (Fig. 5B) or western blot analysis of PARP cleavage and the appearance of LC3-II (Fig. 5C). These results indicate that cetuximab inhibited the growth of these cell lines primarily by inducing cell cycle arrest rather than cell death.
Figure 5.
Rapamycin, but not chloroquine, enhances the effect of cetuximab on inhibiting cell growth and survival without induction of apoptosis. (A–C) Cetuximab inhibits the growth of FaDu and HN5 head and neck cancer cells, without inducing apoptosis or autophagy. (A) Cetuximab inhibited growth of FaDu and HN5 cells measured by an MTT assay after a 5-day cell culture period. DiFi and A431 cells were used as references. (B) Cetuximab did not induce apoptosis in FaDu and HN5 cells measured by an apoptosis ELISA. (C) Cetuximab did not induce PARP cleavage or increase LC3-II in FaDu and HN5 cells measured by western blot. (D) Cetuximab, in combination with either rapamycin or chloroquine, increased LC3-II level but did not cleave PARP in FaDu and HN5 cells. FaDu and HN5 cells were treated for 24 h, as indicated. Cell lysates were analyzed by western blot. (E) Rapamycin, but not chloroquine, enhanced the effect of cetuximab on cell growth and survival of FaDu and HN5 cells. FaDu and HN5 cells were either untreated or treated as indicated for 48 h. The relative number of surviving cells was determined with an MTT assay. The optical density (O.D.) values of the treated groups at wavelength 570 nM were normalized to the O.D. values of the untreated cells (control) and were expressed as a percentage of the O.D. value of the control. Data are shown as means ± SD. The statistical significance of the differences in the O.D. values among the Combo I, II and III groups in FaDu and HN5 cells (column 2 versus columns 5 and 6; column 5 versus columns 11 and 12; column 6 versus columns 13 and 14) was determined with Student's t-test. *: p < 0.05; **: p < 0.01.
We found that cotreatment of FaDu and HN5 cells with cetuximab and either rapamycin or chloroquine led to increases in the LC3-II level (Fig. 5D). Neither combination led to any increase in PARP cleavage. The finding that chloroquine had no proapoptosis effect on FaDu or HN5 cells but enhanced the induction of apoptosis in DiFi (Fig. 2A and B) and A431 cells (Fig. 3F and G) in which apoptosis was either strongly (in DiFi cells) or weakly (in A431 cells) induced by cetuximab treatment further supports our conclusion that the autophagy induced by cetuximab is a response to cetuximab-induced apoptosis when no apoptosis was induced by cetuximab, no autophagy was observed. However, the finding showing that the cetuximab-rapamycin combination led to an increase in the LC3-II level suggests that rapamycin could enhance the effect of cetuximab by inducing autophagic cell death in these cancer cell lines that are resistant to cetuximab-induced cell death through apoptosis.
Figure 5E shows our findings supporting this novel hypothesis. Treatment of FaDu and HN5 cells for a short time (48 h) with cetuximab (10 nM, Fig. 5E, column 2), rapamycin (1 and 10 nM, columns 3 and 4) or chloroquine (12.5 and 25 µM, columns 7 and 8) only modestly affected cell survival; however, cotreatment of the cells with cetuximab and rapamycin (Fig. 5E and combo I) significantly reduced the number of surviving cells (columns 5 and 6 versus column 2). In contrast, cotreatment of the cells with cetuximab and chloroquine (Fig. 5E and combo II) had nearly the same effect as treating the cells with cetuximab alone (columns 9 and 10 versus column 2). Notably, the effect of the rapamycin-cetuximab combination was substantially antagonized by the addition of chloroquine (Fig. 5E and combo III), confirming that the rapamycin-enhanced cell death was mediated by autophagic cell death.
Together, these data are consistent with our finding that autophagy is not induced in the cells in which cetuximab does not induce apoptosis. However, autophagy can be induced by rapamycin; our results show that this is a novel approach to killing cancer cells that are resistant to cetuximab-induced apoptosis.
Discussion
In this paper, we expanded our study that was recently published reporting the induction of autophagy by cetuximab in cancer cells.14 Here, we used several standard experimental techniques to demonstrate that autophagy is induced in cancer cells as a response to cetuximab-induced apoptosis, including fluorescent microscopy and western blot analysis, showing the appearance of the membrane-associated autophagic form of LC3-II and electron microscopy, showing the formation of characteristic autophagosomes after cetuximab treatment. Furthermore, we confirmed that cetuximab induced autophagy with data showing that autophagy-lysosomal inhibition blocked the cetuximab-induced autophagic flux, leading to an accumulation of LC3-II, which strongly indicates that the autophagic flux was activated by cetuximab treatment.
An important question we focused on in this study was the relationship between cetuximab-induced apoptosis and autophagy. We provided three lines of evidence supporting the conclusion that autophagy was induced as a result of cetuximab-induced apoptosis. First, autophagy was detected only in cancer cells in which cetuximab induced either strong or weak apoptosis; inhibition of apoptosis by a caspase inhibitor prevented the induction of autophagy. Second, inhibition of autophagy by knockdown of Atg expression enhanced the cetuximab-induced apoptosis. Third, autophagy was not detected in naturally existing cancer cells in which cetuximab failed to induce apoptosis or in a subline of cetuximab-sensitive cells that become resistant to cetuximab-induced apoptosis. Together, our results indicate that the role of the autophagy induced by cetuximab is to protect or limit the cancer cell death caused by cetuximab-induced apoptosis.
Whether or not cells undergoing apoptosis after EGFR inhibition is an intrinsic property of cancer cells that is determined largely by the extent of the cells' dependence on EGFR-mediated cell signaling for growth and survival and also by the ability of such cells to initiate an apoptotic response through the appropriate cell machinery after EGFR inhibition. In our study, we classified the responses of cancer cells to cetuximab into three types. The first cellular response type occurs when apoptosis is strongly induced by cetuximab treatment and cell death occurs mainly by apoptosis, such as in DiFi cells. In this type of cellular response to cetuximab, which occurs in only a small number of established cancer cell lines, autophagy is induced to protect the cells from or limit cell death. Although the detailed molecular mechanism by which autophagy prevents apoptosis is not fully understood and remains an active area of research, inhibition of the autophagic-lysosomal pathway with chloroquine clearly enhanced the cetuximab-induced apoptosis. The biological and clinical implications of this type of cellular response to cetuximab is that inhibiting autophagy through pharmacological approaches may sensitize the cancer cells to suboptimal doses of cetuximab and may also restore the cetuximab sensitivity of cancer cells with acquired resistance to cetuximab.
The second type of cellular response occurs when only a minimal level of apoptosis is induced after cetuximab treatment. We showed that in this type of cellular response, such as in A431 cells, cell death can be enhanced either by enhancing apoptosis through autophagic-lysosomal inhibition or by further induction of autophagy leading to autophagic cell death with an additional autophagy activator. We found that, in A431 cells, combining cetuximab with either an autophagy inhibitor (such as chloroquine) or an autophagy activator (such as rapamycin) produced more profound cell death than any single agent alone. It is noteworthy that rapamycin inhibits mTOR by binding to the FKBP12 and rapamycin binding domains;35,36 this is mechanistically different from the cetuximab-mediated inhibition of mTOR, which is believed to occur through inhibition of the PtdIns3K/Akt pathway.14
The third type of cell response occurs probably in the majority of cancer cells that respond to cetuximab with various degrees of cytostatic effects, such as in HN5 and FaDu head and neck cancer cells where cetuximab induces cell cycle arrest but not apoptosis. We found that autophagic-lysosomal inhibition had no effect on the ability of cetuximab to induce cell death in these cells. In contrast, rapamycin could induce cell death through the autophagic cell death pathway.
An important caveat of the current study is that we used only one to two cell lines as models of each of the three types of cellular responses that can occur after cetuximab treatment. Additional testing with more cell lines representing each of the cellular response types is needed in order to establish a more general paradigm for understanding the role of autophagy in cetuximab-mediated cancer therapy against EGFR.
An interesting question that is worth pursuing is whether autophagy can be used as a marker of a cancer cell's response to cetuximab monotherapy, which may help in the design of novel therapeutic strategies. For example, if autophagy is induced in the tumor of a patient treated with cetuximab, then autophagy is likely a resistance mechanism; thus, administering an autophagic-lysosomal inhibitor, such as chloroquine, may help to improve the disease response to cetuximab treatment. In addition, in patients who are initially sensitive to cetuximab but later become insensitive owing to the acquisition of resistance mechanisms, inhibition of the autophagic-lysosomal pathway may also help to restore cell death through apoptosis. In contrast, if autophagy is never observed in patients treated with cetuximab, induction of autophagic cell death may be an alternative approach to killing the cancer cells. Several new rapamycin derivatives are currently being tested in clinical trials and may be used in combination with cetuximab in future studies to confirm this hypothesis.
In conclusion, our data indicate that autophagy is induced in cancer cells as a response to cetuximab-induced apoptosis. Novel approaches to regulating the induction of autophagy may be a novel strategy for improving the disease response to EGFR-targeted therapy with cetuximab.
Materials and Methods
Reagents.
Cetuximab was a gift from ImClone Systems, Inc. The following antibodies were used for the western blotting analysis: LC3 (Novus Biologicals, NB100-2331), PARP (Cell Signaling, 9542), caspase 3 (Cell Signaling, 9662), activated (cleaved) caspase 3 (Cell Signaling, 9661), S371-phosphorylated p70S6K (Cell Signaling, 9208), p70SK (Cell Signaling, 9202), Atg5 (Cell Signaling, 2630), beclin 1 (Santa Cruz, sc-11427). All other chemicals were purchased from Sigma-Aldrich, unless otherwise specified.
Cell lines and cell culture.
A431 human vulvar squamous carcinoma cells, DiFi and DiFi5 colorectal adenocarcinoma cells and HN5 and FaDu human head and neck cancer cells have been described previously.5,6,10,12,13,37,38 All cell lines were grown in Dulbecco's modified Eagle's medium and Ham's F12 medium (50:50 by volume) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin and maintained in a humidified 37°C incubator with 95% air and 5% CO2.
cDNA constructs, siRNA and transfection.
The GFP-tagged LC3 cDNA expression construct was a gift from Dr. Noboru Mizushima (Tokyo Medical and Dental University, Tokyo, Japan). siRNA oligonucleotide duplexes (21-mer) include beclin 1 (Ambion, siRNA ID: s16539, sense sequence: CAG AUA CUC UUU UAG ACC ATT; antisense sequence: UGG UCU AAA AGA GUA UCU GTG), Atg5 (Ambion, siRNA ID: s18160, sense sequence: GCU AUA UCA GGA UGA GAU ATT; antisense sequence: UAU CUC AUC CUG AUA UAG CGT) and a negative control siRNA (Santa Cruz, sc-37007). The cDNA construct and the siRNA oligonucleotides were transfected into the targeted cells with Lipofectamine 2000 (Invitrogen, 11668-019) according to the manufacturer's instructions.
Western blot analysis.
Cultured cells were harvested with a rubber scraper and washed twice with cold phosphate-buffered saline (PBS). Cell pellets were lysed and kept on ice for at least 10 min in a buffer containing 50 mM TrisHCl (pH 7.4), 150 mM NaCl, 0.5% Nonidet P-40, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 25 µg/ml leupeptin and 25 µg/ml aprotinin. The lysates were cleared by centrifugation and the supernatants were collected. Cell lysates were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to western blot analysis with the primary antibodies and horseradish peroxidase-labeled secondary antibodies. We visualized the signals with an enhanced chemiluminescence detection kit (Amersham Biosciences, NEL-105001EA).
Cell proliferation assay.
Cells were cultured in 24-well plates with 0.5 ml medium per well at 37°C in a CO2 incubator. After the desired treatment in cell culture, the cells were incubated for an additional 2 h with 50 µl/well of 10 mg/ml MTT and were then lysed with a lysis buffer (500 µl/well) containing 20% SDS in dimethyl formamide/H2O (1:1, v/v; pH 4.7) at 37°C for at least 6 h. We determined the relative number of surviving cells in each group by measuring the optical density (O.D.) of the cell lysates at an absorbance wavelength of 570 nm. The O.D. values of cell lysates in each treatment group were normalized to those of the untreated cells (control) and are given as a percentage of the O.D. values of the control.
Cell viability assay.
We determined cell viability using a trypan blue dye exclusion assay39 and a fluorescence-based cell Live/Dead double staining kit (Invitrogen/Molecular Probes, L-3224).40,41 Briefly, for the trypan blue dye exclusion assay, after specific treatment, cell suspensions (1–2 × 105 cells/ml) were prepared by trypsinization, centrifugation and resuspension. Equal amounts (0.1 ml) of cell suspension and 0.4% trypan blue dye were mixed thoroughly, transferred to the counting chamber of a haemocytometer and left for 5 minutes at room temperature before counting under a microscope. Both the number of stained cells and the total number of cells were then counted. The percentage of viable cells was determined by calculating the percentage of unstained cells. For the cell Live/Dead double staining assay, after specific treatment, the cells were incubated with 1 µM of calcein acetoxymethyl ester and 1 µM of ethidium homodimer-1 (Eth-D) at room temperature for 45 min. The cells were then washed with PBS followed analyzed under a fluorescent microscope for cell viability. Live cells were identified by a green fluorescence that was produced when the nonfluorescent calcein acetoxymethyl ester was converted to alcein, which fluoresces when exposed to 494 nm light; dead cells were identified by a bright red fluorescence that was produced by EthD-1 exposed to 528 nm light.
Apoptosis assay.
After treating cells with various treatments, we measured apoptosis using an ELISA kit (Roche Diagnostics, 11-920-685-001) that quantitatively measures cytoplasmic histone- associated DNA fragments (mononucleosomes and oligonucleosomes) and by western blot analysis using an antibody that recognizes both uncleaved and cleaved PARP and an antibody that recognizes activated caspase 3, as previously reported.9,14,42
Transmission electron microscopy.
After fixing the cells with a solution containing 3% glutaraldehyde plus 2% paraformaldehyde in 0.1 M PBS (pH 7.3) for 1 h, the samples were washed and treated with 0.1% buffered osmium tetroxide for 30 min, stained en bloc with 1% Millipore-filtered uranyl acetate, dehydrated in increasing concentrations of ethanol, infiltrated and embedded in LX-112 medium. The samples were then polymerized in a 70°C oven for 2 d. Ultrathin sections were cut with a Leica Ultracut microtome, stained with uranyl acetate and lead citrate in a Leica EM stainer and examined with a JEM 1010 transmission electron microscope (JEOL USA, Inc.,) at an accelerating voltage of 80 kV. Digital images were obtained using an Advanced Microscopy Techniques imaging system.
Acknowledgements
We thank Kate J. Newberry in the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editorial assistance and Kenneth Dunner in the High Resolution Electron Microscopy core facility of The University of Texas MD Anderson Cancer Center for technical assistance with use of the transmission electron microscope.
Abbreviations
- EGFR
epidermal growth factor receptor
- PtdIns3K
phosphatidylinositol 3-kinase
- mTOR
mammalian target of rapamycin
- Atg
autophagy-related gene
- GFP
green fluorescent protein
- LC3
microtubule-associated 1 light chain 3 (mammalian Atg8)
- ELISA
enzyme-linked immunosorbent assay
- siRNA
small interfering RNA
- PARP
poly (ADP-ribose) polymerase
- Z-VAD-fmk
benzyloxycarbonyl Val-Ala-Asp (O-methyl)-fluoro-methylketone
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13366
Financial Support
This work was supported by National Institutes of Health grant 5R01CA129036 (to Z.F.) and the National Institutes of Health, National Cancer Institute Cancer Center Support Grant CA016672.
References
- 1.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 2.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 3.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 4.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 5.Fan Z, Baselga J, Masui H, Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53:4637–4642. [PubMed] [Google Scholar]
- 6.Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995;95:1897–1905. doi: 10.1172/JCI117871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng D, Fan Z, Lu Y, et al. Anti-epidermal growth factor receptor monoclonal antibody 225 upregulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996;56:3666–3669. [PubMed] [Google Scholar]
- 8.Wu X, Rubin M, Fan Z, et al. Involvement of p27KIP1 in G1 arrest mediated by an anti-epidermal growth factor receptor monoclonal antibody. Oncogene. 1996;12:1397–1403. [PubMed] [Google Scholar]
- 9.Liu B, Fang M, Schmidt M, et al. Induction of apoptosis and activation of the caspase cascade by anti-EGF receptor monoclonal antibodies in DiFi human colon cancer cells do not involve the c-jun N-terminal kinase activity. Br J Cancer. 2000;82:1991–1999. doi: 10.1054/bjoc.2000.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Fang M, Lu Y, Mendelsohn J, Fan Z. Fibroblast growth factor and insulin-like growth factor differentially modulate the apoptosis and G1 arrest induced by anti-epidermal growth factor receptor monoclonal antibody. Oncogene. 2001;20:1913–1922. doi: 10.1038/sj.onc.1204277. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Fan Z. The monoclonal antibody 225 activates caspase-8 and induces apoptosis through a tumor necrosis factor receptor family-independent pathway. Oncogene. 2001;20:3726–3734. doi: 10.1038/sj.onc.1204490. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Liang K, Li X, Fan Z. Responses of cancer cells with wild-type or tyrosine kinase domain-mutated epidermal growth factor receptor (EGFR) to EGFR-targeted therapy are linked to downregulation of hypoxia-inducible factor-1alpha. Mol Cancer. 2007;6:63. doi: 10.1186/1476-4598-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Lu Y, Liang K, et al. Requirement of hypoxia-inducible factor-1alpha downregulation in mediating the antitumor activity of the anti-epidermal growth factor receptor monoclonal antibody cetuximab. Mol Cancer Ther. 2008;7:1207–1217. doi: 10.1158/1535-7163.MCT-07-2187. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Fan Z. The epidermal growth factor receptor antibody cetuximab induces autophagy in cancer cells by downregulating HIF-1α and Bcl-2 and activating the Beclin 1/hVps34 complex. Cancer Res. 2010;70:5942–5952. doi: 10.1158/0008-5472.CAN-10-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 16.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 17.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 18.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 20.Klionsky DJ, Cregg JM, Dunn WA, Jr, et al. A unified nomenclature for yeast autophagy-related genes. Dev Cell. 2003;5:539–545. doi: 10.1016/s1534-5807(03)00296-x. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, Wan F, Dutta S, et al. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boya P, Gonzalez-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Polo RA, Boya P, Pauleau AL, et al. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091–3102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- 24.Ravikumar B, Berger Z, Vacher C, O'Kane CJ, Rubinsztein DC. Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet. 2006;15:1209–1216. doi: 10.1093/hmg/ddl036. [DOI] [PubMed] [Google Scholar]
- 25.Shigemitsu K, Tsujishita Y, Hara K, et al. Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma cells. J Biol Chem. 1999;274:1058–1065. doi: 10.1074/jbc.274.2.1058. [DOI] [PubMed] [Google Scholar]
- 26.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12:1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 28.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu S, Kanaseki T, Mizushima N, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Li X, Liang K, et al. Epidermal growth factor receptor (EGFR) ubiquitination as a mechanism of acquired resistance escaping treatment by the anti-EGFR monoclonal antibody cetuximab. Cancer Res. 2007;67:8240–8247. doi: 10.1158/0008-5472.CAN-07-0589. [DOI] [PubMed] [Google Scholar]
- 32.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slee EA, Zhu H, Chow SC, et al. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996;315:21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 35.Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289 kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA. 1995;92:4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz MC, Heitman J. TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J Biol Chem. 1995;270:27531–27537. doi: 10.1074/jbc.270.46.27531. [DOI] [PubMed] [Google Scholar]
- 37.Fan Z, Masui H, Altas I, Mendelsohn J. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1993;53:4322–4328. [PubMed] [Google Scholar]
- 38.Luwor RB, Lu Y, Li X, Mendelsohn J, Fan Z. The anti-epidermal growth factor receptor monoclonal antibody cetuximab/C225 reduces hypoxia-inducible factor-1 alpha, leading to transcriptional inhibition of vascular endothelial growth factor expression. Oncogene. 2005;24:4433–4441. doi: 10.1038/sj.onc.1208625. [DOI] [PubMed] [Google Scholar]
- 39.Del BG, Lassota P, Darzynkiewicz Z. The S-phase cytotoxicity of camptothecin. Exp Cell Res. 1991;193:27–35. doi: 10.1016/0014-4827(91)90534-2. [DOI] [PubMed] [Google Scholar]
- 40.Imamura K, Takeshima T, Kashiwaya Y, Nakaso K, Nakashima K. D-beta-hydroxybutyrate protects dopaminergic SH-SY5Y cells in a rotenone model of Parkinsons disease. J Neurosci Res. 2006;84:1376–1384. doi: 10.1002/jnr.21021. [DOI] [PubMed] [Google Scholar]
- 41.Pan T, Rawal P, Wu Y, et al. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164:541–551. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Luwor R, Lu Y, Liang K, Fan Z. Enhancement of antitumor activity of the anti-EGF receptor monoclonal antibody cetuximab/C225 by perifosine in PTEN-deficient cancer cells. Oncogene. 2006;25:525–535. doi: 10.1038/sj.onc.1209075. [DOI] [PubMed] [Google Scholar]