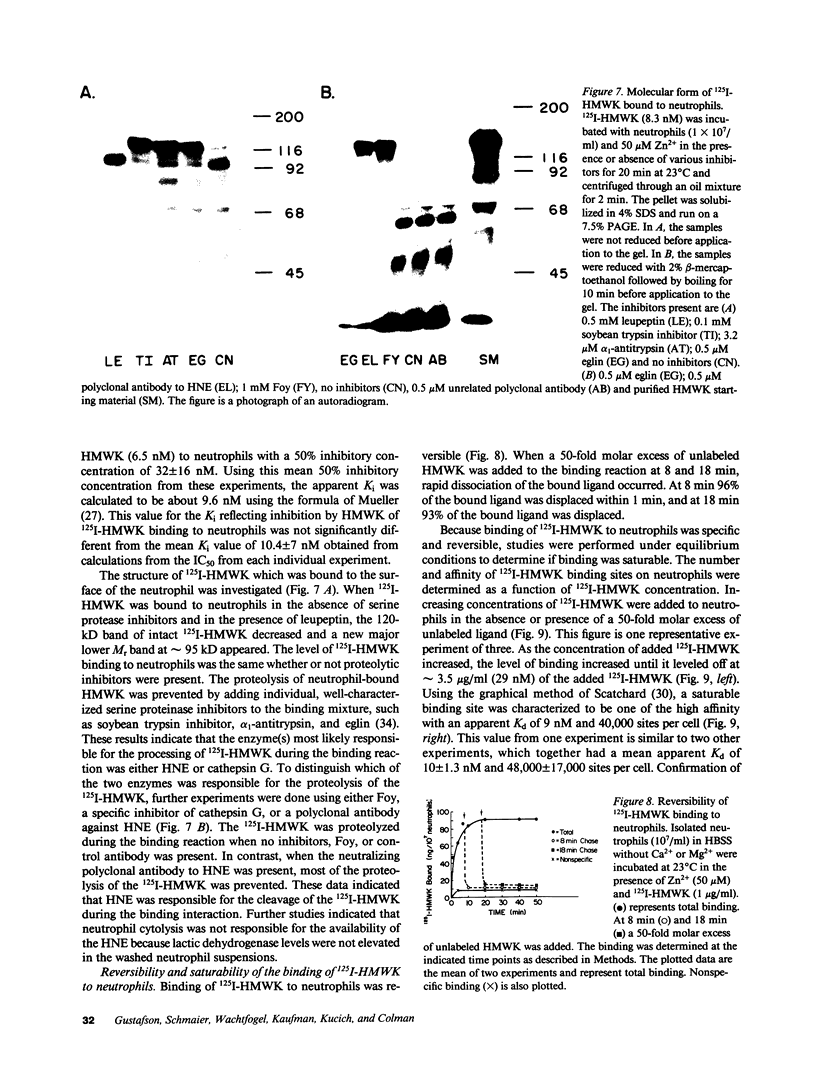
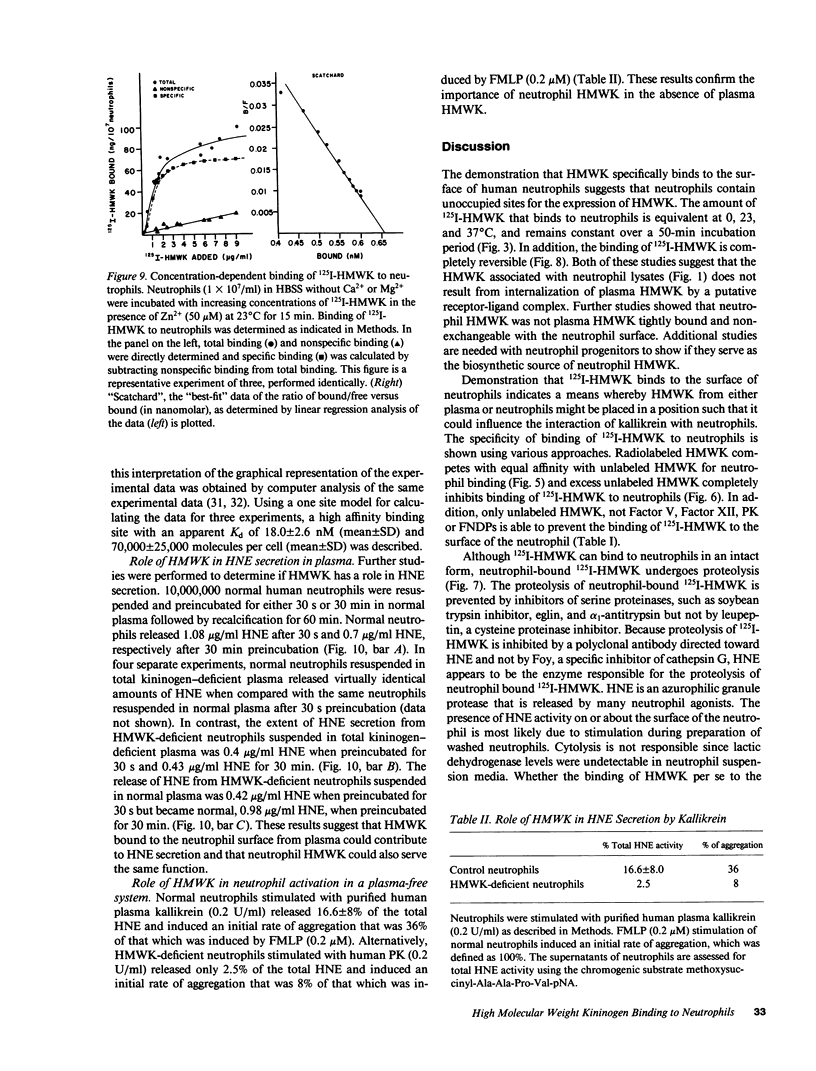
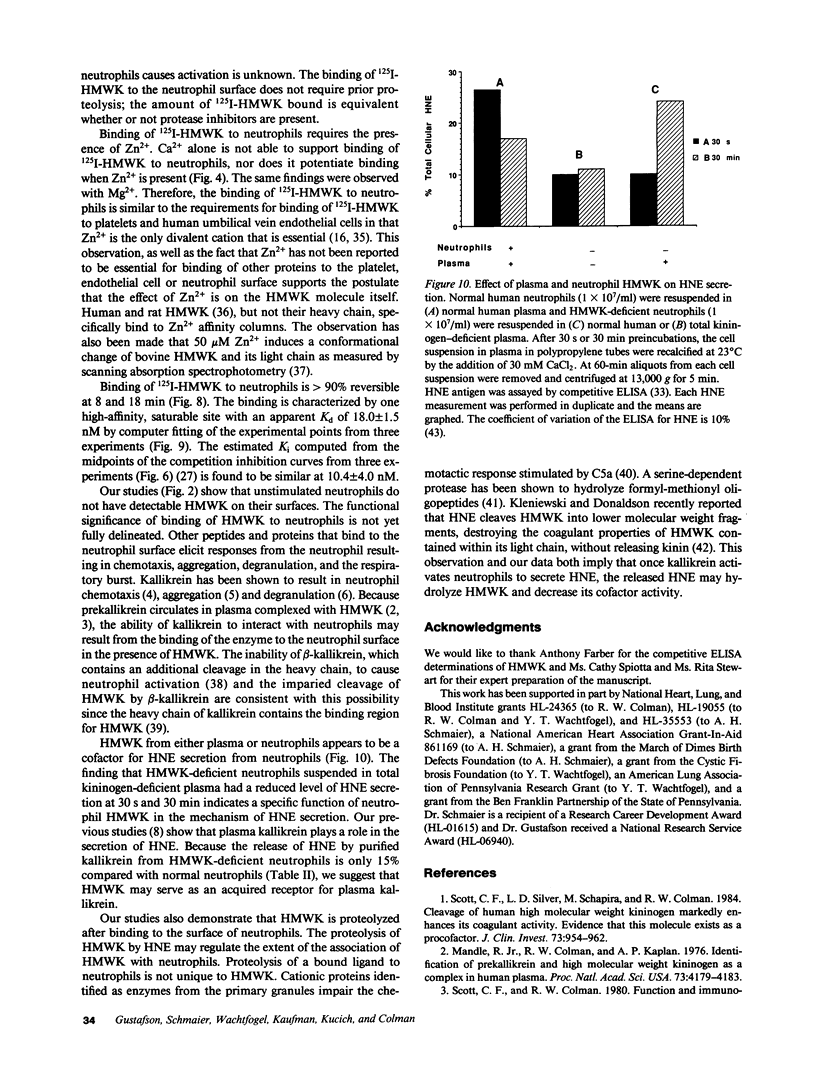
Abstract
Because plasma kallikrein activates human neutrophils, and in plasma prekallikrein (PK) circulates complexed with high molecular weight kininogen (HMWK), we determined whether HMWK could mediate kallikrein's association with neutrophils. HMWK antigen (237 +/- 61 ng HMWK/10(8) neutrophils) was present in lysates of washed human neutrophils. Little if any plasma HMWK was tightly bound and nonexchangeable with the neutrophil surface. Human neutrophils were found to possess surface membrane-binding sites for HMWK but no internalization was detected at 37 degrees C. 125I-HMWK binding to neutrophils was dependent upon Zn2+. Binding of 125I-HMWK to neutrophils was specific and 90% reversible. 125I-HMWK binding to neutrophils was saturable with an apparent Kd of 9-18 nM and 40,000-70,000 sites per cell. Upon binding to neutrophils, 125I-HMWK was proteolyzed by human neutrophil elastase (HNE) into lower relative molecular mass derivatives. Furthermore, HMWK found in neutrophils also served as a cofactor for HNE secretion because neutrophils deficient in HMWK have reduced HNE secretion when stimulated in plasma deficient in HMWK or with purified kallikrein. These studies indicate that human neutrophils contain a binding site for HMWK that could serve to localize plasma or neutrophil HMWK on their surface to possibly serve as a receptor for kallikrein and to participate in HNE secretion by this enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Schiffmann E., Corcoran B. A., Wahl S. M. Role of a peptidase in phagocyte chemotaxis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2439–2442. doi: 10.1073/pnas.73.7.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin G. Methods Enzymol. 1981;80(Pt 100):561–565. doi: 10.1016/s0076-6879(81)80044-4. [DOI] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J. Changes in surface-bound and exchangeable calcium during platelet activation. J Biol Chem. 1982 Dec 10;257(23):14000–14005. [PubMed] [Google Scholar]
- Canellas P. F., Karu A. E. Statistical package for analysis of competition ELISA results. J Immunol Methods. 1981;47(3):375–385. doi: 10.1016/0022-1759(81)90294-5. [DOI] [PubMed] [Google Scholar]
- Castillo M. J., Nakajima K., Zimmerman M., Powers J. C. Sensitive substrates for human leukocyte and porcine pancreatic elastase: a study of the merits of various chromophoric and fluorogenic leaving groups in assays for serine proteases. Anal Biochem. 1979 Oct 15;99(1):53–64. doi: 10.1016/0003-2697(79)90043-5. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R. W., Bagdasarian A., Talamo R. C., Scott C. F., Seavey M., Guimaraes J. A., Pierce J. V., Kaplan A. P. Williams trait. Human kininogen deficiency with diminished levels of plasminogen proactivator and prekallikrein associated with abnormalities of the Hageman factor-dependent pathways. J Clin Invest. 1975 Dec;56(6):1650–1662. doi: 10.1172/JCI108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R. W., Wachtfogel Y. T., Kucich U., Weinbaum G., Hahn S., Pixley R. A., Scott C. F., de Agostini A., Burger D., Schapira M. Effect of cleavage of the heavy chain of human plasma kallikrein on its functional properties. Blood. 1985 Feb;65(2):311–318. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Freise J., Schmidt F. W., Magerstedt P., Schmid K. Gabexate mesilate and camostate: new inhibitors of phospholipase A2 and their influence on the alpha-amylase activity in serum of patients with acute pancreatitis. Clin Biochem. 1985 Aug;18(4):224–229. doi: 10.1016/s0009-9120(85)80044-8. [DOI] [PubMed] [Google Scholar]
- Greengard J. S., Griffin J. H. Receptors for high molecular weight kininogen on stimulated washed human platelets. Biochemistry. 1984 Dec 18;23(26):6863–6869. doi: 10.1021/bi00321a090. [DOI] [PubMed] [Google Scholar]
- Gustafson E. J., Schutsky D., Knight L. C., Schmaier A. H. High molecular weight kininogen binds to unstimulated platelets. J Clin Invest. 1986 Jul;78(1):310–318. doi: 10.1172/JCI112567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I., Kato H., Iwanaga S., Oh-ishi S. Rat plasma high-molecular-weight kininogen. A simple method for purification and its characterization. J Biol Chem. 1985 May 25;260(10):6115–6123. [PubMed] [Google Scholar]
- James H. L., Wachtfogel Y. T., James P. L., Zimmerman M., Colman R. W., Cohen A. B. A unique elastase in human blood platelets. J Clin Invest. 1985 Dec;76(6):2330–2337. doi: 10.1172/JCI112244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. P., Kay A. B., Austen K. F. A prealbumin activator of prekallikrein. 3. Appearance of chemotactic activity for human neutrophils by the conversion of human prekallikrein to kallikrein. J Exp Med. 1972 Jan;135(1):81–97. doi: 10.1084/jem.135.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbiriou-Nabias D. M., Garcia F. O., Larrieu M. J. Radioimmunoassays of human high and low molecular weight kininogens in plasmas and platelets. Br J Haematol. 1984 Feb;56(2):273–286. doi: 10.1111/j.1365-2141.1984.tb03955.x. [DOI] [PubMed] [Google Scholar]
- Kerbiriou D. M., Griffin J. H. Human high molecular weight kininogen. Studies of structure-function relationships and of proteolysis of the molecule occurring during contact activation of plasma. J Biol Chem. 1979 Dec 10;254(23):12020–12027. [PubMed] [Google Scholar]
- Kleniewski J., Donaldson V. Granulocyte elastase cleaves human high molecular weight kininogen and destroys its clot-promoting activity. J Exp Med. 1988 Jun 1;167(6):1895–1907. doi: 10.1084/jem.167.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucich U., Abrams W. R., James H. L. Solid-phase immunoassay of dog neutrophil elastase. Anal Biochem. 1980 Dec;109(2):403–409. doi: 10.1016/0003-2697(80)90668-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandle R. J., Colman R. W., Kaplan A. P. Identification of prekallikrein and high-molecular-weight kininogen as a complex in human plasma. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4179–4183. doi: 10.1073/pnas.73.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Müller R. Determination of affinity and specificity of anti-hapten antibodies by competitive radioimmunoassay. Methods Enzymol. 1983;92:589–601. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
- Schapira M., Despland E., Scott C. F., Boxer L. A., Colman R. W. Purified human plasma kallikrein aggregates human blood neutrophils. J Clin Invest. 1982 May;69(5):1199–1202. doi: 10.1172/JCI110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaier A. H., Schutsky D., Farber A., Silver L. D., Bradford H. N., Colman R. W. Determination of the bifunctional properties of high molecular weight kininogen by studies with monoclonal antibodies directed to each of its chains. J Biol Chem. 1987 Jan 25;262(3):1405–1411. [PubMed] [Google Scholar]
- Schmaier A. H., Smith P. M., Purdon A. D., White J. G., Colman R. W. High molecular weight kininogen: localization in the unstimulated and activated platelet and activation by a platelet calpain(s). Blood. 1986 Jan;67(1):119–130. [PubMed] [Google Scholar]
- Schmaier A. H., Zuckerberg A., Silverman C., Kuchibhotla J., Tuszynski G. P., Colman R. W. High-molecular weight kininogen. A secreted platelet protein. J Clin Invest. 1983 May;71(5):1477–1489. doi: 10.1172/JCI110901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. F., Colman R. W. Function and immunochemistry of prekallikrein-high molecular weight kininogen complex in plasma. J Clin Invest. 1980 Feb;65(2):413–421. doi: 10.1172/JCI109684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. F., Silver L. D., Schapira M., Colman R. W. Cleavage of human high molecular weight kininogen markedly enhances its coagulant activity. Evidence that this molecule exists as a procofactor. J Clin Invest. 1984 Apr;73(4):954–962. doi: 10.1172/JCI111319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg M., Nicoll J. E., Kaplan A. P. The mechanism by which the light chain of cleaved HMW-kininogen augments the activation of prekallikrein, factor XI and Hageman factor. Thromb Res. 1980 Oct 15;20(2):173–189. doi: 10.1016/0049-3848(80)90383-7. [DOI] [PubMed] [Google Scholar]
- Thompson R. E., Mandle R., Jr, Kaplan A. P. Studies of binding of prekallikrein and Factor XI to high molecular weight kininogen and its light chain. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4862–4866. doi: 10.1073/pnas.76.10.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy P. B., Peterson J. M., Nesheim M. E., McDuffie F. C., Mann K. G. Interaction of coagulation factor V and factor Va with platelets. J Biol Chem. 1979 Oct 25;254(20):10354–10361. [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Wachtfogel Y. T., Kucich U., James H. L., Scott C. F., Schapira M., Zimmerman M., Cohen A. B., Colman R. W. Human plasma kallikrein releases neutrophil elastase during blood coagulation. J Clin Invest. 1983 Nov;72(5):1672–1677. doi: 10.1172/JCI111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtfogel Y. T., Pixley R. A., Kucich U., Abrams W., Weinbaum G., Schapira M., Colman R. W. Purified plasma factor XIIa aggregates human neutrophils and causes degranulation. Blood. 1986 Jun;67(6):1731–1737. [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. A functional differentiation of human neutrophil granules: generation of C5a by a specific (secondary) granule product and inactivation of C5a by azurophil (primary) granule products. J Immunol. 1977 Sep;119(3):1068–1076. [PubMed] [Google Scholar]
- van der Graaf F., Tans G., Bouma B. N., Griffin J. H. Isolation and functional properties of the heavy and light chains of human plasma kallikrein. J Biol Chem. 1982 Dec 10;257(23):14300–14305. [PubMed] [Google Scholar]