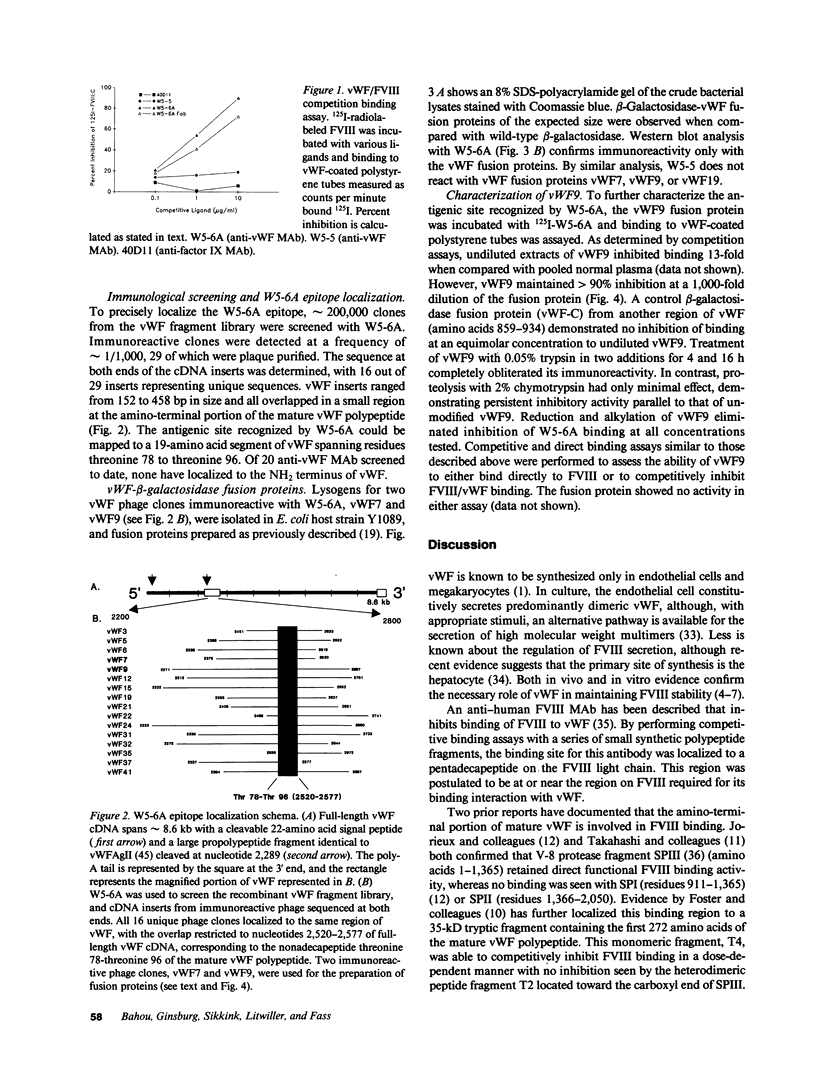
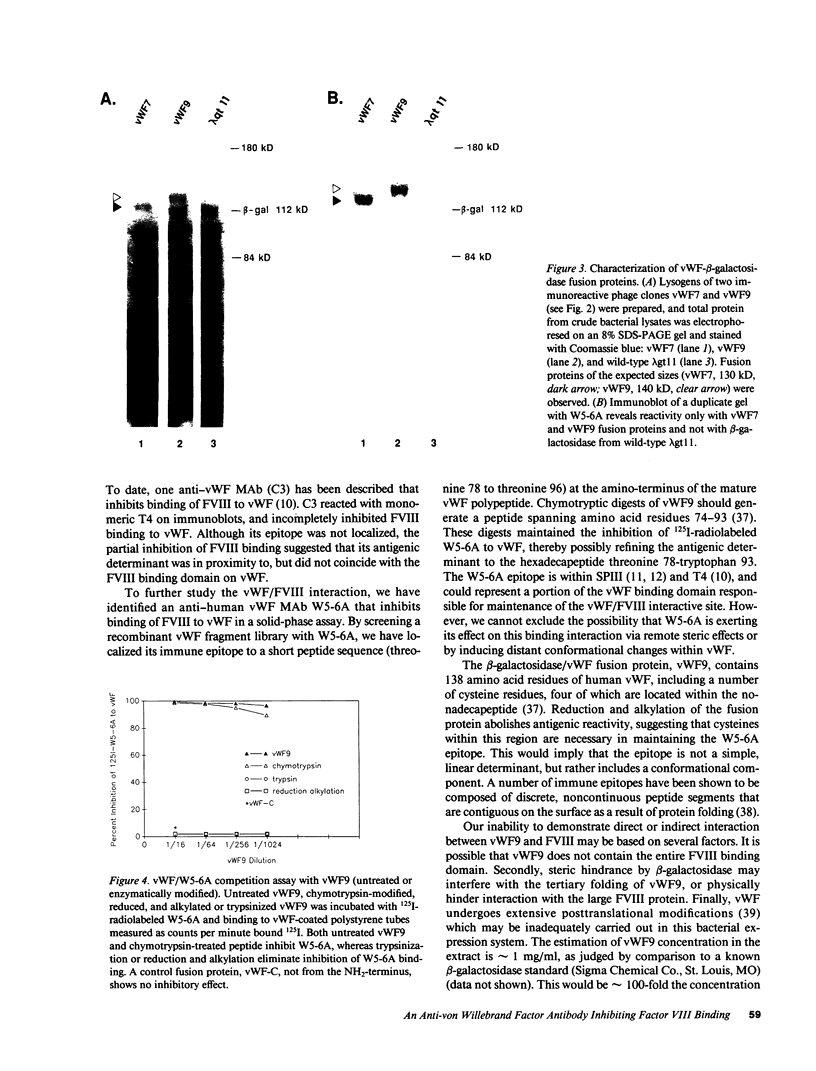
Abstract
vWF is a multimeric glycoprotein that serves as the major carrier in plasma of Factor VIII (FVIII). We have used an anti-human vWF MAb W5-6A to investigate the FVIII binding site on vWF. W5-6A inhibited FVIII binding to vWF-coated polystyrene tubes in a concentration-dependent manner with 90% inhibition of FVIII binding at a concentration of 10 micrograms/ml. The W5-6A epitope was identified by screening a vWF fragment library using the bacteriophage expression vector lambda gt11. DNA sequence analysis of 29 immunoreactive phage clones localized the W5-6A epitope to a nonadecapeptide spanning amino acid residues threonine 78 to threonine 96 at the amino-terminus of the mature vWF polypeptide. Purified beta-galactosidase/vWF fusion protein from one of these clones, vWF9, was incubated with radiolabeled W5-6A and caused near complete inhibition of W5-6A binding to vWF. Inhibitory activity was lost after vWF9 trypsinization or reduction and alkylation. These data indicate that (a) the antigenic determinant recognized by W5-6A localizes to a nonadecapeptide at the NH2 terminus of the mature vWF polypeptide, (b) disulfide bonds within vWF9 may be necessary to maintain the structure required for immunoreactivity with W5-6A, and (c) W5-6A recognizes an immunogenic region on vWF that may be at (or near) the major FVIII binding domain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonthron D., Orr E. C., Mitsock L. M., Ginsburg D., Handin R. I., Orkin S. H. Nucleotide sequence of pre-pro-von Willebrand factor cDNA. Nucleic Acids Res. 1986 Sep 11;14(17):7125–7127. doi: 10.1093/nar/14.17.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhous K. M., Sandberg H., Garris J. B., Mattsson C., Palm M., Griggs T., Read M. S. Purified human factor VIII procoagulant protein: comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8752–8756. doi: 10.1073/pnas.82.24.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R., Sheriff S., Padlan E. A. Antibody-antigen complexes. J Biol Chem. 1988 Aug 5;263(22):10541–10544. [PubMed] [Google Scholar]
- Dewanjee M. K., Solis E., Mackey S. T., Gonzales G., Chesebro J. H., Kaye M. P. Quantitation of platelet and fibrinogen deposition on PTFE and vein grafts in dogs and the effect of vitamin E on graft thrombosis in the acute phase. ASAIO Trans. 1986 Jul-Sep;32(1):187–192. [PubMed] [Google Scholar]
- Engelke D. R., Hoener P. A., Collins F. S. Direct sequencing of enzymatically amplified human genomic DNA. Proc Natl Acad Sci U S A. 1988 Jan;85(2):544–548. doi: 10.1073/pnas.85.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fass D. N., Bowie E. J., Owen C. A., Jr, Mann K. G. Stability of porcine factor VIII. Thromb Res. 1975 Feb;6(2):109–118. doi: 10.1016/0049-3848(75)90016-x. [DOI] [PubMed] [Google Scholar]
- Fay P. J., Kawai Y., Wagner D. D., Ginsburg D., Bonthron D., Ohlsson-Wilhelm B. M., Chavin S. I., Abraham G. N., Handin R. I., Orkin S. H. Propolypeptide of von Willebrand factor circulates in blood and is identical to von Willebrand antigen II. Science. 1986 May 23;232(4753):995–998. doi: 10.1126/science.3486471. [DOI] [PubMed] [Google Scholar]
- Foster P. A., Fulcher C. A., Houghten R. A., Zimmerman T. S. An immunogenic region within residues Val1670-Glu1684 of the factor VIII light chain induces antibodies which inhibit binding of factor VIII to von Willebrand factor. J Biol Chem. 1988 Apr 15;263(11):5230–5234. [PubMed] [Google Scholar]
- Foster P. A., Fulcher C. A., Marti T., Titani K., Zimmerman T. S. A major factor VIII binding domain resides within the amino-terminal 272 amino acid residues of von Willebrand factor. J Biol Chem. 1987 Jun 25;262(18):8443–8446. [PubMed] [Google Scholar]
- Gawryl M. S., Hoyer L. W. Inactivation of factor VIII coagulant activity by two different types of human antibodies. Blood. 1982 Nov;60(5):1103–1109. [PubMed] [Google Scholar]
- Ginsburg D., Handin R. I., Bonthron D. T., Donlon T. A., Bruns G. A., Latt S. A., Orkin S. H. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985 Jun 21;228(4706):1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Zeheb R., Yang A. Y., Rafferty U. M., Andreasen P. A., Nielsen L., Dano K., Lebo R. V., Gelehrter T. D. cDNA cloning of human plasminogen activator-inhibitor from endothelial cells. J Clin Invest. 1986 Dec;78(6):1673–1680. doi: 10.1172/JCI112761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girma J. P., Chopek M. W., Titani K., Davie E. W. Limited proteolysis of human von Willebrand factor by Staphylococcus aureus V-8 protease: isolation and partial characterization of a platelet-binding domain. Biochemistry. 1986 Jun 3;25(11):3156–3163. doi: 10.1021/bi00359a013. [DOI] [PubMed] [Google Scholar]
- Girma J. P., Meyer D., Verweij C. L., Pannekoek H., Sixma J. J. Structure-function relationship of human von Willebrand factor. Blood. 1987 Sep;70(3):605–611. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hawkes R. Identification of concanavalin A-binding proteins after sodium dodecyl sulfate--gel electrophoresis and protein blotting. Anal Biochem. 1982 Jun;123(1):143–146. doi: 10.1016/0003-2697(82)90634-0. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., Gawryl M. S., de la Fuente B. Immunochemical characterization of factor VIII inhibitors. Prog Clin Biol Res. 1984;150:73–85. [PubMed] [Google Scholar]
- Jorieux S., Magallon T., Mazurier C. Evidence that NH2-terminal but not COOH-terminal moiety of plasma von Willebrand factor binds to factor VIII. Thromb Res. 1987 Oct 15;48(2):205–210. doi: 10.1016/0049-3848(87)90417-8. [DOI] [PubMed] [Google Scholar]
- Katzmann J. A., Mujwid D. K., Miller R. S., Fass D. N. Monoclonal antibodies to von Willebrand's factor: reactivity with porcine and human antigens. Blood. 1981 Sep;58(3):530–536. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lollar P., Parker C. G. Stoichiometry of the porcine factor VIII-von Willebrand factor association. J Biol Chem. 1987 Dec 25;262(36):17572–17576. [PubMed] [Google Scholar]
- Lynch D. C., Zimmerman T. S., Collins C. J., Brown M., Morin M. J., Ling E. H., Livingston D. M. Molecular cloning of cDNA for human von Willebrand factor: authentication by a new method. Cell. 1985 May;41(1):49–56. doi: 10.1016/0092-8674(85)90060-1. [DOI] [PubMed] [Google Scholar]
- Mannucci P. M., Mari D. Antibodies to factor VIII-von Willebrand factor in congenital and acquired von Willebrand's disease. Prog Clin Biol Res. 1984;150:109–122. [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R. R., Hathaway W. E., Johnson J., Jacobson L., Muntean W. A variant of von Willebrand's disease with abnormal expression of factor VIII procoagulant activity. Blood. 1982 Jul;60(1):201–207. [PubMed] [Google Scholar]
- Ngo K. Y., Glotz V. T., Koziol J. A., Lynch D. C., Gitschier J., Ranieri P., Ciavarella N., Ruggeri Z. M., Zimmerman T. S. Homozygous and heterozygous deletions of the von Willebrand factor gene in patients and carriers of severe von Willebrand disease. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2753–2757. doi: 10.1073/pnas.85.8.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. D., Brockway W. J., Fass D. N., Magnuson M. A., Bowie E. J. Evaluation of ristocetin-Willebrand factor assay and ristocetin-induced platelet aggregation. Am J Clin Pathol. 1975 Feb;63(2):210–218. doi: 10.1093/ajcp/63.2.210. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Wagner R. H. Antihemophilic factor: separation of an active fragment following dissociation by salts or detergents. Thromb Diath Haemorrh. 1972 Jul 31;27(3):502–515. [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable microcomputer environment for DNA and protein sequence manipulation and analysis. Nucleic Acids Res. 1986 Jan 10;14(1):479–488. doi: 10.1093/nar/14.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. von Willebrand factor and von Willebrand disease. Blood. 1987 Oct;70(4):895–904. [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sporn L. A., Marder V. J., Wagner D. D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986 Jul 18;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Kalafatis M., Girma J. P., Sewerin K., Andersson L. O., Meyer D. Localization of a factor VIII binding domain on a 34 kilodalton fragment of the N-terminal portion of von Willebrand factor. Blood. 1987 Nov;70(5):1679–1682. [PubMed] [Google Scholar]
- Thorell L., Blombäck B. Purification of the factor VIII complex. Thromb Res. 1984 Aug 15;35(4):431–450. doi: 10.1016/0049-3848(84)90235-4. [DOI] [PubMed] [Google Scholar]
- Titani K., Kumar S., Takio K., Ericsson L. H., Wade R. D., Ashida K., Walsh K. A., Chopek M. W., Sadler J. E., Fujikawa K. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986 Jun 3;25(11):3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., de Vries C. J., Distel B., van Zonneveld A. J., van Kessel A. G., van Mourik J. A., Pannekoek H. Construction of cDNA coding for human von Willebrand factor using antibody probes for colony-screening and mapping of the chromosomal gene. Nucleic Acids Res. 1985 Jul 11;13(13):4699–4717. doi: 10.1093/nar/13.13.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984 Dec;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wion K. L., Kelly D., Summerfield J. A., Tuddenham E. G., Lawn R. M. Distribution of factor VIII mRNA and antigen in human liver and other tissues. Nature. 1985 Oct 24;317(6039):726–729. doi: 10.1038/317726a0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. S., Fulcher C. A. Factor VIII procoagulant protein. Clin Haematol. 1985 Jun;14(2):343–358. [PubMed] [Google Scholar]