Abstract
A growing body of work indicates that neural induction may be initiated prior to the establishment of the gastrula mesodermal organizer. Here we examine neural induction in Xenopus embryos in which mesoderm formation has been blocked by Cerberus-short, a reagent that specifically inhibits Nodal-related (Xnr) signals. We find that extensive neural structures with cyclopic eyes and brain tissue are formed despite the absence of mesoderm. This neural induction correlates with the expression of chordin and other BMP inhibitors - such as noggin, follistatin and Xnr3 - at the blastula stage, and requires β-Catenin signaling. Activation of the β-Catenin pathway by mRNA microinjections or by treatment with LiCl leads to differentiation of neurons, as well as neural crest, in ectodermal explants. Xnr signals are required for the maintenance, but not for the initiation, of BMP antagonist expression. Recent work has demonstrated a role for β-Catenin signaling in neural induction mediated by the transcriptional down-regulation of BMP-4 expression. The present results suggest an additional function for β-Catenin, the early activation of expression of secreted BMP antagonists, such as Chordin, in a pre-organizer region in the dorsal side of the Xenopus blastula.
Keywords: Xenopus laevis, neural induction, Spemann's organizer, β-Catenin, Chordin, Noggin, Follistatin, Xnr3, Lefty, Nodal-related
INTRODUCTION
Neural induction in early vertebrate development is a topic of considerable interest (Harland, 2000). Spemann and Mangold (Spemann and Mangold, 1924) provided the initial insight showing that transplantation of dorsal lip mesoderm of the gastrulating amphibian embryo would induce an ectopic secondary axis that included a central nervous system (CNS). This led to the view that neural inducers emanate from dorsal mesoderm, a region also called Spemann's organizer. The molecular dissection of Spemann's organizer has led to the identification of multiple novel secreted proteins. Many of these were found to be antagonists that bind to growth factors in the extracellular space (reviewed by Harland and Gerhart, 1997; De Robertis et al., 2000). Molecules such as Chordin, Noggin and Cerberus bind Bone Morphogenetic Proteins (BMPs) and prevent them from binding to their cognate receptors (Piccolo et al., 1996; Zimmerman et al., 1996; Piccolo et al., 1999). In the case of Follistatin/BMP complexes, receptor binding takes place but activation is inhibited (Iemura et al., 1998). Xnr3 is a member of the TGF-β superfamily that also functions as an antagonist of BMP signaling, perhaps acting as a competitive inhibitor of BMP receptors (Smith et al., 1995; Hansen et al., 1997; Harland, 2000). The expression of this cocktail of BMP antagonists in the organizer has led to the view that neural induction by gastrula organizer grafts is in part mediated by inhibition of BMP signaling in the extracellular space (Harland and Gerhart, 1997; Sasai and De Robertis, 1997).
Evidence is also accumulating that neural tissue might be specified without an absolute requirement for the gastrula organizer. For example, in the mouse, HNF3-β mutants lack a morphological node and node derivatives, but still develop a neural plate (Klingensmith et al., 1999). In zebrafish, microsurgical deletion of the embryonic shield (gastrula organizer) has little effect on the development of the neural plate (Shih and Fraser, 1996; Saude et al., 2000). In Xenopus, there is evidence that a predisposition for neural induction already exists on the dorsal side of the ectoderm prior to its interaction with the gastrula organizer (Sharpe et al., 1987; London et al., 1988). Therefore, the relationship between the undisputed neural inducing activity emanating from the organizer at gastrula stage and the function of earlier signals in the formation of the neural plate is an area of intense interest (Harland, 2000).
An important advance has been the realization that the regulation of BMP expression at the transcriptional level plays an instrumental role in neural patterning. Activation of the β-catenin signaling pathway inhibits BMP-4 transcription in Xenopus ectodermal explants at gastrula and results in the induction of neural markers (Baker et al., 1999). Microinjection of an activated form of β-catenin into the ectoderm of developing embryos greatly expands the neural plate, whereas a dominant-repressive form of the β-Catenin co-factor XTcf-3 (ΔN-XTcf-3) reduces the neural plate (Baker et al., 1999). In Drosophila, dTCF is known to regulate transcription of the BMP homologue dpp in the mesoderm (Yang et al., 2000). In mouse, mutation of β-catenin results in embryos with severe anteroposterior defects that do not express the forebrain markers Hesx1 and Otx2 in the neuroectoderm (Huelsken et al., 2000). In zebrafish, a mutation in Tcf3 (named headless) leads to the loss of forebrain and midbrain structures (Kim et al., 2000). Zebrafish genetics also supports a function for transcriptional regulation of BMP expression in neurogenesis. The early homeobox gene bozozok, which shares sequence similarities to the organizer gene goosecoid, is activated by the β-Catenin pathway (Fekany et al., 1999). In bozozok mutants BMP-2b transcription is not repressed on the dorsal side of the embryo, leading to a moderate reduction of the CNS (Koos and Ho, 1999; Fekany-Lee et al., 2000). In chick, transcriptional downregulation of BMP expression appears to be mediated by a different signaling pathway. FGF-3 and 8 have been implicated in neural induction and are thought to act – at least partially - by inhibiting transcription of BMP-4 and 7 (Wilson et al., 2000; Streit et al., 2000; Harland, 2000).
Treatment of Xenopus embryos with LiCl leads to a dorsalized phenotype with greatly enhanced forebrain structures (Kao and Elinson, 1988). LiCl inhibits the activity of Glycogen Synthase Kinase-3 beta (GSK-3β), preventing the degradation of β-Catenin protein (Klein and Melton, 1996; Schneider et al., 1996). The opposite effect, ventralization, is achieved by irradiation of Xenopus eggs with ultraviolet (UV) light. These ventralized embryos develop all three germ layers, but do not form a CNS, dorsal mesoderm, or Spemann's organizer (Harland and Gerhart, 1997; De Robertis et al., 2000). UV treatment causes depolymerization of microtubule tracks required for the transport of dorsal determinant vesicles to the dorsal side of the embryo (Rowning et al., 1997), and prevents accumulation of β-Catenin protein in cell nuclei of the future dorsal side of the embryo (Scharf and Gerhart, 1980; Schneider et al., 1996; Larabell et al., 1997). An intriguing aspect of the UV experiment is that dorsal development, including a complete CNS, can be restored by microinjection of a surprising variety of gene products, including members of the β-Catenin signaling pathway, Nodal-related proteins and secreted BMP antagonists. This has led to the proposal that these diverse molecular players may be involved in a common dorsal specification pathway (De Robertis et al., 2000).
In zebrafish, genetic studies have shown that Nodal-related factors are required for gastrula organizer formation. The loss of cyclops and squint, or of a co-factor required for Nodal signaling, one-eyed pinhead (oep), results in the lack of expression of the organizer gene goosecoid and in the absence of axial mesendodermal tissues. Surprisingly, embryos lacking Nodal-related signals still express chordino at early stages and later on develop an extensive CNS with a marked expansion of anterior brain located between the cyclopic eye and the auditory vesicle (Feldman et al., 1998; Gritsman et al., 1999; Feldman et al., 2000; Shimizu et al., 2000; Wilson and Rubenstein, 2000). Similarly, mouse cripto mutants, in which Nodal signaling is defective, develop extensive anterior neural tissue, resembling a head without a trunk (Ding et al., 1998).
In Xenopus, five mesoderm-inducing Nodal-related molecules (Xnrs) have been described (Jones et al., 1995; Joseph and Melton, 1997; Takahashi et al., 2000). Their activity can be blocked by overexpression of the Cer-S protein, the C-terminal portion of Cerberus (Bouwmeester et al., 1996), which specifically binds to and inhibits Xnrs (Piccolo et al., 1999; Takahashi et al., 2000). In this paper the term Xnrs refers specifically to mesoderm-inducing Xnrs (1, 2, 4, 5, 6) and not to Xnr3, which has neural-inducing activity, and is not blocked by Cer-S (Smith et al., 1995; Agius et al., 2000; Takahashi et al., 2000). Microinjection of synthetic cer-S mRNA blocks the induction of both dorsal and ventral mesoderm in animal-vegetal Nieuwkoop-type tissue recombinants, suggesting that mesoderm formation is mediated by a gradient of multiple Nodal-related signals released by endoderm at the blastula stage (Agius et al., 2000).
The starting point for the present investigation was the observation that embryos injected with high doses of cer-S mRNA lacked all mesoderm, including Spemann's organizer markers at the gastrula stage, but still developed a CNS containing a cyclopic eye and extensive brain structures. This neural development was sensitive to UV treatment and requires the β-Catenin pathway. A detailed re-investigation of the expression of chordin revealed substantial expression on the dorsal side, including the animal cap, already at the blastula stage. This pre-organizer expression includes other secreted molecules – such as noggin, follistatin and Xnr3 - that are later on also expressed in Spemann's organizer. Cer-S did not block the early expression of these BMP antagonists, but inhibited the maintenance of their expression in mesoderm of the gastrula organizer. LiCl treatment or microinjection of β-catenin was sufficient to ectopically activate this early gene expression program in the animal cap. This pre-organizer center may participate in neural induction by the early β-Catenin pathway.
MATERIALS AND METHODS
Embryo manipulations
Xenopus embryos obtained by in vitro fertilization were maintained in 0.1 × modified Barth medium (Sive et al., 2000) and staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1994). RNA injections were performed into each blastomere at the 4- or 8-cell stage. LiCl treatment and UV irradiation were performed as described (Fainsod et al., 1994). In an effort to limit the perdurance of the LiCl signal on neural tissue we treated embryos between 4-cell and 128-cell stages for 30 min followed by incubation in 1 × Barth solution for 2 hours to compete the effect of LiCl with NaCl. However, no treatments were found that reproducibly enhanced the neural inducing activity of LiCl (measured by both RTPCR for late neural markers as well as by in situ hybridization for β-neurotubulin). Ectodermal explants were excised at stage 9 and cultured in 0.5 × MMR saline until sibling embryos reached the required stage. In situ hybridization was performed on whole embryos or on paraplast sections as described (Lemaire and Gurdon, 1994; Belo et al., 1997; Sive et al., 2000; http://www.hhmi.ucla.edu/derobertis/)
RT-PCR analysis and RNA synthesis
Embryos and explants were processed for RT-PCR analysis as described (Sasai et al., 1995). The following primer sets were used: α-actin, α-globin, Brachyury (Xbra), dkk-1, EF1α, follistatin, frzb-1, gsc, NCAM, noggin, Ornithine decarboxylase (ODC) and Xnr3 (Agius et al., 2000), cerberus (Bouwmeester et al., 1996), chordin (Sasai et al., 1994), En-2, Krox-20 and Otx-2 (Sasai et al., 1995). To generate synthetic mRNAs, the plasmids pCS2-cer-S, pCS2-XtAlk4, pCS2-antivin/lefty, pCS2-dnGSK3, pCS2-b-catenin and pCS2-caBR were linearized with Not I, and pSP64-XtBR was linearized with EcoR I. In this study cer-S was always injected at high doses (150 pg). At lower doses residual Xnr activity causes cyclopia and anterior defects instead of the head-like structures analyzed here (Piccolo et al., 1999). All mRNAs were transcribed with SP6 RNA polymerase as described (Piccolo et al., 1999). The pCS2-antivin/lefty construct was cloned during a screen for proteins secreted at the gastrula stage (Pera and De Robertis, 2000), using a cDNA library in the pCS2+ vector prepared from stage 11 Xenopus embryos treated with LiCl.
RESULTS
Embryos lacking mesoderm develop a CNS
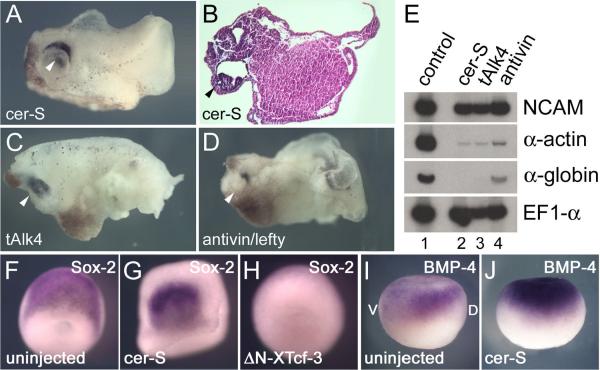
Embryos injected vegetally into each blastomere at the 4-cell stage with 150 pg of cer-S mRNA develop into head-like structures with a cyclopic eye and brain tissue that lack mesoderm, except for a small remaining tail-like structure (Figs. 1A and 1B). The presence of neural tissue was confirmed by RT-PCR analyses at stage 26, which showed expression of the pan-neural marker NCAM, and the absence of α-actin and α-globin, which mark dorsal and ventral mesoderm (Fig. 1E, lanes 1 and 2). The same phenotype was observed when two other mesoderm inhibitors were tested. A truncated version of a Xenopus Activin/Nodal receptor (tAlk4; Agius et al., 2000) and Antivin/Lefty, an extracellular Activin/Nodal receptor antagonist (Cheng et al., 2000), displayed a similar phenotype (CNS with cyclopic eye, decreased α-actin and α-globin expression) when injected radially (Figs. 1C–1E).
FIG. 1.
Inhibition of Nodal signaling does not prevent CNS formation. (A-D) External and histological views of embryos injected radially into the vegetal pole of each blastomere at the 4-cell stage with either 150 pg cer-S (n=167), 1.5 ng tAlk4 (n=21) or 1.5 ng antivin mRNA (n=89) at stage 32. The cyclopic eyes are indicated by arrowheads. (E) RT-PCR analysis of the same embryos showing expression of NCAM, but a decrease of the mesodermal markers α-actin and α-globin caused by the three anti-mesodermal agents. EF1-α serves as a loading control. (F-J) Whole-mount in situ hybridization analyses of control, cer-S and ΔN-XTcf-3 injected embryos with the neural plate marker Sox-2 at stage 12.5 (F-H; dorsal view) and BMP-4 at stage 10.5 (I, J; lateral view). D, dorsal; V, ventral.
In situ hybridization analyses showed that the pan-neural marker Sox-2 was expressed on one side of the marginal zone in cer-S injected embryos at the neural plate stage (Figs. 1F and 1G). Due to the lack of mesoderm, these embryos did not undergo epiboly but still formed a neural plate. To test whether BMP-4 was regulated at the transcriptional level in cer-S injected embryos, in situ hybridizations were performed at gastrula (stage 10.5). At this stage BMP-4 transcripts are expressed in the animal cap and ventral mesoderm (Fainsod et al., 1994). In whole embryos in which mesoderm formation was blocked by cer-S, BMP-4 expression was cleared from the entire marginal zone but was still present, at somewhat elevated levels, in animal cap ectoderm (Fig. 1J). We conclude that mesoderm formation and Xnr signaling are not required for neural induction in Xenopus.
Neural induction in cer-S injected embryos requires β-Catenin signaling
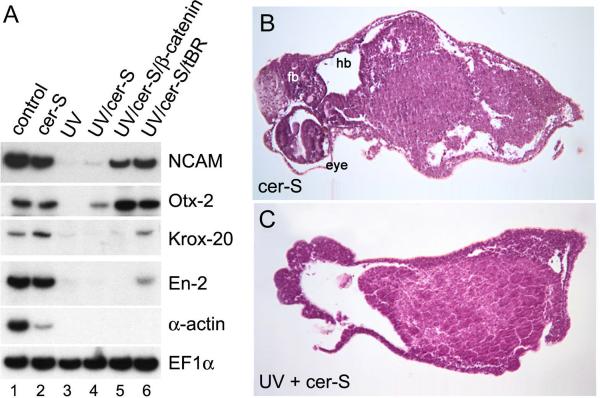
The asymmetric expression of Sox-2 at the neurula stage in the marginal zone of cer-S injected embryos (Fig. 1G) provided the first clue that dorsal β-Catenin signaling might be involved in neural induction in the absence of mesoderm. To test this, we examined whether neural induction would still take place in embryos in which cortical rotation of dorsal determinants was prevented by UV treatment. As shown in Fig. 2A, the neural markers NCAM, Otx-2, Krox-20 and En-2 were expressed in cer-S injected embryos at levels comparable to those of uninjected embryos (Fig. 2A, lanes 1 and 2), but were absent after UV irradiation (Fig. 2A, lanes 1–4). Suppression of neural plate formation was also observed when ΔNXTcf-3 mRNA (Molenaar et al., 1996) was used to block transcriptional activation by β-Catenin (Fig. 1H). Importantly, NCAM and Otx-2 expression could be restored in UV-treated embryos injected with cer-S and β-catenin mRNAs (Fig. 2A, lane 5). This indicates that β-Catenin is sufficient to restore, at least partially, neural differentiation. This effect of β-Catenin does not require the formation of dorsal mesoderm, since it takes place in cer-S embryos. In agreement with the prevailing view that inhibition of BMP signaling is required for neural induction, a dominant-negative version of the BMP receptor (tBR) also restored neural tissue when injected with cer-S together into UV-treated embryos (Fig. 2A, lane 6). In histological sections, the formation of cyclopic eyes and forebrain/hindbrain tissues in cer-S injected embryos was prevented by UV treatment, confirming the molecular marker analyses (Figs. 2B and 2C). We conclude that neural induction in the absence of mesoderm is dependent on a functional β-Catenin dorsal signaling pathway.
FIG. 2.
Neural induction is dependent on cortical rotation. (A) RT-PCR analysis of embryos that have been irradiated with UV light (lane 3–6) and injected radially in the marginal zone at the 4-cell stage with cer-S mRNA (lane 2, 4), cer-S and β-catenin (150 pg each) mRNA (lane 5), or cer-S and tBR (1.5 ng) mRNA (lane 6). Note that expression of the neural markers NCAM, Otx-2, Krox-20 and En-2 is inhibited by UV irradiation, but is restored after injection of either by β-catenin or a dominant-negative BMP receptor (3 embryos per sample, 3 independent analyses). (B, C) Histological analysis of embryos injected with cer-S mRNA at stage 42. UV treatment results in the loss of neural structures in cer-S injected embryos (n=81). fb, forebrain; hb, hindbrain.
A β-Catenin-dependent blastula pre-organizer
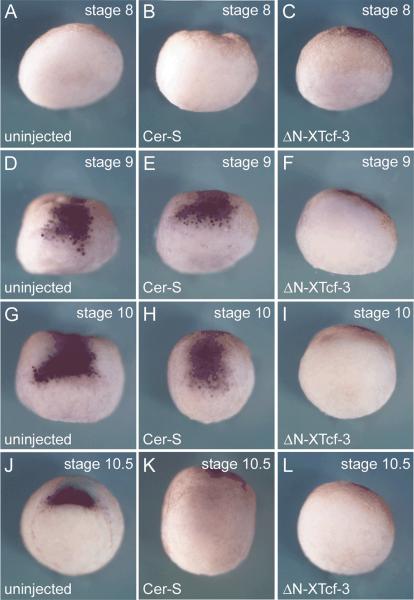
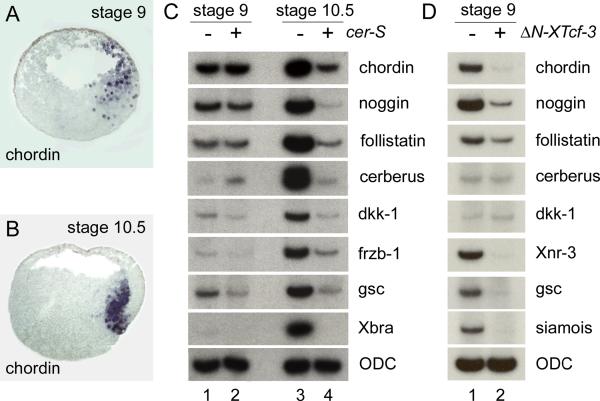
Neural induction in cer-S injected embryos was puzzling, since we had observed that this treatment eliminated the expression of most organizer genes when embryos were examined at the gastrula stage 10.5 (Agius et al., 2000). A helpful clue came from earlier work on blastula stage embryos. Smith and Harland (1992) showed that expression of noggin at the dorsal side of the stage 9 blastula. Expression of the neural inducer Xnr3 had also been reported in the dorsal surface of stage 9 blastula embryos (Smith et al., 1995). Similarly, expression of chordin had been noted before the start of gastrulation in Xenopus (Mizuseki et al., 1998). In zebrafish, early chordino expression was observed even in Nodal signaling deficient embryos (Grinblat et al., 1998; Gritsman et al., 1999; Shimizu et al., 2000). We therefore re-investigated the onset of chordin expression. Embryos were collected at 2 hour intervals at stages 8, 9, 10 and 10.5, using pigmented embryos in order to time accurately the onset of dorsal lip formation (stage 10). As shown in Fig. 3D, a patch of zygotic chordin expression was detected on the animal cap and marginal zone 2 hours before appearance of the blastopore lip. This early expression had been missed in our earlier studies (Sasai et al., 1994). Expression intensifies with the onset of gastrulation and by stage 10.5 involutes with the mesoderm (Fig. 3J). In situ hybridization on paraffin sections showed that at blastula (stage 9) chordin transcripts are expressed in the entire dorsal side, including deep cells of the animal cap, marginal zone and vegetal regions (Fig. 4A). This pattern differs from that of Xnr3 at blastula, which is localized in the surface layer (Smith et al., 1995). Upon epiboly, chordin expression moved vegetally and by stage 10.5 was found in the involuting dorsal blastopore lip (Fig. 4B).
FIG. 3.
Pre-organizer expression of chordin requires the early β-Catenin pathway for its initiation, and Xnrs for its maintenance in the gastrula organizer. Whole-mount in situ hybridizations with chordin probe of embryos injected with cer-S (600pg), ΔN-XTcf-3 (600pg) or uninjected controls at stage 8 (A–C), 9 (D–F), 10 (G–I) and 10.5 (J–L). A patch of chordin expression is detectable at least two hours before the external dorsal lip is seen at stage 10 (three independent experiments). All embryos are shown in dorsal view; pigmented embryos with strong dorsalventral polarity were used in these experiments.
FIG. 4.
Expression of organizer marker genes at the blastula and gastrula. (A, B) In situ hybridization on paraffin sections with a chordin probe at stages 9 and 10.5. Embryos were sectioned sagittally along the dorsal-ventral axis. Note the broad expression domain at stage 9, resembling the area of nuclear localization of β-Catenin (Schneider et al., 1996). (C) RT-PCR analysis of embryos at stage 9 and 10.5 in the presence or absence of microinjected cer-S mRNA. Many classical organizer genes can be detected as early as stage 9 (lane 1) and chordin, noggin, follistatin and cerberus continue to be expressed in the absence of Xnr signaling (lane 2). At stage 10.5 all organizer markers, as well as Xbra, are inhibited by the anti-Xnr reagent Cer-S (lanes 3, 4). The lack of Xbra expression at stage 9 indicates that at this early blastula stage mesoderm induction has not yet taken place. (D) RT-PCR analysis of embryos injected with ΔN-XTcf-3 (lane 2) and untreated controls (lane 1) at stage 9, showing that the induction of many organizer genes is dependent on an early β-Catenin signal. ODC serves as an RNA loading control.
Microinjection experiments with cer-S or ΔN-XTcf-3 showed that the early expression of chordin was independent of Xnr signaling, but dependent on an active β-Catenin pathway (Figs. 3D–3F). However, Xnr signaling was required for the maintenance of chordin expression in the mesoderm of Spemann's organizer at stage 10.5 (Figs. 3J–3L). To examine the full spectrum of genes affected by cer-S mRNA, embryos were injected and harvested at stage 9 and 10.5 by RT-PCR analyses. Interestingly, many organizer genes were expressed at early blastula stages even before the mesodermal marker Xbra was detectable (Fig. 4C, lane 1). Microinjection of cer-S mRNA did not affect the expression levels of chordin, noggin, follistatin and cerberus, while the expression of frzb-1 and goosecoid, and perhaps dkk-1, was decreased by inhibiting Xnr signaling (Fig. 4C, compare lanes 1 and 2). At the gastrula 10.5 stage all organizer markers tested failed to be maintained in the presence of Cer-S (Fig. 4C, compare lanes 3 and 4). Having shown that chordin requires β-Catenin signaling for its expression at blastula, we next tested the wider spectrum of organizer genes that are dependent on this signaling pathway. As shown in Fig. 4D, the transcription of chordin, noggin, follistatin, Xnr-3, goosecoid and siamois was inhibited by injection of ΔN-XTcf-3 mRNA in whole embryos cultured until blastula (stage 9, 7.5 hours post fertilization).
We conclude that BMP antagonists secreted by the mesoderm of Spemann's organizer at the gastrula stage, such as Chordin, Noggin, Follistatin and Cerberus, are also expressed at the blastula stage. This expression takes place for at least two hours before any external signs of blastopore formation are visible and before mesoderm, marked by Xbra, is formed. This pre-organizer expression requires an active β-Catenin pathway. Xnr signaling is required for the maintenance of organizer-specific gene expression at gastrula, but not for its initiation.
The β-Catenin pathway is sufficient to induce pre-organizer factors
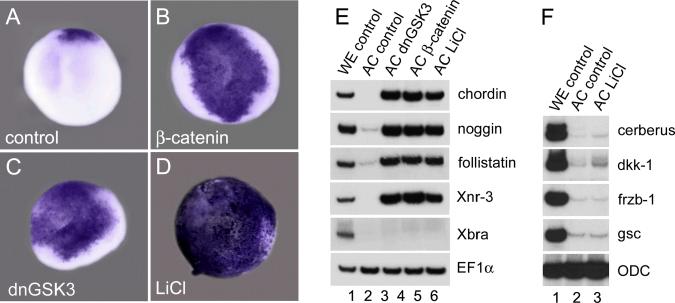
We next investigated whether activation of the early β-Catenin pathway is sufficient to induce BMP antagonists at the blastula stage. To this end, Xenopus embryos were radially injected into the animal cap region at the 4-cell stage either with synthetic mRNA encoding β-catenin, or a dominant-negative version of GSK3 (dnGSK3) that acts upstream of β-catenin preventing its degradation (He et al., 1995). In addition, embryos were treated with LiCl, which leads to the accumulation and nuclear translocation of β-Catenin throughout the embryo (Schneider et al., 1996). When these embryos were examined at stage 9 (7 hours post-fertilization), whole mount in situ hybridization revealed abundant ectopic expression of chordin throughout the animal cap (Figs. 5A–5D). RT-PCR analysis of animal cap explants excised at stage 8 and harvested two hours later at stage 9 showed a robust induction of the neural inducers chordin, noggin, follistatin and Xnr3 (Fig. 5E, compare to whole embryo controls in lane 1). This induction was specific, since not all organizer genes were induced in animal cap explants: LiCl treatment was unable to activate cerberus, dkk-1, frzb-1 and gsc at blastula (Fig. 5F). Upregulation of β-Catenin in the presence of cer-S mRNA also led to the expression of chordin, noggin and follistatin in animal cap explants excluding a requirement for nodal signaling for this early phase of organizer gene expression (data not shown). The results suggest that activation of the β-Catenin pathway is sufficient to induce expression of multiple neural-inducing BMP antagonists already at the blastula stage.
FIG. 5.
The β-Catenin pathway is sufficient to induce pre-organizer gene expression program at the blastula stage. (A–D). Embryos were injected into the animal pole at 4-cell stage with synthetic mRNA for β-catenin (150 pg per blastomere), dnGSK-3 (150 pg per blastomere) or were treated with LiCl and analyzed at stage 9 for chordin expression by in situ hybridization. The patch of expression of chordin in panel A marks the position of the pre-organizer on the dorsal margin of blastula embryos. All embryos are shown in animal view. (E,F) Ectodermal explants of the embryos treated the same way as above were isolated at stage 8 and the expression levels of (E) chordin, noggin, follistatin and Xnr3 or (F) cerberus, dkk-1, frzb-1 and gsc were determined by RT-PCR at stage 9. EF1α and ODC serve as control for equal loading. Note that organizer gene markers in E, but not those in F, were induced by the β-Catenin pathway in ectodermal explants at blastula.
CNS and neural crest induction by the β-catenin pathway
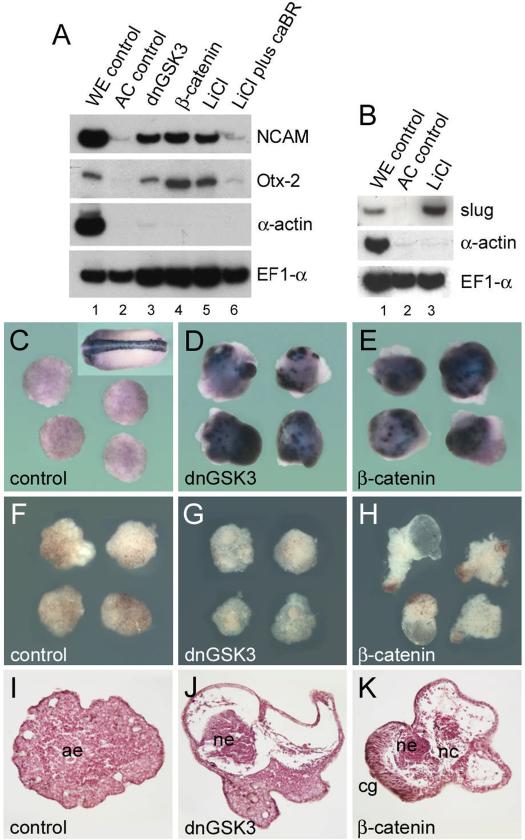
Injection of synthetic chordin mRNA allows differentiation of mature neurons to occur in animal caps (Sasai et al., 1995) and components of the β-catenin pathway induce neural marker genes such as Nrp-1 (Baker et al., 1999). To test whether mature neurons were formed by activating the β-Catenin pathway, animal cap explants from embryos injected with dnGSK3 or β-catenin mRNA, or treated with LiCl, were cultured until stage 24. The pan-neural marker NCAM was induced in these caps in the absence of mesoderm, although at lower levels than those found in whole embryo controls (Fig. 6A, lanes 1–5). This neuralization was prevented by microinjection of a constitutively active BMP receptor (Fig 6A, lane 6), once again demonstrating the importance of the inhibition of BMP signaling in neural induction in Xenopus. To identify mature neurons the β-neurotubulin marker (Richter et al., 1988) was used in in situ hybridizations. Whereas microinjection of chordin leads to uniform and abundant neuronal differentiation in ectodermal explants at stage 25 (Sasai et al., 1994), microinjection of dnGSK3 or β-catenin mRNA lead to the appearance of isolated patches of neuronal cells (Figs. 6C–6E). Morphological and histological examination of the injected explants at stage 42 (Figs. 6F–6K) did not reveal the massive anterior neural induction observed when chordin is injected (Sasai et al., 1995). The explants developed fluid-filled spaces (Fig. 6H) with patches of neural tissue surrounded by whorls of loose mesenchyme containing melanocytes, i.e., tissue with the histological appearance of neural crest (Figs. 6J and 6K). The presence of neural crest in explants was confirmed by the expression of the neural crest marker slug in explants treated with LiCl (Fig. 6B). These data are in line with those of others showing that the Wnt signaling pathway promotes neural crest formation and CNS posterization (McGrew et al., 1995; Saint-Jeannet et al., 1997; LaBonne and Bronner-Fraser, 1998). Thus, the persistent activation of the β-Catenin pathway in our experimental conditions may cause some neural cells specified to become anterior neural tissue to subsequently adopt other fates such as neural crest. In conclusion, the results suggest a pathway in which β-Catenin activates the expression of BMP antagonists already at the blastula stage. These secreted factors, perhaps in concert with transcriptional downregulation of BMPs, may participate in neural induction.
FIG. 6.
β-Catenin signaling promotes neural differentiation and neural crest formation. (A) RT-PCR analysis of ectodermal explants injected with dnGSK3 (lane 3; 150 pg/blastomere), β-catenin (lane 4; 150 pg/blastomere), treated with LiCl (lane 5; 120 mM in 0.1 × Barth for 30 min at 32-cell stage), or injected with a constitutive active BMP receptor (caBR; 1 ng/blastomere) and treated with LiCl (lane 6) and harvested at stage 24. Note that induction of the neural marker genes NCAM and Otx-2 is activated by the β-Catenin pathway and requires inhibition of BMP signaling. (B) Ectodermal explants of embryos treated with LiCl express the neural crest marker Slug by RT-PCR analysis. (C–K) Ectodermal explants microinjected with dnGSK3 or β–catenin mRNA and analyzed by in situ hybridization using β-neurotubulin as a marker for differentiated neurons at stage 24 (C–E) or by morphological (F–H) and histological (I–K) criteria at stage 42. The inset in panel C shows the expression of β-neurotubulin in a control embryo. Note that in explants of dnGSK3 and β-catenin injected embryos β-neurotubulin expression is patchy and that in histological sections neural crest-like tissues with melanocytes are observed. ae, atypical epidermis; ne, neural tissue; nc, neural crest; cg, cement gland.
DISCUSSION
Neural induction and mesoderm formation have traditionally been thought to be associated during development (Sasai and De Robertis, 1997). However, recent findings have questioned this interpretation. Zebrafish and mouse mutants lacking mesendoderm develop with extensive anterior neural structures (Ding et al., 1998; Gritsman et al., 1999; Wilson and Rubenstein, 2000). We now show that Xenopus embryos injected with the Nodal-specific antagonist cer-S, that do not form mesoderm and lack Spemann's organizer at gastrula stage 10.5 (Agius et al., 2000), develop extensive brain structures with large cyclopic eyes (Figs. 1A and 1B). Inhibition of CNS formation by UV irradiation or by the dominant-repressive ΔN-XTcf-3 construct indicate that neural induction in the absence of mesoderm requires early signals mediated by the β-Catenin pathway in the ectoderm. We find that many of the organizer-specific BMP antagonists (Chordin, Noggin, Follistatin and Cerberus) are already expressed at blastula stage 9, at least two hours before the first sign of a dorsal lip appears at gastrula stage 10. This early phase of expression, in a region designated the blastula pre-organizer, is Xnr-independent but requires an active β-Catenin pathway (Fig. 7). Thus, the β-Catenin signal could facilitate neural induction in part through secreted BMP antagonists.
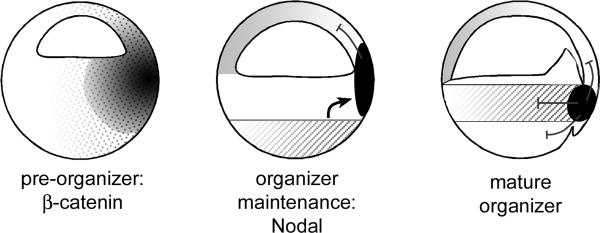
FIG. 7.
Model for organizer induction. The diagram indicates three steps in the establishment of a dorsal signaling center. At the blastula stage nuclear β-Catenin (dotted area) induces the early zygotic expression of organizer-specific genes (black) such as chordin, noggin, follistatin and Xnr3 in the pre-organizer region. These BMP antagonists may participate in the pre-determination of the neural plate. Later, Nodal signals originating from vegetal cells (hatched area) are required for the induction of mesoderm and for maintenance of organizer gene expression. At the gastrula stage, the same cocktail of factors secreted by the mature Spemann's organizer will pattern all three germ layers and is maintained by Nodal-related signals produced from within the mesoderm (hatched area).
The existence of a blastula organizer precursor has been suggested earlier in Xenopus (Gerhart et al., 1991; Heasman, 1997), zebrafish (Grinblat et al., 1998) and mouse (Tam and Steiner, 1999). We recently proposed a simplified pathway of dorsal development to explain how such diverse molecules as β-Catenin, Xnrs and BMP antagonists can rescue the effect of UV irradiation in Xenopus (De Robertis et al., 2000). In this model, β-Catenin (together with the endodermal determinants VegT and Vg1) would induce Xnr expression in the endoderm. A gradient of multiple Xnrs would subsequently induce mesoderm and establish Spemann's organizer, which in turn secretes BMP and Wnt antagonists, promoting dorso-anterior cell fates. While the experimental data presented here still support this model for the patterning of the mesodermal germ layer, neural induction can take place in the ectoderm in the absence of Nodal signaling. Thus, the pathway should be modified for neural development, since it is not linear. The β-Catenin pathway, directly or indirectly, activates a blastula pre-organizer region that expresses many neural-inducing secreted factors that are later on found in the mature organizer. As shown in Fig. 7, mesoderm induction is required for the maintenance of the expression of secreted BMP antagonists in Spemann's gastrula organizer. However, expression of these secreted antagonists is initiated via an earlier β-Catenin-dependent UV-sensitive pathway.
The expression domain of chordin in the pre-organizer (Fig. 4A) encompasses a dorsal region that, in the animal cap, includes cells fated to become brain tissue (Dale and Slack, 1987; Bauer et al., 1994). The expression of multiple BMP antagonists at blastula may contribute to the predisposition of dorsal ectoderm to neural induction (Sharpe et al., 1987; London et al., 1988). By the gastrula stage, expression of chordin (Fig. 4B) is found in mesendoderm caudal to the future forebrain region in Xenopus (Bauer et al., 1994). An interesting modification of the Nieuwkoop activation/transformation model of neural patterning (reviewed by Sasai and De Robertis, 1997) has been proposed by Stern and colleagues (Foley et al., 2000). In the chick, early signals would generate a proneural region that, although unable to differentiate by itself into forebrain, is later on stabilized by signals from underlying mesendoderm giving rise to the future forebrain. Hensen's node itself would serve as a source of caudalizing signals that posteriorize the CNS. The forebrain would escape caudalization due to morphogenetic movements that separate it from the gastrula organizer. Additional insulation of the forebrain from caudalization would be assured by inhibitory factors secreted by prechordal mesendoderm (Foley et al., 2000). In zebrafish, Nodal-related factors emanating from the organizer and marginal zone have been proposed to play an important role in caudalizing the CNS (Thisse et al., 2000). It is interesting to speculate that the pre-organizer region of the Xenopus blastula could correspond to a region of neural predisposition that is subsequently maintained and patterned by signals from prechordal mesendoderm and organizer, as was proposed by Foley et al. (2000) for the chick embryo. Regardless of the early signals, the fact remains that a graft of dorsal mesodermal tissue at the gastrula stage, as in Spemann's experiment, can induce a complete CNS. It is interesting to note that the early and the late events share common molecules secreted by the blastula pre-organizer and by the mature organizer. In both cases, a decrease in BMP signaling levels would facilitate the formation of a region in which neural induction and dorsal development can take place.
The existence of a β-Catenin-dependent pre-organizer region may help understand not only neural formation in the absence of mesoderm, but also another unresolved issue in neural induction, the origin of planar signals. In Xenopus embryos, neural tissue can still be formed when the juxtaposition of mesoderm and ectoderm is prevented (Ruiz i Altaba, 1992). This has led to the proposal that neural inducing factors do not only derive from the underlying mesoderm (vertical signals), but can also migrate in a planar fashion in the ectoderm. Since many anti-BMP factors are expressed at the blastula stage in CNS precursor cells (Bauer et al., 1994), the early source of neural inducing molecules may reside in the neural ectoderm itself.
Organizer gene expression in Xenopus and zebrafish
We recently reported that in Xenopus embryos loss of mesoderm resulted in a loss of organizer markers at gastrula stage 10.5, including chordin and goosecoid (Agius et al., 2000). However, in zebrafish mutants lacking axial mesoderm such as cyclops;squint double homozygotes or maternal/zygotic one-eyed pinhead (MZoep), expression of the organizer gene chordino could still be detected, whereas goosecoid was not (Feldman et al., 1998; Gritsman et al., 1999; Shimizu et al., 2000). This implied that differences in the regulation of gene expression between these two vertebrates might exist. The concept of a blastula pre-organizer may now help resolve this issue. In agreement with zebrafish, we show that in Xenopus, chordin, as well as other BMP antagonists, are initially expressed independently of Xnr signaling at the blastula stage. Early activation of these secreted factors is initially dependent only on β-Catenin signaling, whereas goosecoid is expressed in mesendoderm and is strongly dependent on Nodal-related signaling.
Multiple Regulation of BMP Activity
The data presented here do not definitively prove that the BMP antagonists present in the pre-organizer region in fact mediate CNS induction. This is a difficult issue because of the multiple factors involved, all of which would have to be inhibited simultaneously. The participation of multiple factors in neural induction has recently been underscored by the inactivation of the BMP antagonists chordin and noggin in mouse: neural plate induction takes place normally, but the forebrain fails to develop subsequently (Bachiller et al., 2000). In Xenopus and zebrafish, inhibiting BMP activity seems to be a prerequisite for neural formation (Harland, 2000). Recent work indicates that this is achieved at two levels: by antagonizing BMP activity in the extracellular space and by repression of BMP transcription (Harland, 2000). In Xenopus, microinjections of a stabilized form of β-catenin or other components of the Wnt signaling pathway inhibit BMP-4 transcription in gastrula ectodermal explants (Baker et al., 1999). The down-regulation of BMP-4 transcription cannot be mimicked by microinjection of noggin mRNA and may be a direct transcriptional effect (Baker et al., 1999). However, we have shown here that noggin, chordin, follistatin and Xnr3 are all activated in the blastula pre-organizer. Perhaps a combination of secreted BMP antagonists might be able to indirectly down-regulate BMP-4 expression. In cer-S injected embryos the clearing of BMP-4 transcripts, that is normally restricted to the dorsal side at the gastrula stage (Fainsod et al., 1994), is extended to the entire marginal zone. The neural plate appears to develop on one side of this region free of BMP-4 transcripts. Thus, BMP-4 transcriptional down-regulation (Baker et al., 1999) and asymmetric expression of BMP antagonists might cooperate in neural plate formation in the embryo.
Zebrafish genetics supports the idea that multiple inputs are required for neural plate development. Loss-of-function of chordino, the zebrafish homologue of Chordin, results in a reduced neural plate (Hammerschmidt et al., 1996; Schulte-Merker et al., 1997). The homeobox gene bozozok, a transcriptional repressor acting downstream of β-Catenin signaling, inhibits transcription of BMP-2b/4 on the dorsal side of the embryo (Fekany et al., 1999; Koos and Ho, 1999; Fekany-Lee et al., 2000). Its mutation also causes a modest decrease of neural fates. Interestingly, in chordino;bozozok double mutant embryos synergistic effects are observed, resulting in a dramatic loss of head and trunk neuroectoderm (Gonzalez et al., 2000). This strongly supports the view that BMP antagonism by Chordin and regulation of BMP transcription by the β-Catenin/bozozok pathway cooperate in neural development. However, the presence of a rudimentary tail argues that even in double mutant embryos the anti-BMP function may not be completely eliminated and that additional pathways are involved in neural induction. The data from Xenopus, in which ΔN-XTcf-3 or UV treatment lead to a complete loss of neural structures, suggest that most of these pathways are triggered by the initial β-Catenin activation that takes place after fertilization.
In summary, our results suggest, but do not prove, that a blastula preorganizer dependent on the initial β-Catenin signal may participate in CNS specification. Taken together with previous investigations (Baker et al., 1999), the resuts lend support to the emerging concept that neural induction may start very early in development with signals mediated by the β-Catenin pathway.
ACKNOWLEDGMENTS
We are indebted to Drs. I. Dawid, D. Kimelman, H. Clevers and N. Ueno for generous gifts of plasmids. We thank Drs. J. Smith and F. Pituello (Toulouse), and C. Coffinier, J. Larraín, and N. Ketpura (UCLA) for critically reviewing the manuscript, as well as U. Tran, S.Y. Li and A. Cuellar for technical assistance. O. W., M. O. and E. P. were supported by HFSPO long term postdoctoral fellowships. This work was supported by grant R37 HD-21502-15 from NIH. E. M. D. R. is an HHMI investigator.
REFERENCES
- Agius E, Oelgeschläger M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DV, Huang S, Moody SA. The cleavage stage origin of Spemann's Organizer: analysis of the movements of blastomere clones before and during gastrulation in Xenopus. Development. 1994;120:1179–1189. doi: 10.1242/dev.120.5.1179. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech. Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Cheng AM, Thisse B, Thisse C, Wright CV. The lefty-related factor Xatv acts as a feedback inhibitor of nodal signaling in mesoderm induction and L-R axis development in xenopus. Development. 2000;127:1049–1061. doi: 10.1242/dev.127.5.1049. [DOI] [PubMed] [Google Scholar]
- Dale L, Slack JM. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99:527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Larraín J, Oelgeschläger M, Wessely O. The Establishment of Spemann's Organizer and Patterning of the Vertebrate Embryo. Nature Reviews Genetics. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anteriorposterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekany K, Yamanaka Y, Leung T, Sirotkin HI, Topczewski J, Gates MA, Hibi M, Renucci A, Stemple D, Radbill A, Schier AF, Driever W, Hirano T, Talbot WS, Solnica-Krezel L. The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development. 1999;126:1427–1438. doi: 10.1242/dev.126.7.1427. [DOI] [PubMed] [Google Scholar]
- Fekany-Lee K, Gonzalez E, Miller-Bertoglio V, Solnica-Krezel L. The homeobox gene bozozok promotes anterior neuroectoderm formation in zebrafish through negative regulation of BMP2/4 and Wnt pathways. Development. 2000;127:2333–2345. doi: 10.1242/dev.127.11.2333. [DOI] [PubMed] [Google Scholar]
- Feldman B, Dougan ST, Schier AF, Talbot WS. Nodal-related signals establish mesendodermal fate and trunk neural identity in zebrafish. Curr. Biol. 2000;10:531–534. doi: 10.1016/s0960-9822(00)00469-3. [DOI] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal- related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Foley AC, Skromne I, Stern CD. Reconciling different models of forebrain induction and patterning: a dual role for the hypoblast. Development. 2000;127:3839–3854. doi: 10.1242/dev.127.17.3839. [DOI] [PubMed] [Google Scholar]
- Gerhart J, Doniach T, Steward R. Organizing the Xenopus organizer. In: Keller R, Clark WH, Griffin F, editors. Gastrulation: Movements, Patterns, and Molecules. Plenum Press; New York: 1991. pp. 57–76. [Google Scholar]
- Gonzalez EM, Fekany-Lee K, Carmany-Rampey A, Erter C, Topczewski J, Wright CV, Solnica-Krezel L. Head and trunk in zebrafish arise via coinhibition of BMP signaling by bozozok and chordino. Genes Dev. 2000;14:3087–3092. doi: 10.1101/gad.852400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinblat Y, Gamse J, Patel M, Sive H. Determination of the zebrafish forebrain: induction and patterning. Development. 1998;125:4403–4416. doi: 10.1242/dev.125.22.4403. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nusslein-Volhard C. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development. 1996;123:95–102. doi: 10.1242/dev.123.1.95. [DOI] [PubMed] [Google Scholar]
- Hansen CS, Marion CD, Steele K, George S, Smith WC. Direct neural induction and selective inhibition of mesoderm and epidermis inducers by Xnr3. Development. 1997;124:483–492. doi: 10.1242/dev.124.2.483. [DOI] [PubMed] [Google Scholar]
- Harland R. Neural induction. Curr. Opin. Genet. Dev. 2000;10:357–362. doi: 10.1016/s0959-437x(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann's organizer. Ann. Rev. Cell Dev. Biol. 1997;13:611–67. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- Heasman J. Patterning the Xenopus blastula. Development. 1997;124:4179–91. doi: 10.1242/dev.124.21.4179. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J. Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemura S, Yamamoto TS, Takagi C, Uchiyama H, Natsume T, Shimasaki S, Sugino H, Ueno N. Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc. Natl. Acad. Sci. USA. 1998;95:9337–9342. doi: 10.1073/pnas.95.16.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Kuehn MR, Hogan BL, Smith JC, Wright CV. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–62. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- Joseph EM, Melton DA. Xnr4: a Xenopus nodal-related gene expressed in the Spemann organizer. Dev. Biol. 1997;184:367–372. doi: 10.1006/dbio.1997.8510. [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev. Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J, Ang SL, Bachiller D, Rossant J. Neural induction and patterning in the mouse in the absence of the node and its derivatives. Dev. Biol. 1999;216:535–549. doi: 10.1006/dbio.1999.9525. [DOI] [PubMed] [Google Scholar]
- Koos DS, Ho RK. The nieuwkoid/dharma homeobox gene is essential for bmp2b repression in the zebrafish pregastrula. Dev. Biol. 1999;215:190–207. doi: 10.1006/dbio.1999.9479. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J. Cell Biol. 1997;136:1123–1136. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P, Gurdon JB. A role for cytoplasmic determinants in mesoderm patterning: cell-autonomous activation of the goosecoid and Xwnt-8 genes along the dorsoventral axis of early Xenopus embryos. Development. 1994;120:1191–9. doi: 10.1242/dev.120.5.1191. [DOI] [PubMed] [Google Scholar]
- London C, Akers R, Phillips C. Expression of Epi 1, an epidermis-specific marker in Xenopus laevis embryos, is specified prior to gastrulation. Dev. Biol. 1988;129:380–389. doi: 10.1016/0012-1606(88)90385-5. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Lai CJ, Moon RT. Specification of the anteroposterior neural axis through synergistic interaction of the Wnt signaling cascade with noggin and follistatin. Dev. Biol. 1995;172:337–342. doi: 10.1006/dbio.1995.0027. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis. Garland Publishing, Inc.; New York: 1994. [Google Scholar]
- Pera EM, De Robertis EM. A direct screen for secreted proteins in xenopus embryos identifies distinct activities for the wnt antagonists crescent and frzb-1. Mech. Dev. 2000;96:183–195. doi: 10.1016/s0925-4773(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–10. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Grunz H, Dawid IB. Gene expression in the embryonic nervous system of Xenopus laevis. Proc. Natl. Acad. Sci. USA. 1988;85:8086–8090. doi: 10.1073/pnas.85.21.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowning BA, Wells J, Wu M, Gerhart JC, Moon RT, Larabell CA. Microtubule-mediated transport of organelles and localization of beta- catenin to the future dorsal side of Xenopus eggs. Proc. Natl. Acad. Sci. USA. 1997;94:1224–1229. doi: 10.1073/pnas.94.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Planar and vertical signals in the induction and patterning of the Xenopus nervous system. Development. 1992;116:67–80. doi: 10.1242/dev.116.1.67. [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet JP, He X, Varmus HE, Dawid IB. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc. Natl. Acad. Sci. USA. 1997;94:13713–13718. doi: 10.1073/pnas.94.25.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer- specific homeobox genes. Cell. 1994;79:779–90. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–6. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Sasai Y, De Robertis EM. Ectodermal patterning in vertebrate embryos. Dev. Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. [DOI] [PubMed] [Google Scholar]
- Saude L, Woolley K, Martin P, Driever W, Stemple DL. Axis-inducing activities and cell fates of the zebrafish organizer. Development. 2000;127:3407–3417. doi: 10.1242/dev.127.16.3407. [DOI] [PubMed] [Google Scholar]
- Scharf SR, Gerhart JC. Determination of the dorsal-ventral axis in eggs of Xenopus laevis: complete rescue of uv-impaired eggs by oblique orientation before first cleavage. Dev. Biol. 1980;79:181–198. doi: 10.1016/0012-1606(80)90082-2. [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech. Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Sharpe CR, Fritz A, De Robertis EM, Gurdon JB. A homeobox-containing marker of posterior neural differentiation shows the importance of predetermination in neural induction. Cell. 1987;50:749–558. doi: 10.1016/0092-8674(87)90333-3. [DOI] [PubMed] [Google Scholar]
- Shih J, Fraser SE. Characterizing the zebrafish organizer: microsurgical analysis at the early-shield stage. Development. 1996;122:1313–1322. doi: 10.1242/dev.122.4.1313. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamanaka Y, Ryu SL, Hashimoto H, Yabe T, Hirata T, Bae YK, Hibi M, Hirano T. Cooperative roles of Bozozok/Dharma and Nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech. Dev. 2000;91:293–303. doi: 10.1016/s0925-4773(99)00319-6. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis: A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, McKendry R, Ribisi S, Jr., Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Über Induktion von Embryoanlagen durch Implantation Artfremder Organisatoren. Roux' Arch. Entw. Mech. 1924;100:599–638. [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yokota C, Takano K, Tanegashima K, Onuma Y, Goto JI, Asashima M. Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development. 2000;127:5319–5329. doi: 10.1242/dev.127.24.5319. [DOI] [PubMed] [Google Scholar]
- Tam PP, Steiner KA. Anterior patterning by synergistic activity of the early gastrula organizer and the anterior germ layer tissues of the mouse embryo. Development. 1999;126:5171–5179. doi: 10.1242/dev.126.22.5171. [DOI] [PubMed] [Google Scholar]
- Thisse B, Wright CV, Thisse C. Activin- and Nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature. 2000;403:425–428. doi: 10.1038/35000200. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr. Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Rubenstein LR. Induction and Dorsoventral Patterning of the Telencephalon. Neuron. 2000;28:641–651. doi: 10.1016/s0896-6273(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Yang X, van Beest M, Clevers H, Jones T, Hursh DA, Mortin MA. decapentaplegic is a direct target of dTcf repression in the Drosophila visceral mesoderm. Development. 2000;127:3695–3702. doi: 10.1242/dev.127.17.3695. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]