Abstract
The development of multicellular organisms, as well as maintenance of organ architecture and function, requires robust regulation of cell fates. This is in part achieved by conserved signaling pathways through which cells process extracellular information and translate this information into changes in proliferation, differentiation, migration, and cell shape. Gene deletion studies in higher eukaryotes have assigned critical roles for components of the extracellular matrix (ECM) and their cellular receptors in a vast number of developmental processes, indicating that a large proportion of this signaling is regulated by cell-ECM interactions. In addition, genetic alterations in components of this signaling axis play causative roles in several human diseases. This review will discuss what genetic analyses in mice and lower organisms have taught us about adhesion signaling in development and disease.
Integrins anchor cells to the extracellular matrix but also transmit “inside-out” and “outside-in” signals. These are relayed by proteins such as talin and kindlin, mutations which lead to disorders such as Kindler syndrome.
Almost all cells in multicellular organisms are surrounded by a three-dimensional organized meshwork of macromolecules that constitute the extracellular matrix (ECM). The ECM is a dynamic structure that is generated and constantly remodeled by cells that secrete and manipulate its components into a precise configuration. It functions as a structural framework that provides cells with positional and environmental information, but also forms specialized structures such as cartilage, tendons, basement membranes (BM), bone, and teeth. In addition to its structural properties, the ECM acts as a signaling platform that regulates a large number of cellular functions. It is capable of binding growth factors, chemokines, and cytokines thereby modulating their bioavailability and activity. On the other hand, the ECM is recognized by multiple cell surface receptors that transmit information from the extracellular environment by propagating intracellular signals (for a review, see Hynes 2009).
The major cell surface receptors that recognize and assemble the ECM are integrins. Integrins are heterodimeric transmembrane proteins composed of α and β subunits. Eighteen α subunits and eight β subunits can assemble in 24 different combinations with overlapping substrate specificity and cell-type-specific expression patterns (Hynes 2002; Humphries et al. 2006). This, together with the ability of different heterodimers to assemble specific intracellular signaling complexes, provides multiple layers of signaling specificity to these receptors. Conversely, the integrin expression profile of a given cell type determines which ECM components it can bind. Signals arising from integrins regulate virtually all aspects of cell behavior, including cell migration, survival, cell cycle progression, and differentiation.
Genetics has proven to be a powerful tool to dissect the functions of ECM–cell interactions in complex organisms. To date, all of the integrin subunits and their major ligands have been deleted in mice. Given the large variety of cellular processes regulated by adhesion signaling, it is not surprising that a significant subset of these proteins has proven to be essential for embryonic development and/or tissue maintenance. However, in addition to underlining the importance of cell-ECM interactions in development, genetic studies also revealed critical roles for tissue- and cell-type-specific modes of adhesion signaling and provided important insights into human disease.
THE INTEGRIN-INTERMEDIATE FILAMENT AXIS
Basement membranes (BMs) are dense sheets of extracellular matrix that function as structural barriers that separate epithelial and endothelial cells as well as peripheral nerve axons, fat cells, and muscle cells from the underlying tissue stroma. BMs provide structural support, separate tissues into compartments, and regulate cell behavior. All cell types are known to produce components of BMs, which include type IV collagen, laminin, fibronectin, heparan sulfate proteoglycans, and nidogen/entactin as well as several minor components (Erickson and Couchman 2000; Yurchenco 2010). The molecular composition of the BM varies among different tissues, conferring signaling specificity important for defining the specialized functions of epithelial and endothelial cells in different organs.
All BMs contain laminins, a family of 16 heterotrimeric glycoproteins generated by the combination of 5α, 4β, and 3γ chains. When present in sufficient concentrations, laminin networks can self-assemble into polymers that interact with other ECM components, as well as cell surface receptors such as integrins and dystroglycan. The laminin isoforms present in BMs are regulated in a tissue-specific manner, as well as temporally during development (Miner 2008; Yurchenco 2010). The central role of laminins in BM assembly is illustrated by gene deletion studies in mice. Laminin-111 (containing α1, β1, and γ1 chains) and laminin-511 (containing α5, β1, and γ1 chains) are the central constituents of peri-implantation stage BMs. Mice deficient in γ1 or β1 subunits are unable to generate laminin trimers, and therefore lack BMs (Smyth et al. 1999; Miner et al. 2004).
Laminin-332 (containing α3, β3, and γ2 chains; previously termed laminin-5) is a component of epithelial BMs and thus present in skin, stratified squamous mucosa, amnion, and cornea. Its main task is to maintain epithelial integrity and epithelial-mesenchymal cohesion in tissues exposed to high mechanical forces. This function is facilitated by the unique ability of laminin-322 to interact with two distinct integrin heterodimers. Its interaction with α3β1 integrin results in the assembly of canonical focal adhesions (FA), whereas the interaction with α6β4 integrin results in the formation of specialized adhesion complexes termed hemidesmosomes (Tsuruta et al. 2008).
Hemidesmosomes—Structure and Assembly
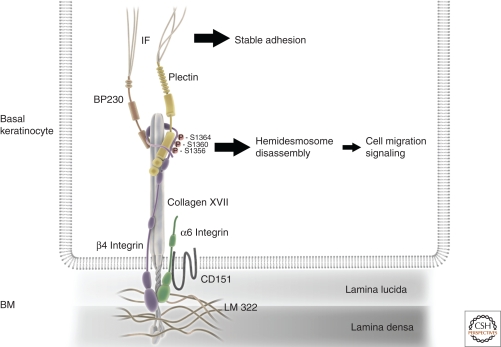
Hemidesmosomes were first characterized at the ultrastructural level as electron-dense clusters at the plasma membrane-ECM interphase (Weiss and Ferris 1954). Further studies identified them as multiprotein adhesion complexes present in stratified and simple epithelia. Two types of hemidesmosomes can be distinguished based on their molecular composition. Type I or classical hemidesmosomes are found in stratified epithelium such as the skin and contain three transmembrane proteins: α6β4 integrin, tetraspanin CD151, and type XVII collagen (also called bullous pemphigoid [BP] antigen 180) (Fig. 1). Type II hemidesmosomes are found in simple epithelia such as intestine, and contain only integrin α6β4 (Uematsu et al. 1994; reviewed in Litjens et al. 2006).
Figure 1.
Molecular architecture of type I hemidesmosomes. Schematic depiction of a type I hemidesmosome found in stratified epithelia such as in basal skin keratinocytes. The core component is α6β4 integrin, which binds the basement membrane (BM) component laminin (LM)-322. α6β4 integrin recruits the plakin protein plectin through multiple interactions with the β4 integrin cytoplasmic tail, which initiates the formation of hemidesmosomes. This is followed by the recruitment of collagen XVII, which interacts both with β4 integrin and plectin as well as with LM 322. Collagen XVII in turn mediates the recruitment of another plakin, bullous pemphigoid antigen 230 (BP 230), which together with plectin provides the connection to intermediate filaments (IF). This linkage is essential to stabilize the hemidesmosome and to provide stable adhesion of the basal keratinocyte to the BM. Also, the transmembrane protein tetraspanin CD151 that interacts with α6 integrin is found in type I hemidesmosomes. Phosphorylation of serines (S) 1356, 1360, and 1364 on the cytoplasmic tail of β4 integrin by growth factors induces disassembly of hemidesmosomes, which promotes cell migration and signaling. The molecules are not drawn to scale.
The unique feature of hemidesmosomes is that they connect the ECM to the intermediate filament (IF) network. This interaction is established by two plakin proteins, plectins and BP230 (also called BPAG1), of which plectin is present both in type I and II hemidesmosomes. Plectins are large cytoplasmic proteins, which at their carboxyl terminus contain six plakin repeats. A stretch of basic residues linking the fourth and fifth plakin repeat mediates the interaction of plectin with IFs. The amino terminus contains two calponin-homology (CH) domains that constitute an actin-binding domain. Through the actin-binding domain, plectins can associate with either the cytoplasmic domain of the β4 integrin subunit or actin filaments in a mutually exclusive manner, which explains the absence of actin in hemidesmosomes (Rezniczek et al. 1998; Geerts et al. 1999; Litjens et al. 2003). The interaction of plectin with the cytoplasmic tail of β4 integrin is considered as the initial step of hemidesmosome assembly (Geerts et al. 1999; Koster et al. 2004). The cytoplasmic tail of β4 integrin is unusually large and shares no homology with other β integrin subunits. Its interaction with plectin has been shown to induce a conformational change in the integrin tail (de Pereda et al. 2009). Because the recruitment of type XVII collagen and the plakin BP230 to hemidesmosomes requires prior interaction of plectin with β4, this conformational change might facilitate the interactions of both proteins with the integrin (Koster et al. 2004).
Deletion of α6β4 leads to the loss of hemidesmosomes and epithelial detachment in mice (Dowling et al. 1996; van der Neut et al. 1996), indicating that BP180, despite its ability to bind laminin-332 and plectin in vitro, is not sufficient to maintain adhesion of cells to laminin-332 in the absence of α6β4 (Tasanen et al. 2004) and identifying α6β4 as the core component of hemidesmosomes. Another critical event in hemidesmosome assembly is the interaction between β4 integrin tails and plectin. Blocking this interaction compromises hemidesmosome assembly in vitro (Geerts et al. 1999; Koster et al. 2001). In plectin-deficient mice hemidesmosomes appear ultrastructurally normal, but their number and mechanical stability are reduced (Andra et al. 1997). The fact that type II hemidesmosomes lack both type XVII collagen and BP230 further shows that α6β4 and plectin alone are sufficient to initiate the formation of these structures and to maintain their integrity.
Although α6β4 is the only integrin found in hemidesmosomes, other integrins can indirectly contribute to their assembly. Integrin α3β1 has been shown to cluster in “pre-hemidesmosomal” structures on the basal cell surface of human keratinocytes together with CD151. However, as hemidesmosomes mature, α3β1 integrins become recruited to cell-cell or focal contacts, whereas CD151 remains in the hemidesmosomes (Sterk et al. 2000). The initial α3β1-containing adhesions have been proposed to contribute to the recruitment of α6β4 to the plasma membrane, and thereby increase the efficacy with which hemidesmosomes are formed. This is supported by the observation that β1-deficient mice have reduced numbers of hemidesmosomes in the skin, although it should be noted that the expression level of α6β4 is also reduced in these mice, suggesting that the mechanism could also be more indirect (Brakebusch et al. 2000). Polarity proteins have also been shown to play a role in hemidesmosome assembly through the regulation of polarized targeting of integrins. In the larval epidermis of the zebrafish, the basolateral identity protein Lgl2 is required for hemidesmosome formation through recruitment of β4 integrin to the basal plasma membrane (Sonawane et al. 2005, 2009). Taken together, the formation of hemidesmosomes requires the establishment of a basolateral membrane identity through polarity proteins and possibly also other integrin-based adhesion structures to recruit α6β4 integrin to these sites. α6β4 integrin then interacts with BM laminin and together with plectin nucleates stable, IF-bound hemidesmosomes. Interestingly, however, keratinocytes expressing a β4 integrin subunit unable to bind laminin can still form structures that contain all components of type I hemidesmosomes, which implies that ligand binding is not necessary for their formation (Nievers et al. 1998, 2000). These complexes, however, differ in their morphology, density, and dynamics from normal hemidesmosomes, suggesting that the integrin-laminin interaction plays a role in the stability and structural organization of hemidesmosomes (Geuijen and Sonnenberg 2002).
Hemidesmosomes—Guardians of Epidermal Integrity
A central function of the skin is to act as a bidirectional barrier to prevent dehydration and to protect against injury and pathogens. In addition, it is exposed to constant mechanical stress. On the other hand, this tissue is continuously renewed through a terminal differentiation program, in which basal keratinocytes detach from the BM and move to the upper layers of the epithelium where they slough off. Finally, in case of injury, keratinocytes need to rapidly acquire a motile phenotype and migrate to close the wound. These functions require mechanically stable but dynamic modes of cell adhesion. Hemidesmosomes are found in the basal plasma membrane of basal keratinocytes, where they function to attach these cells firmly to the BM separating the epidermis from the dermis (Fig. 1). Interestingly, α6β4 integrins and thus also hemidesmosomes are not required for skin morphogenesis or developmental homeostasis (DiPersio et al. 2000). The importance of hemidesmosomes in maintaining epidermal integrity is, however, shown by multiple lines of genetic evidence. Ablation of genes encoding α6 integrin, β4 integrin, or plectin in mice results in severe blistering of the skin causing neonatal death because of a severe epithelial barrier defect (Dowling et al. 1996; Georges-Labouesse et al. 1996a; van der Neut et al. 1996; Andra et al. 1997). In line with their dispensable role in hemidesmosome assembly, mice lacking type XVII collagen or BP230 display only mild forms of skin blistering (Guo et al. 1995; Nishie et al. 2007), whereas deletion of CD151 is dispensable for both hemidesmosome stability and the integrity of skin (Wright et al. 2004).
No experimental data exist on the dynamic behavior of hemidesmosomes in vivo, but it has been analyzed in keratinocyte culture, in which hemidesmosomes have been shown to display a certain dynamics also in nonmigratory cells (Tsuruta et al. 2003). When cells are stimulated to migrate, α6β4 integrin is mobilized from hemidesmosomes to actin-based structures such as lamellipodia and filopodia to promote cell motility, resulting in hemidesmosome disassembly (Geuijen and Sonnenberg 2002; Tsuruta et al. 2003). This type of relocalization has also been visualized in human wound edges, where β4 integrins can be seen in trailing-edge hemidesmosomes as well as in lamellipodia of the leading edge (Underwood et al. 2009). In vitro analyses have shown that the mobilization of α6β4 integrin from hemidesmosomes is facilitated by growth factor-mediated phosphorylation of multiple residues in the cytoplasmic tail of β4. Both serines and tyrosines can be phosphorylated, but the exact sites and their relevance remain controversial. The current data suggest that serine phosphorylation might be more relevant under physiological conditions, leading to hemidesmosome disassembly through the release of plectin from the integrin (Margadant et al. 2008) (Fig. 1)
α6β4 Integrin and Cancer
β4 integrin was originally identified as a “tumor-specific” protein (Tumor Surface Protein 180) up-regulated in metastatic variants of mouse lung carcinoma and melanoma cell lines (Kennel et al. 1981), and has later been shown to be up-regulated in several human cancers, suggesting that the expression of β4 integrin is beneficial for tumor cells (Giancotti 2007). The role of α6β4 integrin signaling in tumorigenesis has been studied in mice carrying a truncated cytoplasmic tail of β4 lacking the tyrosine phosphorylation sites as well as other potential interaction motifs. In contrast to mice lacking the entire β4 cytoplasmic domain, which display extensive skin blistering because of the absence of hemidesmosomes (Murgia et al. 1998), mice with a more restricted truncation do not show these defects. However, they show delayed wound healing and defective neoangiogenesis in tumor xenografts (Nikolopoulos et al. 2004, 2005). In addition, when crossed to the MMTV-Neu mice that carry an activated form of epidermal growth factor receptor-2 (ErbB2) driven by a mouse mammary tumor virus (MMTV) promoter leading to mammary tumors, the β4 mutant mice display delayed tumor onset, impaired tumor growth and decreased metastatic potential (Guo et al. 2006). Whether this applies to other tumor types as well remains to be shown.
The tumor-promoting properties of α6β4 integrin seem to result from both promigratory and signaling functions. β4 integrin has been shown to cross-talk with several receptor tyrosine kinases (RTK), including ErbB2, epidermal growth factor receptor (EGF-R), Met and Ron (Mariotti et al. 2001; Trusolino et al. 2001; Santoro et al. 2003; Guo et al. 2006). Following stimulation, these RTKs induce activation of phosphatidylinositol 3-kinase (PI3-K) or Src family kinases (SFK), leading to phosphorylation of the β4 integrin tail, disassembly of hemidesmosomes, and induction of cell motility (Mariotti et al. 2001; Santoro et al. 2003). On the other hand, β4 integrin has been shown to promote SFK-dependent phosphorylation of the catalytic sites of RTKs and their substrates (Bertotti et al. 2006; Guo et al. 2006), thereby amplifying their signaling ability. Taken together, α6β4 integrin seems to have a proinvasive and proangiogenic activity in tumors, but additional genetic studies are needed to determine whether this role is limited to malignancies involving hyperactivation of specific RTKs. In addition, several groups have failed to detect significant tyrosine phosphorylation of β4 integrin in keratinocytes or transformed cell lines in response to stimulation with EGF and hemidesmosome disassembly, making the relevance of tyrosine phosphorylation events controversial (Rabinovitz et al. 1999, 2004; Alt et al. 2001; Wilhelmsen et al. 2007). Knockin mice carrying mutations in specific phosphorylation sites of the β4 integrin subunit would provide more conclusive information on the in vivo significance of the various phosphorylation events in α6β4 integrin signaling, hemidesmosome turnover, and invasion.
THE INTEGRIN-ACTIN AXIS
In contrast to α6β4, most integrins engage the actin cytoskeleton following ligand binding. A central ligand of actin-associated integrins is FN, which is recognized by 11 integrin heterodimers in mice and humans, and it will be used in this section as an example to discuss the principles of ECM-integrin interactions leading to engagement and remodeling of the actin cytoskeleton. FN is a large, modular glycoprotein that exists in two forms; cellular FN which is present in tissues where it is assembled into a fibrillar matrix, and plasma FN, which is produced by hepatocytes and is secreted into the blood where it remains in a nonfibrillar, soluble form, or following entry into tissues becomes incorporated into the ECM (Leiss et al. 2008). FN is found only in vertebrates, and it has co-evolved together with the cardiovascular system. Consistently, FN is critical for the development of the vasculature, where it localizes between the endothelium and perivascular cells. Deletion of FN in mice results in embryonic lethal cardiovascular defects, which vary depending on the genetic background. FN-null embryos from 129S4 mice are unable to form the dorsal aorta, indicative of an early defect already in vasculogenesis, whereas this structure is present in C57BL/6 derived embryos that display defects in vascular lumen formation (George et al. 1993, 1997; Georges-Labouesse et al. 1996b).
FN is secreted as a disulfide-bonded dimer, and its deposition into a fibrillar matrix is a cell-driven process that critically depends on integrins. So far α5β1, αvβ3, α4β1, and αIIbβ3 integrins have been shown to induce fibrillogenesis in vitro. These integrins bind FN that is secreted as a compact globular structure where binding sites for other FN molecules are buried within the protein (see Schwarzbauer and DeSimone 2011). Binding is followed by the activation of integrin signaling, leading to the recruitment of cytoplasmic proteins to form focal complexes (FCs). Several FC components are actin-binding and modulatory proteins, allowing the recruitment and reorganization of the actin cytoskeleton at these sites and their maturation into FAs (Geiger and Yamada 2011). The major FN-fibril-forming integrin α5β1 leaves FAs and moves along F-actin to the cell center to form fibrillar adhesions and to facilitate the generation of mechanical tension via the actin cytoskeleton, leading to stretching of the bound FN molecule, unraveling of the cryptic self-association sites, and finally the binding to other FN molecules resulting in fibril formation (Mao and Schwarzbauer 2005; Leiss et al. 2008; Schwarzbauer and DeSimone 2011).
Interactions via the RGD Motif
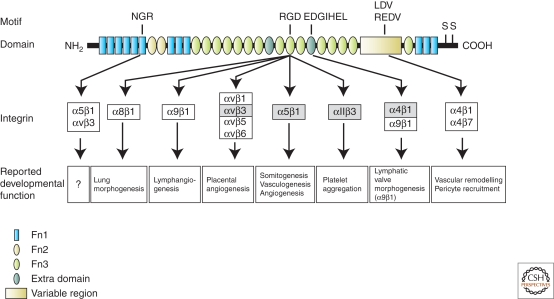
Cell adhesion to FN critically depends on the Arg-Gly-Asp (RGD) motif of FN, which is recognized by α5β1, the αv family, α8β1, α9β1, and the platelet-specific αIIbβ3 integrins (Fig. 2). α5β1 is considered the major integrin responsible for FN assembly, and its interaction with the RGD motif is required for this function. However, although deletion of α5 integrin in mice also leads to embryonic lethality and vascular defects, these mice develop significantly further than the FN-null mice (Yang et al. 1993). This is attributable to the ability of cells to assemble FN using other integrins, mainly of the αv subfamily. The deletion of all five αv heterodimers also leads to late embryonic defects in vascular development mainly in the placenta. A subset of animals can even proceed through embryonic development and die after birth because of hemorrhages, indicating that, unlike α5, αv integrins are dispensable for vasculogenesis and partly also for angiogenesis (Bader et al. 1998). Only a double knockout of the αv and α5 integrin genes results in a loss of FN fibrillogenesis (Yang et al. 1999). Interestingly, although the RGD motif is central for the interaction of FN with α5β1 and αvβ3, the inactivation of this motif by a RGD to RGE point mutation also allows FN fibrillogenesis in vivo. The knockin mice carrying this mutation display a phenotype closely resembling that of the α5-null mice (Takahashi et al. 2007). This highlights the importance of this motif in FN signaling through α5β1, but also shows the ability of other integrin interaction sites on FN to take over the role of RGD in FN fibrillogenesis. Whether these sites play a role in FN assembly when the RGD sequence is intact, remains open. Together, these results indicate that FN fibrillogenesis is not sufficient for it to carry out its functions during development. They further illustrate that the majority of FN signaling in embryogenesis occurs through α5β1 and that αv and α5β1 integrins relay distinct signals on FN binding. The severity of the FN-null phenotype in contrast to the integrin deletions also suggests that the functions of FN during development extend beyond the FN-integrin signaling axis. These functions could be related to its mechanical role in the ECM, or its ability to induce integrin-independent signaling, for example, through sequestering growth factors such as vascular endothelial growth factor (VEGF) or transforming growth factor-β (TGF-β).
Figure 2.
Developmental functions of fibronectin-integrin interactions. Fibronectin (FN) is a dimeric glycyoprotein consisting of type I, type II, and type III modular repeats. Dimerization is achieved by disulfide bonds mediated by the two cysteines (S) in the COOH-terminus of the protein. The binding domains and motifs for integrins, as well as mouse developmental functions reported to depend on the particular interaction are indicated. Integrins that have been shown to mediate FN fibrillogenesis in vitro are marked by gray boxes.
Non-RGD Binding Integrins
The FN gene can be alternatively spliced allowing the expression of up to 20 possible monomeric isoforms in humans and up to 12 in mouse, potentially giving rise to a larger variety of FN dimers (Pankov and Yamada 2002). Some of these splice variants can generate additional integrin interaction sites on FN. One of them is the variable region (v-region) that can interact with two non-RGD-binding integrins, α4β1 and α4β7 (Wayner et al. 1989; Guan and Hynes 1990; Fig. 2). These integrins are mainly expressed by hematopoietic cells, although α4β1 is also found in several other cell types, such as neural crest cells and cellular components of the cardiovascular and peripheral nervous systems. The two α4 integrins have many important roles during development. Deletion of the α4 integrin gene results in embryonic lethality because of early placental and cardiovascular defects (Yang et al. 1995). However, these defects are probably not attributed to the FN- α4β1/α4β7 integrin interaction, as these two integrins also bind cell counter receptors such as vascular-cell adhesion molecule-1 (VCAM-1) and MadCAM, respectively. The developmental defects in the α4 integrin-null mice are very similar to those found in mice lacking VCAM-1, indicating that the observed defects are caused by an abrogated interaction between α4β1 integrin and VCAM-1 (Kwee et al. 1995; Yang et al. 1995). From a clinical point of view, it is particularly interesting that the interaction between α4β1 and VCAM-1 is important for the homing of autoreactive T cells to the central nervous system during the pathogenesis of an autoimmune disease called multiple sclerosis (Yednock et al. 1992; Vajkoczy et al. 2001; Bauer et al. 2009). This disease is characterized by axon demyelination and damage, leading to numbness, weakness, paresis as well as cognitive problems. Blocking antibodies against α4 integrin have proven to be an effective treatment of multiple sclerosis (Steinman 2009), and genetic analysis in mice showed that α4β1 is required for the arrest of T cells on the brain endothelial surface (Bauer et al. 2009).
α4β1 integrins can also interact with the alternative spliced extra domain A (EDA) of FN (Fig. 2), which is highly expressed during development and during pathological conditions such as tumorigenesis. This “reactivation” of the embryonic splice pattern is interesting, as α4β1 has recently been shown to play a critical role in tumor lymphangiogenesis in a FN-dependent manner. Both α4β1 and FN are highly expressed in lymphatic endothelial cells of tumors, whereas other integrin subunits seem not to be up-regulated (Garmy-Susini et al. 2010). Whether this function really depends on the EDA domain was, however, not analyzed. In addition, it is not clear whether α4β1 integrin is also regulating developmental lymphangiogenesis, as the constitutive knockout mice die before the initiation of this process. The EDA domain itself, however, plays a role in developmental lymphangiogenesis through integrin α9β1. Interestingly, this integrin is highly expressed in lymphatic but not blood endothelial cells (Huang et al. 2000). Deletion of α9 integrin gene leads to a severe chylothorax caused by defects in lymphatic valve morphogenesis and subsequent respiratory failure (Huang et al. 2000; Bazigou et al. 2009). This phenotype was partially recapitulated by deletion of EDA. Furthermore, both α9β1 integrin as well as the EDA domain were shown to be critical for FN assembly in lymphatic endothelial cells in vitro (Bazigou et al. 2009), in contrast with previous studies showing that EDA is not required for FN assembly or mouse development because of compensation by EDB (Tan et al. 2004; Astrof et al. 2007). This study suggests that matrix assembly might be regulated by integrins that are expressed in a tissue-specific manner to allow more stringent control of distinct anatomical structures.
Linkage to the Actin Cytoskeleton
In addition to catalyzing matrix assembly, the binding of integrins to FN or other ligands leads to the induction of multiple modes of intracellular signaling. These include activation of classical signaling pathways through tyrosine and serine phosphorylation of specific substrates, resulting ultimately in the regulation of cell survival, growth and differentiation. This signaling operates in close collaboration with growth factor signaling, making it difficult to dissect which signals specifically emanate from ligated integrins (Legate et al. 2009; Ivaska and Heino 2010). The other central signaling mode is the recruitment of filamentous (F-) actin to integrin adhesion sites, a process tightly coupled to the active remodeling of the actin cytoskeleton. The integrin-actin linkage is central to integrin function in several respects. First, active actin polymerization, crosslinking, and subsequent force generation are critical for the maturation of nascent and short-lived integrin adhesions into signaling-competent and stable FAs. Second, it is required for the precise spatiotemporal control of cell protrusion and retraction during cell migration. Third, it allows cells to adopt or change their characteristic shape, for example to polarize. Finally, it is critical for FN matrix assembly. Integrins do not bind actin directly, but regulate this linkage by recruiting a large number of actin-binding and regulatory proteins. A recent study based on database mining identified 156 signaling, structural and adaptor molecules that can be found in integrin adhesions (Zaidel-Bar et al. 2007). Only a small subset of these proteins binds directly to integrins, and the large majority is recruited through protein-protein interactions between the various scaffold proteins (for reviews, see Legate and Fässler 2009; Geiger and Yamada 2011). A large body of structural, biochemical, and genetic evidence points to talin, kindlin, and the integrin-linked kinase (ILK)-pinch-parvin (IPP) complex as some of the central FA components regulating the integrin-actin linkage. All of these proteins are ubiquitously expressed and regulate a wide range of integrin heterodimers. Deletion of any of these proteins leads to early embryonic lethality, which results from compromised integrin function and defects in their connection to the cytoskeleton. On the cell-biological level these defects span the entire range of functions attributed to the integrin-actin linkage. Hence, they can be viewed as global regulators of integrin function. The precise functions of these proteins will be discussed in the next section.
IN VIVO MECHANISMS OF INTEGRIN SIGNALING THROUGH INTRACELLULAR EFFECTORS AND SCAFFOLDS
Talin and kindlin are two FA proteins that directly bind integrins and thereby are involved in the very early events of integrin signaling. They are distinguished from the large group of FA proteins by their ability to regulate both integrin activation (inside-out signaling) and intracellular signaling downstream of ligand binding (outside-in signaling). Talin is present in all multicellular eukaryotes; lower eukaryotes encode only a single talin isoform, whereas vertebrates have two talin isoforms, talin-1 and talin-2. Talin consists of a large carboxy-terminal rod and an amino-terminal head domain composed of a FERM domain with four subdomains: F0, F1, F2, and F3. The F3 subdomain contains a phosphotyrosine-binding motif, which harbors a high affinity binding site for β-integrin tails. The rod domain contains multiple binding sites for the actin–binding protein vinculin as well as for actin itself. In addition it contains a second integrin-binding site (Critchley and Gingras 2008). The talin head has been shown to be sufficient for integrin binding and activation, whereas the rod is required for the scaffolding function of talin in outside-in signaling (Garcia-Alvarez et al. 2003; Tanentzapf and Brown 2006; Tanentzapf et al. 2006).
Kindlins are an evolutionarily conserved family of multidomain proteins, which in mammals consists of three members: kindlin-1 (also known as FERMT1), kindlin-2 (also known as FERMT2 or MIG-2), and kindlin-3 (also known as FERMT3 or URP2). Although encoded by separate genes, they show identical domain architecture and high sequence similarity (Ussar et al. 2006). Like talin, kindlins contain a FERM domain through which they interact with β1, β2, and β3 integrins, but this FERM domain is unique in that it is interrupted by a pleckstrin homology (PH) domain, which provides a putative binding motif for membrane lipids.
Consequences of Loss of Integrin Inside-Out Signaling
Integrins are present on the plasma membrane in low-, intermediate-, and high-affinity states. Structural studies suggest that a low-affinity state is characterized by a bent, “closed” conformation of the extracellular domains and a high-affinity state by an extended, “open” conformation (Campbell and Humphries 2011). The extended conformation is thought to be achieved by separation of the α and β cytoplasmic tails through binding of talin to the β subunit (Moser et al. 2009b; Shattil et al. 2010). Studies in cultured cells have shown that the binding of talin-1 to the cytoplasmic domain of the β-integrin subunit is a common step in β1 and β3 integrin activation in vitro (Tadokoro et al. 2003). In contrast, kindlin alone is not sufficient to activate these integrins, but its co-operation with talin is required to generate fully active integrins (Montanez et al. 2008; Moser et al. 2008, 2009a). The molecular details of this cooperation are not clear.
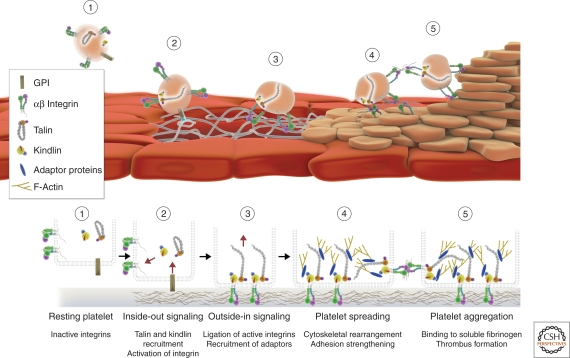
Regulation of the ligand binding affinity is fundamental for various cellular functions. For example, the platelet integrin αIIbβ3 needs to be maintained inactive to prevent it from binding fibrinogen present in the blood stream thus prohibiting platelet aggregation and thrombus formation. On the other hand, this integrin needs to be rapidly activated to mediate platelet adhesion on vascular injury to prevent bleeding (Fig. 3). Similarly, leukocytes require integrin activation to adhere to and migrate across the endothelium to combat pathogens. The importance of this process is illustrated by the phenotypes of talin- and kindlin-deficient mice. Deletion of talin-1 or kindlin-3 in platelets blocks activation of β1 and β3 integrins, leading to the inability of platelets to bind fibrinogen and to form clots. As a result, these mice suffer from severe bleeding (Nieswandt et al. 2007; Petrich et al. 2007; Moser et al. 2008). Similarly, neutrophils lacking kindlin-3 are unable to activate β2 integrins, resulting in loss of neutrophil adhesion to activated endothelial cells (Moser et al. 2009a). Talin has also been shown to bind and activate neutrophil integrin β2 in vitro (Simonson et al. 2006). The importance of integrin affinity regulation in adherent cell types is not as clear. Although deletion of talin and kindlin in mesenchymal or epithelial cells leads to defects in integrin activation, these cells are still capable of adhering, albeit to a reduced extent (Ussar et al. 2008; Zhang et al. 2008), suggesting that under conditions of less stringent requirements for rapid induction of adhesion, high concentrations of ligands, and the absence of high shear flow, avidity regulation might be the predominant mechanism of integrin regulation. The fact that both talin and kindlin are also important in regulation of outside-in signaling makes the in vivo dissection of these two processes difficult.
Figure 3.
Talin and kindlin regulate bidirectional integrin signaling. Bidirectional integrin signaling is essential for platelets (tan) to seal the injured blood vessel endothelium (red) and stop bleeding. Integrins in circulating, resting platelets exist in a low affinity state indicated by a bent confirmation (1). Following vessel injury, von Willebrand factor (vWF) and collagen are exposed to bind their receptors GPIb and GPVI that are expressed on the surface of platelets. Together with locally produced thrombin these receptors trigger the activation of αIIbβ3 integrin. This is achieved by promoting the association of talin-1 and kindlin-3 with the cytoplasmic tail of β3 integrin, facilitating a conformational change in the integrin (inside-out signaling) (2). The conformational change allows integrins to bind fibrinogen, vWF, and fibronectin with high affinity. As a result, platelets adhere to the vessel wall. Integrin ligation subsequently initiates signaling through kindlin and talin (outside-in signaling) (3) resulting in the recruitment of adaptor proteins and rearrangement of the cytoskeleton to promote cell spreading (4). This, together with integrin-mediated binding of soluble fibrinogen, results in platelet aggregation and formation of a stable clot (5). The molecules are not drawn to scale.
Consequences of Loss of Outside-In Signaling
A central piece of evidence that talin has important functions besides integrin activation comes from studies performed with talin-null flies, which show that in the absence of talin, integrins are able to associate with the ECM, but are unable to connect to the cytoskeleton, leading to muscle detachment at the integrin-actin interphase (Brown et al. 2002). Consistent with this finding, mouse fibroblasts lacking both talin-1 and talin-2 are able to adhere, but not to link integrins to the cytoskeleton (Priddle et al. 1998; Zhang et al. 2008). This function requires the talin rod domain, which contains binding sites for F-actin and vinculin indicating that talin provides an essential linkage to the cytoskeleton independent of its ability to activate integrins. Kindlins do not bind actin directly, but connect to the actin cytoskeleton via a migfilin-filamin interaction as well as through the IPP complex (Montanez et al. 2008). Platelets lacking kindlin-3 expression are unable to organize their cytoskeleton or to establish stable lamellipodia (Moser et al. 2008), which probably contributes to their inability to spread and form a crosslinked clot (see Fig. 3). Loss of kindlin-2 impairs actin organization and FA formation in endoderm cells, which together with defective integrin activation leads to early embryonic lethality. Interestingly, the rudimentary FAs in kindlin-2-deficient cells do not contain ILK, suggesting that kindlin acts as a nucleator of FA formation (Montanez et al. 2008).
Despite the central role of talin and kindlin in outside-in signaling, they are not sufficient to establish a stable integrin-actin connection. An important scaffold also required for this function is the IPP complex, which is a constituent of at least β1 and β3 integrin-containing adhesion sites. The core component of this complex is ILK, a pseudokinase that is also capable of binding these integrins directly, at least in vitro (Wickström et al. 2010). Whether the binding is actually relevant for the function of this complex, or whether it occurs in vivo, has not been conclusively shown. The other two members of this complex are pinch and parvin. Pinch, which interacts with the amino-terminal ankyrin repeats of ILK, is a family of proteins containing only LIM domains and consisting of two members, pinch-1 and pinch-2 (Tu et al. 1999, 2001; Chiswell et al. 2008). The parvins, which interact with the carboxy-terminal kinase-homology domain of ILK, are a family of CH-domain-containing proteins with three members; the ubiquitously expressed α-parvin (also known as actopaxin or CH-ILKBP), β-parvin (also known as affixin), which is highly expressed in heart and skeletal muscle, and γ-parvin, which is restricted to the hematopoietic system (Nikolopoulos and Turner 2000; Olski et al. 2001; Tu et al. 2001; Yamaji et al. 2001; Chu et al. 2006). The IPP complex is thought to be preassembled in the cytoplasm and is subsequently recruited to integrin adhesions in an ILK-dependent manner (Zhang et al. 2002). The stability of each individual component depends on the assembly of the complex, as depletion of ILK or pinch leads to a decrease in the protein levels of the other two complex members (Fukuda et al. 2003; Li et al. 2005).
The central biological function of the IPP complex is the regulation of the cytoskeleton downstream of integrins, although a number of additional functions for this complex as well as its individual components have been assigned by in vitro studies. The importance of these proteins in the integrin-actin connection is highlighted by deletion studies in mice, where deletion of ILK or pinch-1 results in peri-implantation lethality caused by severe defects in F-actin organization at adhesion sites, leading to a failure in epiblast polarization (Sakai et al. 2003; Li et al. 2005). The role of parvins is more complex because of the presence of three structurally very similar isoforms. Mice lacking β- or γ-parvin show no obvious phenotypes, whereas α-parvin-null mice survive up to embryonic day 14.5 and die because of defects in cardiovascular development (Chu et al. 2006; Montanez et al. 2009; I Thievessen and R Fässler, unpubl.). The α/β-parvin double knock-out results in early embryonic lethality, suggesting that parvins can compensate for each other during early development (E. Montanez and R. Fässler, unpubl.). Caenorhabditis elegans expresses only single orthologs for pinch and parvin, and is thus a suitable model organism for a more straightforward analysis of the IPP complex. Studies in C. elegans have shown that ILK (PAT-4), pinch (UNC-97), and parvin (PAT-6) colocalize with β integrin (PAT-3) at muscle attachment sites, where a robust connection of cells to the cytoskeleton is required during muscle contraction. Deletion of β integrin or any member of the IPP complex leads to detachment of muscles from the body wall and embryonic lethality (Mackinnon et al. 2002; Lin et al. 2003; Norman et al. 2007).
On the cellular level, ILK-deficiency leads to compromised cell adhesion, spreading and migration. This is because of defective recruitment of the cytoskeleton to adhesion sites, resulting in defects in FA maturation and cytoskeletal remodeling. The defective maturation of ILK-deficient FAs into fibrillar adhesions subsequently leads to impaired deposition of the FN matrix (Sakai et al. 2003; Stanchi et al. 2009). The precise molecular mechanism by which ILK regulates the cytoskeleton is not clear. Parvins are capable of interacting with F-actin through their two CH domains, but as these domains also regulate the interaction of parvins with ILK and paxillin, they might not be available for actin binding. Therefore, it is likely that the IPP complex requires additional downstream partners for the regulation of the actin cytoskeleton, and the identification of these proteins is a central focus of future research. It is, however, clear that the presence of parvin in the IPP complex is critical for the regulation of the actin cytoskeleton. The absence of α-parvin in mice causes impaired migration of vascular smooth muscle cells (vSMC) toward developing vessels resulting in defective stabilization of the vasculature and subsequent dilation of vessels, formation of microaneurysms and vessel rupture. The migration defect is caused by aberrant actomyosin contractility (Montanez et al. 2009), and can be phenocopied by vSMC-specific ablation of ILK (Kogata et al. 2009). However, this function seems to be restricted to certain cell types, suggesting that although the central role of the IPP complex in integrin outside-in signaling is ubiquitous, the precise molecular mechanisms might be cell type-specific to accommodate the specialized needs of various cell and tissue types.
GENETIC DISEASES OF CELL–MATRIX INTERACTIONS
Aberrant cell–matrix interactions are involved in a large number of pathological conditions such as cancer and various inflammatory diseases. Altered function of this signaling axis is however rarely the actual cause of these disorders. In contrast, recent studies have identified a panel of genetic diseases whose etiology can be pinpointed to a mutation in a component of the adhesive machinery. The high degree of conservation in the components of this pathway has allowed the generation of mouse models for these diseases that can be analyzed to generate mechanistic insights and therapeutic modalities for clinical applications.
Integrin Activation Diseases
As discussed earlier, leukocyte and platelet adhesion are cellular events in which integrin affinity regulation is of key importance. Inherited human diseases with defects in these processes have been described more than 25 years ago, but only recently, after the discovery of the central role of talin and kindlin in integrin activation, the causes for these diseases have been identified.
There are currently three distinct syndromes that together constitute the leukocyte adhesion deficiency (LAD) family of diseases. They affect distinct phases of the leukocyte adhesion cascade and therefore cause symptoms of variable severity. LAD I, a disease that affects several hundreds of patients worldwide, results from impaired firm adhesion of leukocytes, leading to recurrent severe infections and impaired wound healing. This disease is caused by a range of mutations in the β2 integrin (ITGB2) gene, including deletions, truncations, substitutions, frame shifts, and intronic mutations, resulting in loss of protein expression or expression of a truncated protein. LAD III (also known as LAD I/variant), which has been more recently described, is characterized by similar symptoms as LAD I. Interestingly, these patients also suffer from a bleeding tendency, indicating that additional β subunits are involved. Indeed, these patients show defects in β1, β2, and β3 integrin activation (Kuijpers et al. 1997; Alon and Etzioni 2003). In contrast to LAD I, no mutations in integrin genes have been identified, and the cause of this disease remained unknown until the role of kindlin in integrin activation was discovered. Subsequently, genetic sequencing of LAD III patients has revealed mutations in the kindlin 3 (FRMT3) gene in all patients tested so far, leading to expression of a truncated protein, or reduction or loss of protein expression (Mory et al. 2008; Kuijpers et al. 2009; Malinin et al. 2009; Svensson et al. 2009). Transfection of patient’s lymphocytes with wild-type kindlin-3 was shown to restore integrin activation (Malinin et al. 2009; Svensson et al. 2009). In addition, kindlin-3-deficient mice recapitulate all symptoms of LAD III, firmly identifying defective kindlin-3 as the cause of LAD III (Moser et al. 2008; Moser et al. 2009a). In this respect, reconstitution of kindlin-3 expression provides a possible therapeutic modality for this disease. Interestingly, the naturally occurring mutations have also provided information on the structure-function aspects of kindlin-3. A homozygous stop codon identified in three patients occurs distal to the PTB-containing integrin-binding subdomain of kindlin-3, suggesting that the carboxyl terminus of kindlin is required for its function in integrin activation (Mory et al. 2008). A point mutation leading to a truncation in the middle of the PH-domain was further shown to impair membrane localization of kindlin-3 and thereby block both lymphocyte adhesion and migration, whereas a point mutation in the F2 subdomain inhibited only the migration of these cells, demonstrating that the functions of kindlin in inside-out and outside-in signaling can be uncoupled (McDowall et al. 2010).
The bleeding tendency of LAD III patients closely resembles that of Glanzmann’s thrombasthenia (GT), a rare, autosomal recessive bleeding disorder caused by mutations leading to quantitative or qualitative defects in platelet αIIbβ3 integrin. The genetic background is very heterogeneous: more than 100 different mutations in either the αIIb (ITGA2B) or β3 integrin (ITGB3) genes have been reported so far (Kannan and Saxena 2009). Genetic ablation of the β3 integrin in mice almost fully recapitulates the human disease, including gastrointestinal and cutaneous hemorrhage, increased bleeding time, reduced platelet aggregation, and clot retraction (Hodivala-Dilke et al. 1999). However, the heterogeneity in the mutation spectrum of the integrins found in GT patients also applies to the symptoms; siblings sharing the same mutation can display symptoms of varying severity, suggesting that polymorphisms in other genes involved in hemostasis could influence the phenotype. In addition, several GT patients lacking a mutation in the αIIbβ3 integrin have been identified (Kannan and Saxena 2009). As the bleeding phenotype of the kindlin-3-deficient mice and the LADIII patients closely resemble GT, it is possible that altered function of this protein might play a role in this disease as well. However, no polymorphisms in the kindlin-3 gene in GT patients have been reported so far. Taken together, integrin activation plays a central role in immune function and hemostasis. The prominent role of kindlin-3 in these processes together with its restricted expression pattern makes it a promising candidate for anti-inflammatory and antithrombotic therapies.
Adhesion Strengthening Diseases
Another “class” of diseases involving impaired cell–matrix interactions can be found affecting tissues that are subjected to high levels of mechanical stress such as the skin and skeletal muscle. The common denominator of these disorders, namely muscular dystrophies and skin-blistering diseases, is that the symptoms are caused by weakened interactions of cells with their extracellular environment, leading to tissue dysfunction and disruption. Another common feature is that causative mutations for a single disorder can be found in either components of the ECM, their integrin receptors, or adhesion-strengthening scaffold proteins. In the case of congenital muscular dystrophies, although the dystrophin-dystroglycan-laminin/agrin axis is a common target (Yurchenco 2010), other mutations affect the laminin-211/α7β1 integrin/plectin axis, leading to defects in organization and migration of myoblasts, subsequent myofiber degeneration and progressive muscle weakness (for more detailed review see Kanagawa and Toda 2006; Mendell et al. 2006). Clinical features, cellular defects and their corresponding causative mutations of muscular dystrophies resulting from defective cell-ECM interaction are summarized in Table 1.
Table 1.
Selected mutations causing muscular dystrophy and their corresponding mouse models
| Human disease | Central clinical features | Affected gene (protein) | Cellular defect | Mouse model | Reference |
|---|---|---|---|---|---|
| Limb-Girdle muscular dystrophy | Early adulthood onset proximal muscle weakness, nasal pattern of speech | MYOT (myotilin) | Sarcomeric organization | N/A | – |
| Severe, early onset muscle weakness | SGCA (α sarcoglycan) | Muscle membrane integrity | KO | (Duclos et al. 1998) | |
| SGCB (β sarcoglycan) | KO | (Durbeej et al. 2000) | |||
| SGCG (γ sarcoglycan) | KO | (Sasaoka et al. 2003) | |||
| SGCD (δ sarcoglycan) | KO | (Coral-Vazquez et al. 1999) | |||
| Late onset muscle weakness and atrophy, cardiomyopathy | TTN (titin) | Sarcomere contraction /relaxation | N/A | – | |
| Relatively mild muscle weakness, variable clinical features | TCAP (telethonin) | Sarcomere stability | N/A | – | |
| Congenital muscular dystrophy | Early onset with variable symptoms, hypotonia, torticollis | ITGA7 (integrin α7) | Impaired muscle cell–matrix interactions | KO | (Mayer et al. 1997) |
| Early onset hypotonia and muscle weakness |
COL6A1 COL6A2 COL6A3 (type VI collagen) |
Stability of extracellular matrix | KOa | (Bonaldo et al. 1998) | |
| Severe, early onset muscle weakness, white matter hypodensity, mental retardation | LAMA2 (laminin 211) | Attachment, migration and organization of myoblasts | Dy/Dy (naturally occurring mutant) | (Sunada et al. 1994) | |
| Dy2j/Dy2j (naturally occurring mutant) | (Xu et al. 1994) | ||||
| DyPas/DyPas (naturally occurring null allele) | (Besse et al. 2003) | ||||
| Dy3K/Dy3K (KO) | (Miyagoe et al. 1997) | ||||
| DyW/DyW (hypomorph) | (Kuang et al. 1998) | ||||
| Duchenne muscular dystrophy | Severe, early onset muscle weakness with respiratory, orthopedic and cardiac complications | DMD (dystrophin) | Stabilization of membrane during contraction | Mdx (naturally occurring null allele) | (Bulfield et al. 1984) |
| Mdx 2Cv, Mdx 3Cv Mdx 4Cv, Mdx 5Cvb |
(Chapman et al. 1989) | ||||
| Mdx 52 (deletion of exon 52) | (Araki et al. 1997) |
aAblation of the col6a1 gene.
bMutants recovered from ENU chemical mutagenesis screen.
Abbreviations: N/A, not analyzed; KO, knockout mouse; Dy, Dystrophin; mdx, X chromosome-linked muscular dystrophy.
The same principles are true for epidermolysis bullosa (EB), the prototype of human skin-blistering diseases (Uitto 2009). EB is a group of highly variable disorders characterized by fragility of skin leading to blistering and erosions on mechanical trauma. Genetic studies on different variants of EB have so far shown mutations in 12 distinct genes encoding structural components of the BM and adhesion proteins (Table 2). Interestingly, some of the EB patients also develop muscular dystrophy, underlining the pathophysiological link between these two diseases. As the histopathology of these diseases is very complex, the identification of the causative mutation has become a central diagnostic tool of EB (Nagy and McGrath 2010). Genetic mouse models for the different EB variants are available, and they have been used to work out therapeutic strategies for these diseases (Natsuga et al. 2010). As a result, gene delivery, ectopic protein replacement as well as cellular therapies have already been tested on EB patients (Uitto 2009).
Table 2.
Mutations causing epidermolysis bullosa (EB) and their corresponding mouse models
| Human disease | Site of tissue separation | Affected gene (protein) | Mouse model | Mouse skin phenotype | Reference |
|---|---|---|---|---|---|
| Epidermolysis bullosa simplex (EBS) | Intraepidermal | PKP1 (plakophilin-1) | N/A | – | – |
| DSP (desmoplakin) | N/A | – | – | ||
| KRT5 (keratin-5) | KOa | Severe blistering, loss of keratin filaments | (Peters et al. 2001) | ||
| KRT14 (keratin-14) | KO | Blistering, decreased keratin filaments | (Lloyd et al. 1995) | ||
| K14-R131C KIa | Severe blistering | (Cao et al. 2001) | |||
| K14-ΔCT TG | Blistering | (Vassar et al. 1991) | |||
| PLEC1 (plectin) | KOa | Blistering, reduced stability and number of hemidesmosomes | (Andra et al. 1997) | ||
| Conditional KO | Blistering, skin fragility | (Ackerl et al. 2007) | |||
| Junctional epidermolysis bullosa (JBS) | Lamina lucida | ITGA6 (integrin α6) | KOa | Severe blistering, loss of hemidesmosomes | (Georges-Labouesse et al. 1996a) |
| ITGB4 (integrin β4) | KOa | Severe blistering, loss of hemidesmosomes | (Dowling et al. 1996; van der Neut et al. 1996) | ||
| ITGB4-ΔCT KIa | Severe blistering, loss of hemidesmosomes, hypoproliferation | (Murgia et al. 1998) | |||
| Conditional KO | Blistering, loss of hemidesmosomes | (Raymond et al. 2005) | |||
| LAMA3 (laminin-332) | KOa | Severe blistering, abnormal hemidesmosomes, reduced cell survival | (Ryan et al. 1999) | ||
| LAMB3 (laminin-332) | Spontaneous null allele | Blistering, abnormal hemidesmosomes | (Kuster et al. 1997) | ||
| LAMC2 (laminin-332) | KOa | Blistering, rudimentary hemidesmosomes, apoptosis | (Meng et al. 2003) | ||
| Spontaneous hypomorphic allele | Blistering, ulcerations, hyperkeratosis, rudimentary hemidesmosomes | (Bubier et al. 2010) | |||
| Dystrophic epidermolysis bullosa (DBS) | Sub-lamina densa | COL7A1 (type VII collagen) | KOa | Severe blistering, absence of anchoring fibrils | (Heinonen et al. 1999) |
| COL7 hypomorph | Severe blistering, decreased anchoring fibrils | (Fritsch et al. 2008) | |||
| Kindler syndrome | Primarily lamina lucida | FERMT1 (kindlin-1) | KOa | Skin atrophy | (Ussar et al. 2008) |
aLeads to early postnatal lethality.
Abbreviations used: N/A, not analyzed; KO, knock-out mouse; K14-R131C, Arginine-131 to Cysteine substitution in the Keratin-14 gene; KI, knock in mouse; K14-ΔCT, carboxy-terminal truncation of Keratin-14; ITGB4-ΔCT, deletion of the entire cytoplamsic domain of integrin β4; TG, transgenic mouse.
CONCLUDING REMARKS
Two decades of genetic studies on cell-ECM interactions have illustrated the importance of adhesion signaling in the development of multicellular organisms as well as in disease. It is now clear that these interactions not only provide structural support and positional cues to guide morphogenesis, but also act as signaling platforms to regulate cell fate in a highly tissue-specific manner. It is also evident that the relationship between ECM proteins and their integrin receptors is complex: Integrins transmit information from the ECM to the cell, but also regulate the deposition and remodeling of the matrix itself. In addition, the ECM functions as a reservoir for growth factors, whereas integrins cross talk with growth factor signaling on various levels. Because of this complexity, mechanistic interpretations of the various phenotypes caused by genetic ablation of individual components of the adhesion signaling machinery have proven to be difficult. Thus, a major challenge for the future is to understand which types of intracellular signals directly emanate from integrin adhesions, and how these signals are propagated. This requires generation of more sophisticated mouse models, such as knock-in mice carrying point mutations that only partially disrupt protein function together with reporter mice for specific signaling events, transcriptional changes, or changes in protein-protein interactions or conformations. In addition, a large majority of the cell-biological analyses on integrin signaling are still performed on rigid 2D-surfaces, which very poorly recapitulate the more compliant and complex 3D environment of tissues. As our understanding of the biophysical properties of the ECM in tissues steadily increases, a major goal of future research is to translate this knowledge into generation of more relevant in vitro models for cell biological studies of integrin signaling.
ACKNOWLEDGMENTS
The authors apologize to all those whose work could not be cited because of space restrictions and for not always citing all the primary literature. The authors would like to thank Max Iglesias for artwork. The work of the Wickström and Fässler laboratories are funded by the Max Planck Society.
Footnotes
Editors: Richard Hynes and Kenneth Yamada
Additional Perspectives on Extracellular Matrix Biology available at www.cshperspectives.org
REFERENCES
- Ackerl R, Walko G, Fuchs P, Fischer I, Schmuth M, Wiche G 2007. Conditional targeting of plectin in prenatal and adult mouse stratified epithelia causes keratinocyte fragility and lesional epidermal barrier defects. J Cell Sci 120: 2435–2443 [DOI] [PubMed] [Google Scholar]
- Alon R, Etzioni A 2003. LAD-III, a novel group of leukocyte integrin activation deficiencies. Trends Immunol 24: 561–566 [DOI] [PubMed] [Google Scholar]
- Alt A, Ohba M, Li L, Gartsbein M, Belanger A, Denning MF, Kuroki T, Yuspa SH, Tennenbaum T 2001. Protein kinase Cδ-mediated phosphorylation of α6β4 is associated with reduced integrin localization to the hemidesmosome and decreased keratinocyte attachment. Cancer Res 61: 4591–4598 [PubMed] [Google Scholar]
- Andra K, Lassmann H, Bittner R, Shorny S, Fässler R, Propst F, Wiche G 1997. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev 11: 3143–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki E, Nakamura K, Nakao K, Kameya S, Kobayashi O, Nonaka I, Kobayashi T, Katsuki M 1997. Targeted disruption of exon 52 in the mouse dystrophin gene induced muscle degeneration similar to that observed in Duchenne muscular dystrophy. Biochem Biophys Res Commun 238: 492–497 [DOI] [PubMed] [Google Scholar]
- Astrof S, Crowley D, Hynes RO 2007. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol 311: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO 1998. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 95: 507–519 [DOI] [PubMed] [Google Scholar]
- Bauer M, Brakebusch C, Coisne C, Sixt M, Wekerle H, Engelhardt B, Fässler R 2009. Beta1 integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity. Proc Natl Acad Sci 106: 1920–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Mäkinen T 2009. Integrin-α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell 17: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertotti A, Comoglio PM, Trusolino L 2006. β4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J Cell Biol 175: 993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse S, Allamand V, Vilquin JT, Li Z, Poirier C, Vignier N, Hori H, Guenet JL, Guicheney P 2003. Spontaneous muscular dystrophy caused by a retrotransposal insertion in the mouse laminin α2 chain gene. Neuromuscul Disord 13: 216–222 [DOI] [PubMed] [Google Scholar]
- Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM 1998. Collagen VI deficiency induces early onset myopathy in the mouse: An animal model for Bethlem myopathy. Hum Mol Genet 7: 2135–2140 [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, et al. 2000. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J 19: 3990–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NH, Gregory SL, Rickoll WL, Fessler LI, Prout M, White RA, Fristrom JW 2002. Talin is essential for integrin function in Drosophila. Dev Cell 3: 569–579 [DOI] [PubMed] [Google Scholar]
- Bubier JA, Sproule TJ, Alley LM, Webb CM, Fine JD, Roopenian DC, Sundberg JP 2010. A mouse model of generalized non-Herlitz junctional epidermolysis bullosa. J Invest Dermatol 130: 1819–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA, Moore KJ 1984. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci 81: 1189–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ID, Humphries MJ 2011. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T, Longley MA, Wang XJ, Roop DR 2001. An inducible mouse model for epidermolysis bullosa simplex: Implications for gene therapy. J Cell Biol 152: 651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman VM, Miller DR, Armstrong D, Caskey CT 1989. Recovery of induced mutations for X chromosome-linked muscular dystrophy in mice. Proc Natl Acad Sci 86: 1292–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiswell BP, Zhang R, Murphy JW, Boggon TJ, Calderwood DA 2008. The structural basis of integrin-linked kinase-PINCH interactions. Proc Natl Acad Sci 105: 20677–20682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Thievessen I, Sixt M, Lammermann T, Waisman A, Braun A, Noegel AA, Fässler R 2006. gamma-Parvin is dispensable for hematopoiesis, leukocyte trafficking, and T-cell-dependent antibody response. Mol Cell Biol 26: 1817–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coral-Vazquez R, Cohn RD, Moore SA, Hill JA, Weiss RM, Davisson RL, Straub V, Barresi R, Bansal D, Hrstka RF, et al. 1999. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: A novel mechanism for cardiomyopathy and muscular dystrophy. Cell 98: 465–474 [DOI] [PubMed] [Google Scholar]
- Critchley DR, Gingras AR 2008. Talin at a glance. J Cell Sci 121: 1345–1347 [DOI] [PubMed] [Google Scholar]
- de Pereda JM, Lillo MP, Sonnenberg A 2009. Structural basis of the interaction between integrin α6β4 and plectin at the hemidesmosomes. EMBO J 28: 1180–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, van der Neut R, Georges-Labouesse E, Kreidberg JA, Sonnenberg A, Hynes RO 2000. αa3β1 and α6β4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J Cell Sci 113: 3051–3062 [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E 1996. βa4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol 134: 559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos F, Straub V, Moore SA, Venzke DP, Hrstka RF, Crosbie RH, Durbeej M, Lebakken CS, Ettinger AJ, van der Meulen J, et al. 1998. Progressive muscular dystrophy in α-sarcoglycan-deficient mice. J Cell Biol 142: 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M, Cohn RD, Hrstka RF, Moore SA, Allamand V, Davidson BL, Williamson RA, Campbell KP 2000. Disruption of the β-sarcoglycan gene reveals pathogenetic complexity of limb-girdle muscular dystrophy type 2E. Mol Cell 5: 141–151 [DOI] [PubMed] [Google Scholar]
- Erickson AC, Couchman JR 2000. Still more complexity in mammalian basement membranes. J Histochem Cytochem 48: 1291–1306 [DOI] [PubMed] [Google Scholar]
- Fritsch A, Loeckermann S, Kern JS, Braun A, Bosl MR, Bley TA, Schumann H, von Elverfeldt D, Paul D, Erlacher M, et al. 2008. A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J Clin Invest 118: 1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Chen K, Shi X, Wu C 2003. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J Biol Chem 278: 51324–51333 [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC 2003. Structural determinants of integrin recognition by talin. Mol Cell 11: 49–58 [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, Feral C, Papayannopoulou T, Lowy A, Blair SL, et al. 2010. Integrin α4β1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res 70: 3042–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts D, Fontao L, Nievers MG, Schaapveld RQ, Purkis PE, Wheeler GN, Lane EB, Leigh IM, Sonnenberg A 1999. Binding of integrin α6β4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J Cell Biol 147: 417–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Yamada KM 2011. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Baldwin HS, Hynes RO 1997. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood 90: 3073–3081 [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO 1993. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119: 1079–1091 [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse EN, George EL, Rayburn H, Hynes RO 1996b. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn 207: 145–156 [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M 1996a. Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 13: 370–373 [DOI] [PubMed] [Google Scholar]
- Geuijen CA, Sonnenberg A 2002. Dynamics of the alpha6beta4 integrin in keratinocytes. Mol Biol Cell 13: 3845–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG 2007. Targeting integrin β4 for cancer and anti-angiogenic therapy. Trends Pharmacol Sci 28: 506–511 [DOI] [PubMed] [Google Scholar]
- Guan JL, Hynes RO 1990. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor α4 β1. Cell 60: 53–61 [DOI] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, Fuchs E 1995. Gene targeting of BPAG1: Abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell 81: 233–243 [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG 2006. β4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126: 489–502 [DOI] [PubMed] [Google Scholar]
- Heinonen S, Mannikko M, Klement JF, Whitaker-Menezes D, Murphy GF, Uitto J 1999. Targeted inactivation of the type VII collagen gene (Col7a1) in mice results in severe blistering phenotype: A model for recessive dystrophic epidermolysis bullosa. J Cell Sci 112: 3641–3648 [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO 1999. β4-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 103: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV Jr, Sheppard D 2000. Fatal bilateral chylothorax in mice lacking the integrin α9β1. Mol Cell Biol 20: 5208–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ 2006. Integrin ligands at a glance. J Cell Sci 119: 3901–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO 2002. Integrins: Bidirectional, allosteric signaling machines. Cell 110: 673–687 [DOI] [PubMed] [Google Scholar]
- Hynes RO 2009. The extracellular matrix: Not just pretty fibrils. Science 326: 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Heino J 2010. Interplay between cell adhesion and growth factor receptors: From the plasma membrane to the endosomes. Cell Tissue Res 339: 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Toda T 2006. The genetic and molecular basis of muscular dystrophy: Roles of cell-matrix linkage in the pathogenesis. J Hum Genet 51: 915–926 [DOI] [PubMed] [Google Scholar]
- Kannan M, Saxena R 2009. Glanzmann’s thrombasthenia: An overview. Clin Appl Thromb Hemost 15: 152–165 [DOI] [PubMed] [Google Scholar]
- Kennel SJ, Foote LJ, Lankford PK 1981. Analysis of surface proteins of mouse lung carcinomas using monoclonal antibodies. Cancer Res 41: 3465–3470 [PubMed] [Google Scholar]
- Kogata N, Tribe RM, Fässler R, Way M, Adams RH 2009. Integrin-linked kinase controls vascular wall formation by negatively regulating Rho/ROCK-mediated vascular smooth muscle cell contraction. Genes Dev 23: 2278–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster J, Kuikman I, Kreft M, Sonnenberg A 2001. Two different mutations in the cytoplasmic domain of the integrin β4 subunit in nonlethal forms of epidermolysis bullosa prevent interaction of β4 with plectin. J Invest Dermatol 117: 1405–1411 [DOI] [PubMed] [Google Scholar]
- Koster J, van Wilpe S, Kuikman I, Litjens SH, Sonnenberg A 2004. Role of binding of plectin to the integrin β4 subunit in the assembly of hemidesmosomes. Mol Biol Cell 15: 1211–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vachon PH, Liu L, Loechel F, Wewer UM, Engvall E 1998. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J Clin Invest 102: 844–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Van Lier RA, Hamann D, de Boer M, Thung LY, Weening RS, Verhoeven AJ, Roos D 1997. Leukocyte adhesion deficiency type 1 (LAD-1)/variant. A novel immunodeficiency syndrome characterized by dysfunctional beta2 integrins. J Clin Invest 100: 1725–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, van de Vijver E, Weterman MA, de Boer M, Tool AT, van den Berg TK, Moser M, Jakobs ME, Seeger K, Sanal O, et al. 2009. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood 113: 4740–4746 [DOI] [PubMed] [Google Scholar]
- Kuster JE, Guarnieri MH, Ault JG, Flaherty L, Swiatek PJ 1997. IAP insertion in the murine LamB3 gene results in junctional epidermolysis bullosa. Mamm Genome 8: 673–681 [DOI] [PubMed] [Google Scholar]
- Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA 1995. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development 121: 489–503 [DOI] [PubMed] [Google Scholar]
- Legate KR, Fässler R 2009. Mechanisms that regulate adaptor binding to {β}-integrin cytoplasmic tails. J Cell Sci 122: 187–198 [DOI] [PubMed] [Google Scholar]
- Legate KR, Wickström SA, Fässler R 2009. Genetic and cell biological analysis of integrin outside-in signaling. Genes & Dev 23: 397–418 [DOI] [PubMed] [Google Scholar]
- Leiss M, Beckmann K, Giros A, Costell M, Fässler R 2008. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr Opin Cell Biol 20: 502–507 [DOI] [PubMed] [Google Scholar]
- Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fässler R 2005. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci 118: 2913–2921 [DOI] [PubMed] [Google Scholar]
- Lin X, Qadota H, Moerman DG, Williams BD 2003. C. elegans PAT-6/actopaxin plays a critical role in the assembly of integrin adhesion complexes in vivo. Curr Biol 13: 922–932 [DOI] [PubMed] [Google Scholar]
- Litjens SH, de Pereda JM, Sonnenberg A 2006. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol 16: 376–383 [DOI] [PubMed] [Google Scholar]
- Litjens SH, Koster J, Kuikman I, van Wilpe S, de Pereda JM, Sonnenberg A 2003. Specificity of binding of the plectin actin-binding domain to β4 integrin. Mol Biol Cell 14: 4039–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C, Yu QC, Cheng J, Turksen K, Degenstein L, Hutton E, Fuchs E 1995. The basal keratin network of stratified squamous epithelia: Defining K15 function in the absence of K14. J Cell Biol 129: 1329–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD 2002. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol 12: 787–797 [DOI] [PubMed] [Google Scholar]
- Malinin NL, Zhang L, Choi J, Ciocea A, Razorenova O, Ma YQ, Podrez EA, Tosi M, Lennon DP, Caplan AI, et al. 2009. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med 15: 313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE 2005. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 24: 389–399 [DOI] [PubMed] [Google Scholar]
- Margadant C, Frijns E, Wilhelmsen K, Sonnenberg A 2008. Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol 20: 589–596 [DOI] [PubMed] [Google Scholar]
- Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG 2001. EGF-R signaling through Fyn kinase disrupts the function of integrin α6β4 at hemidesmosomes: Role in epithelial cell migration and carcinoma invasion. J Cell Biol 155: 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fässler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K 1997. Absence of integrin α7 causes a novel form of muscular dystrophy. Nat Genet 17: 318–323 [DOI] [PubMed] [Google Scholar]
- McDowall A, Svensson L, Stanley P, Patzak I, Chakravarty P, Howarth K, Sabnis H, Briones M, Hogg N 2010. Two mutations in the KINDLIN3 gene of a new leukocyte adhesion deficiency-III patient reveal distinct effects on leukocyte function in vitro. Blood 115: 4834–4842 [DOI] [PubMed] [Google Scholar]
- Mendell JR, Boue DR, Martin PT 2006. The congenital muscular dystrophies: Recent advances and molecular insights. Pediatr Dev Pathol 9: 427–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Klement JF, Leperi DA, Birk DE, Sasaki T, Timpl R, Uitto J, Pulkkinen L 2003. Targeted inactivation of murine laminin γ2-chain gene recapitulates human junctional epidermolysis bullosa. J Invest Dermatol 121: 720–731 [DOI] [PubMed] [Google Scholar]
- Miner JH 2008. Laminins and their roles in mammals. Microsc Res Tech 71: 349–356 [DOI] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE 2004. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 131: 2247–2256 [DOI] [PubMed] [Google Scholar]
- Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Nabeshima Y, Takeda S 1997. Laminin α2 chain-null mutant mice by targeted disruption of the Lama2 gene: A new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett 415: 33–39 [DOI] [PubMed] [Google Scholar]
- Montanez E, Ussar S, Schifferer M, Bosl M, Zent R, Moser M, Fässler R 2008. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev 22: 1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez E, Wickström SA, Altstätter J, Chu H, Fässler R 2009. αa-parvin controls vascular mural cell recruitment to vessel wall by regulating RhoA/ROCK signalling. EMBO J 28: 3132–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mory A, Feigelson SW, Yarali N, Kilic SS, Bayhan GI, Gershoni-Baruch R, Etzioni A, Alon R 2008. Kindlin-3: A new gene involved in the pathogenesis of LAD-III. Blood 112: 2591. [DOI] [PubMed] [Google Scholar]
- Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, Wang HV, Sperandio M, Fässler R 2009a. Kindlin-3 is required for β2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med 15: 300–305 [DOI] [PubMed] [Google Scholar]
- Moser M, Legate KR, Zent R, Fässler R 2009b. The tail of integrins, talin, and kindlins. Science 324: 895–899 [DOI] [PubMed] [Google Scholar]
- Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R 2008. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med 14: 325–330 [DOI] [PubMed] [Google Scholar]
- Murgia C, Blaikie P, Kim N, Dans M, Petrie HT, Giancotti FG 1998. Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin beta4 cytoplasmic domain. EMBO J 17: 3940–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N, McGrath JA 2010. Blistering skin diseases: A bridge between dermatopathology and molecular biology. Histopathology 56 91–99 [DOI] [PubMed] [Google Scholar]
- Natsuga K, Shinkuma S, Nishie W, Shimizu H 2010. Animal models of epidermolysis bullosa. Dermatol Clin 28: 137–142 [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Moser M, Pleines I, Varga-Szabo D, Monkley S, Critchley D, Fässler R 2007. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med 204: 3113–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievers MG, Kuikman I, Geerts D, Leigh IM, Sonnenberg A 2000. Formation of hemidesmosome-like structures in the absence of ligand binding by the (α)6(β)4 integrin requires binding of HD1/plectin to the cytoplasmic domain of the (β)4 integrin subunit. J Cell Sci 113: 963–973 [DOI] [PubMed] [Google Scholar]
- Nievers MG, Schaapveld RQ, Oomen LC, Fontao L, Geerts D, Sonnenberg A 1998. Ligand-independent role of the β4 integrin subunit in the formation of hemidesmosomes. J Cell Sci 111: 1659–1672 [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Turner CE 2000. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol 151: 1435–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG 2004. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell 6: 471–483 [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Puri C, Tacchetti C, Giancotti FG 2005. Targeted deletion of the integrin β4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-κB, causing defects in epidermal growth and migration. Mol Cell Biol 25: 6090–6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, Sakai K, Nakamura H, Olasz E, Yancey KB, et al. 2007. Humanization of autoantigen. Nat Med 13: 378–383 [DOI] [PubMed] [Google Scholar]
- Norman KR, Cordes S, Qadota H, Rahmani P, Moerman DG 2007. UNC-97/PINCH is involved in the assembly of integrin cell adhesion complexes in Caenorhabditis elegans body wall muscle. Dev Biol 309: 45–55 [DOI] [PubMed] [Google Scholar]
- Olski TM, Noegel AA, Korenbaum E 2001. Parvin, a 42 kDa focal adhesion protein, related to the α-actinin superfamily. J Cell Sci 114: 525–538 [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM 2002. Fibronectin at a glance. J Cell Sci 115: 3861–3863 [DOI] [PubMed] [Google Scholar]
- Peters B, Kirfel J, Bussow H, Vidal M, Magin TM 2001. Complete cytolysis and neonatal lethality in keratin 5 knockout mice reveal its fundamental role in skin integrity and in epidermolysis bullosa simplex. Mol Biol Cell 12: 1775–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR, et al. 2007. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med 204: 3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddle H, Hemmings L, Monkley S, Woods A, Patel B, Sutton D, Dunn GA, Zicha D, Critchley DR 1998. Disruption of the talin gene compromises focal adhesion assembly in undifferentiated but not differentiated embryonic stem cells. J Cell Biol 142: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I, Toker A, Mercurio AM 1999. Protein kinase C-dependent mobilization of the α6β4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol 146: 1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I, Tsomo L, Mercurio AM 2004. Protein kinase C-α phosphorylation of specific serines in the connecting segment of the β4 integrin regulates the dynamics of type II hemidesmosomes. Mol Cell Biol 24: 4351–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond K, Kreft M, Janssen H, Calafat J, Sonnenberg A 2005. Keratinocytes display normal proliferation, survival and differentiation in conditional β4-integrin knockout mice. J Cell Sci 118: 1045–1060 [DOI] [PubMed] [Google Scholar]
- Rezniczek GA, de Pereda JM, Reipert S, Wiche G 1998. Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeleton: Direct interaction between the β4 subunit and plectin at multiple molecular sites. J Cell Biol 141: 209–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Lee K, Miyashita Y, Carter WG 1999. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol 145: 1309–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fässler R 2003. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes & Dev 17: 926–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MM, Gaudino G, Marchisio PC 2003. The MSP receptor regulates α6β4 and α3β1 integrins via 14–3-3 proteins in keratinocyte migration. Dev Cell 5: 257–271 [DOI] [PubMed] [Google Scholar]
- Sasaoka T, Imamura M, Araishi K, Noguchi S, Mizuno Y, Takagoshi N, Hama H, Wakabayashi-Takai E, Yoshimoto-Matsuda Y, Nonaka I, et al. 2003. Pathological analysis of muscle hypertrophy and degeneration in muscular dystrophy in gamma-sarcoglycan-deficient mice. Neuromuscul Disord 13: 193–206 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE, DeSimone DW 2011. Deciphering the mechanisms of fibronectin matrix assembly. Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Kim C, Ginsberg MH 2010. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 11: 288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson WT, Franco SJ, Huttenlocher A 2006. Talin1 regulates TCR-mediated LFA-1 function. J Immunol 177: 7707–7714 [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D 1999. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol 144: 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane M, Carpio Y, Geisler R, Schwarz H, Maischein HM, Nuesslein-Volhard C 2005. Zebrafish penner/lethal giant larvae 2 functions in hemidesmosome formation, maintenance of cellular morphology and growth regulation in the developing basal epidermis. Development 132: 3255–3265 [DOI] [PubMed] [Google Scholar]
- Sonawane M, Martin-Maischein H, Schwarz H, Nusslein-Volhard C 2009. Lgl2 and E-cadherin act antagonistically to regulate hemidesmosome formation during epidermal development in zebrafish. Development 136: 1231–1240 [DOI] [PubMed] [Google Scholar]
- Stanchi F, Grashoff C, Nguemeni Yonga CF, Grall D, Fässler R, Van Obberghen-Schilling E 2009. Molecular dissection of the ILK-PINCH-parvin triad reveals a fundamental role for the ILK kinase domain in the late stages of focal-adhesion maturation. J Cell Sci 122: 1800–1811 [DOI] [PubMed] [Google Scholar]
- Steinman L 2009. A molecular trio in relapse and remission in multiple sclerosis. Nat Rev Immunol 9: 440–447 [DOI] [PubMed] [Google Scholar]
- Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A 2000. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin α6β4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol 149: 969–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunada Y, Bernier SM, Kozak CA, Yamada Y, Campbell KP 1994. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J Biol Chem 269: 13729–13732 [PubMed] [Google Scholar]
- Svensson L, Howarth K, McDowall A, Patzak I, Evans R, Ussar S, Moser M, Metin A, Fried M, Tomlinson I, et al. 2009. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med 15: 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA 2003. Talin binding to integrin β tails: a final common step in integrin activation. Science 302: 103–106 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, Heckmann D, Pfeifer A, Kessler H, Takagi J, Erickson HP, et al. 2007. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol 178: 167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL 2004. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood 104: 11–18 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Brown NH 2006. An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nat Cell Biol 8: 601–606 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Martin-Bermudo MD, Hicks MS, Brown NH 2006. Multiple factors contribute to integrin-talin interactions in vivo. J Cell Sci 119: 1632–1644 [DOI] [PubMed] [Google Scholar]
- Tasanen K, Tunggal L, Chometon G, Bruckner-Tuderman L, Aumailley M 2004. Keratinocytes from patients lacking collagen XVII display a migratory phenotype. Am J Path 164: 2027–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM 2001. A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell 107: 643–654 [DOI] [PubMed] [Google Scholar]
- Tsuruta D, Hopkinson SB, Jones JC 2003. Hemidesmosome protein dynamics in live epithelial cells. Cell Motil Cytoskeleton 54: 122–134 [DOI] [PubMed] [Google Scholar]
- Tsuruta D, Kobayashi H, Imanishi H, Sugawara K, Ishii M, Jones JC 2008. Laminin-332-integrin interaction: A target for cancer therapy? Curr Med Chem 15: 1968–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Huang Y, Zhang Y, Hua Y, Wu C 2001. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol 153: 585–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Li F, Goicoechea S, Wu C 1999. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol 19: 2425–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu J, Nishizawa Y, Sonnenberg A, Owaribe K 1994. Demonstration of type II hemidesmosomes in a mammary gland epithelial cell line, BMGE-H. J Biochem 115: 469–476 [DOI] [PubMed] [Google Scholar]
- Uitto J 2009. Progress in heritable skin diseases: Translational implications of mutation analysis and prospects of molecular therapies. Acta Derm Venereol 89: 228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood RA, Carter WG, Usui ML, Olerud JE 2009. Ultrastructural localization of integrin subunits β4 and α3 within the migrating epithelial tongue of in vivo human wounds. J Histochem Cytochem 57: 123–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussar S, Moser M, Widmaier M, Rognoni E, Harrer C, Genzel-Boroviczeny O, Fässler R 2008. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet 4: e1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussar S, Wang HV, Linder S, Fässler R, Moser M 2006. The kindlins: Subcellular localization and expression during murine development. Exp Cell Res 312: 3142–3151 [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Laschinger M, Engelhardt B 2001. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest 108: 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A 1996. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat Genet 13: 366–369 [DOI] [PubMed] [Google Scholar]
- Vassar R, Coulombe PA, Degenstein L, Albers K, Fuchs E 1991. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell 64: 365–380 [DOI] [PubMed] [Google Scholar]
- Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG 1989. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol 109: 1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P, Ferris W 1954. Electron-microscopic study of the texture of the basement membrane of larval amphibian skin. Proc Natl Acad Sci 40: 528–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickström SA, Lange A, Montanez E, Fässler R 2010. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 29: 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, Kuikman I, Margadant C, van Rheenen J, Sonnenberg A 2007. Serine phosphorylation of the integrin β4 subunit is necessary for epidermal growth factor receptor induced hemidesmosome disruption. Mol Biol Cell 18: 3512–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MD, Geary SM, Fitter S, Moseley GW, Lau LM, Sheng KC, Apostolopoulos V, Stanley EG, Jackson DE, Ashman LK 2004. Characterization of mice lacking the tetraspanin superfamily member CD151. Mol Cell Biol 24: 5978–5988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu XR, Wewer UM, Engvall E 1994. Murine muscular dystrophy caused by a mutation in the laminin α2 (Lama2) gene. Nat Genet 8: 297–302 [DOI] [PubMed] [Google Scholar]
- Yamaji S, Suzuki A, Sugiyama Y, Koide Y, Yoshida M, Kanamori H, Mohri H, Ohno S, Ishigatsubo Y 2001. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J Cell Biol 153: 1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO 1993. Embryonic mesodermal defects in α5 integrin-deficient mice. Development 119: 1093–1105 [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO 1995. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development 121: 549–560 [DOI] [PubMed] [Google Scholar]
- Yang JT, Bader BL, Kreidberg JA, Ullman-Cullere M, Trevithick JE, Hynes RO 1999. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev Biol 215: 264–277 [DOI] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N 1992. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4 β1 integrin. Nature 356: 63–66 [DOI] [PubMed] [Google Scholar]
- Yurchenco PD 2010. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a004911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B 2007. Functional atlas of the integrin adhesome. Nat Cell Biol 9: 858–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Tu Y, Velyvis A, Yang Y, Qin J, Wu C 2002. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci 115: 4777–4786 [DOI] [PubMed] [Google Scholar]