Abstract
The extrapancreatic actions of sulfonylureas on the glucose transport system were studied in the L6 line of cultured rat skeletal muscle cells. Insulin (10(-7) M) increased 2-deoxyglucose uptake in differentiated L6 myotubes by 30-40% after 8 h of incubation. The sulfonylurea tolazamide (0.6 mg/ml, 22 h) had no effect on glucose uptake in the absence of insulin, but increased insulin-stimulated 2-deoxyglucose uptake twofold. The total cellular content of glucose transporters was assessed with a monoclonal anti-transporter antibody by a solid-phase ELISA method. Insulin (8 h) increased the quantity of glucose transporters, with a maximal twofold increase at 10(-7) M and a dose-response curve similar to that for insulin stimulation of glucose uptake. In spite of its lack of effect on glucose uptake, tolazamide alone (0.6 mg/ml) increased the cellular content of transporters by 70%. The effects of insulin and tolazamide on transporter gene expression were studied with probes derived from Hep G2 glucose transporter cDNA. Insulin increased the transporter mRNA level 1.7-fold, tolazamide increased it 1.5-fold, and the combination of insulin and tolazamide increased transporter mRNA 3-fold. It is concluded that sulfonylureas, together with insulin, enhance glucose uptake in L6 skeletal muscle cells by increasing the number of functioning glucose transport molecules. The long-term regulation of the glucose transport system in skeletal muscle by insulin and sulfonylureas in vivo may involve similar changes in transporter function, number, and gene expression.
Full text
PDF
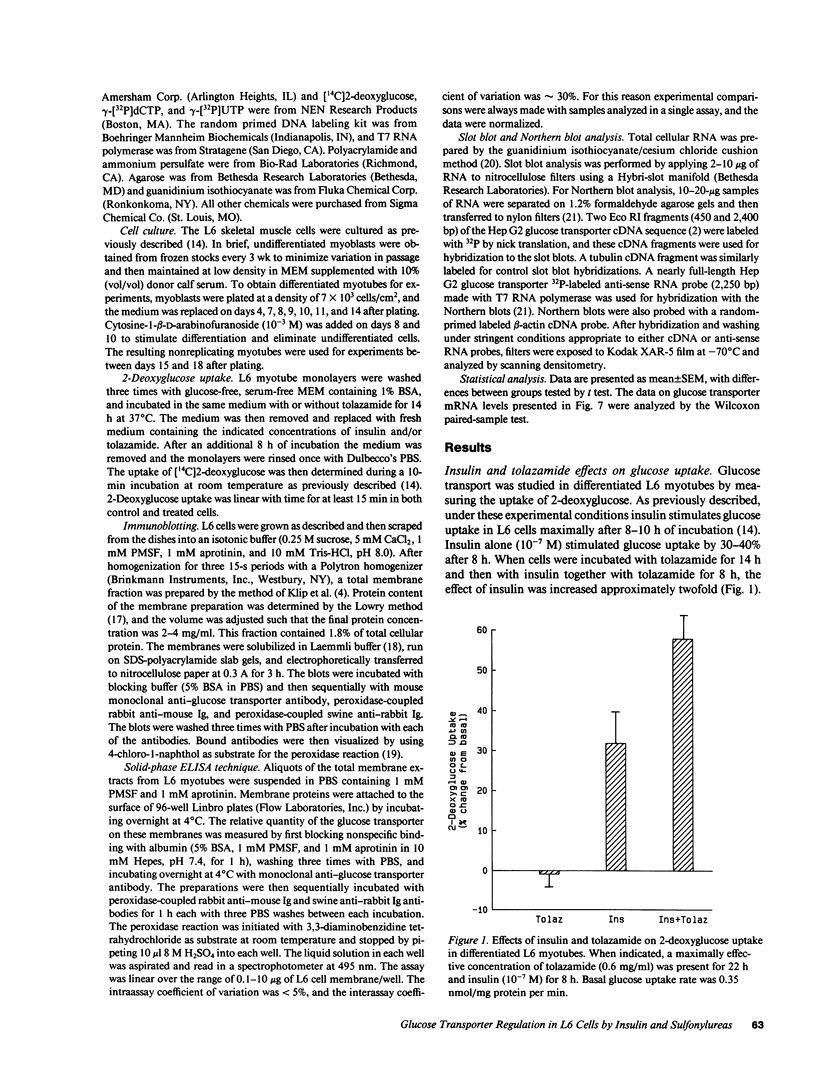
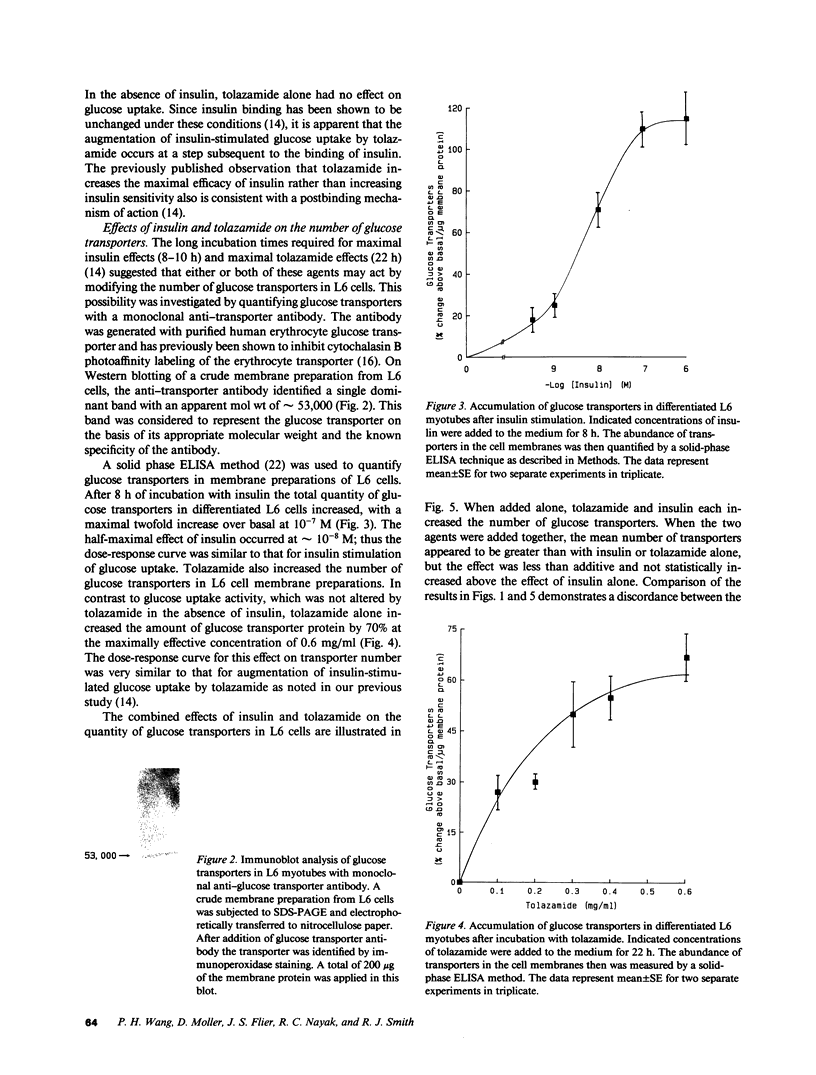
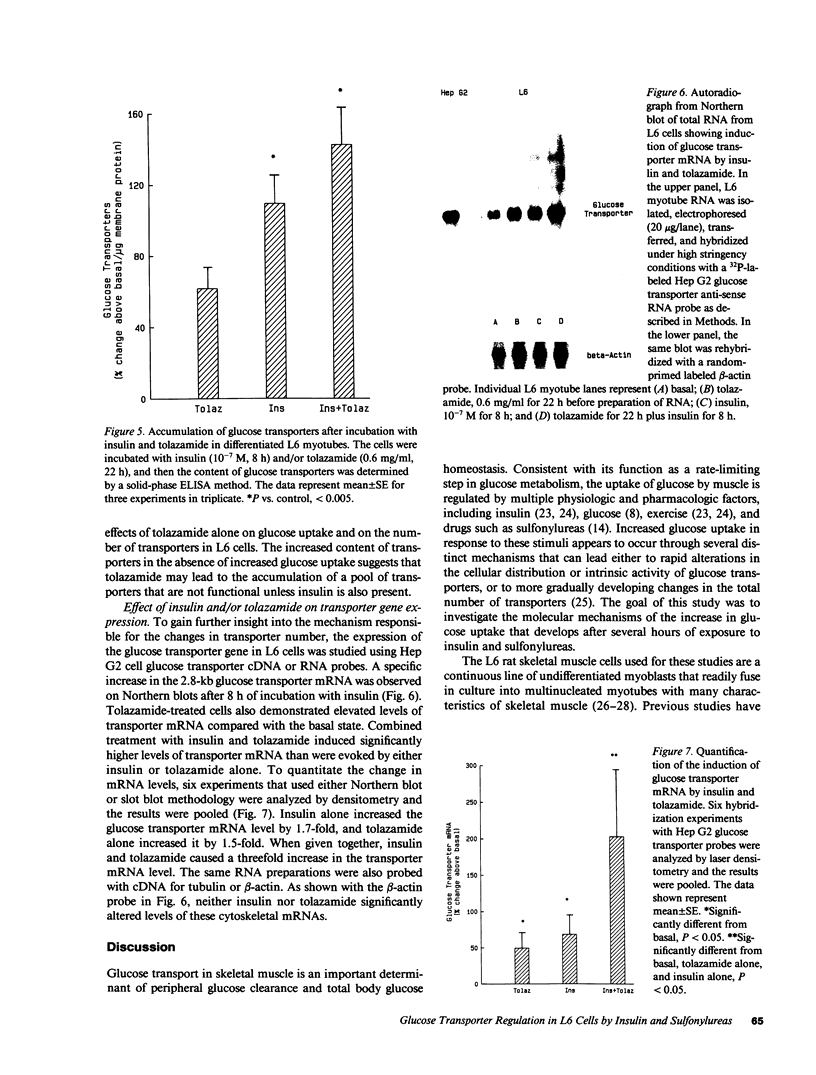


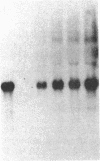
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beguinot F., Kahn C. R., Moses A. C., Smith R. J. The development of insulin receptors and responsiveness is an early marker of differentiation in the muscle cell line L6. Endocrinology. 1986 Jan;118(1):446–455. doi: 10.1210/endo-118-1-446. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Interaction of insulin and exercise on glucose uptake. Biochem J. 1975 Jan;146(1):231–238. doi: 10.1042/bj1460231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Chen C. C., Kurokawa T., Shaw S. Y., Tillotson L. G., Kalled S., Isselbacher K. J. Human erythrocyte glucose transporter: normal asymmetric orientation and function in liposomes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2652–2656. doi: 10.1073/pnas.83.8.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Davies A., Meeran K., Cairns M. T., Baldwin S. A. Peptide-specific antibodies as probes of the orientation of the glucose transporter in the human erythrocyte membrane. J Biol Chem. 1987 Jul 5;262(19):9347–9352. [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- Feldman J. M., Lebovitz H. E. An insulin dependent effect of chronic tolbutamide administration on the skeletal muscle carbohydrate transport system. Diabetes. 1969 Feb;18(2):84–95. doi: 10.2337/diab.18.2.84. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M., McCall A. L., Lodish H. F. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987 Feb;79(2):657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrel D. R., Picq R., Bajard L., Harfouche M., Tourniaire J. Acute effect of glyburide on insulin sensitivity in type I diabetic patients. J Clin Endocrinol Metab. 1987 Nov;65(5):896–900. doi: 10.1210/jcem-65-5-896. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Mechanism for markedly hyperresponsive insulin-stimulated glucose transport activity in adipose cells from insulin-treated streptozotocin diabetic rats. Evidence for increased glucose transporter intrinsic activity. J Biol Chem. 1987 Apr 15;262(11):5118–5124. [PubMed] [Google Scholar]
- Karnieli E., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell in streptozotocin-induced diabetes mellitus. Depletion of intracellular glucose transport systems. J Clin Invest. 1981 Sep;68(3):811–814. doi: 10.1172/JCI110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Klip A., Walker D., Ransome K. J., Schroer D. W., Lienhard G. E. Identification of the glucose transporter in rat skeletal muscle. Arch Biochem Biophys. 1983 Oct 1;226(1):198–205. doi: 10.1016/0003-9861(83)90285-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maloff B. L., Lockwood D. H. In vitro effects of a sulfonylurea on insulin action in adipocytes. Potentiation of insulin-stimulated hexose transport. J Clin Invest. 1981 Jul;68(1):85–90. doi: 10.1172/JCI110257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Rinninger F., Kirsch D., Häring H. U., Kemmler W. Extrapancreatic action of the sulphonylurea gliquidone: post-receptor effect on insulin-stimulated glycogen synthesis in rat hepatocytes in primary culture. Diabetologia. 1984 Jun;26(6):462–465. doi: 10.1007/BF00262222. [DOI] [PubMed] [Google Scholar]
- Schubert D., Tarikas H., Humphreys S., Heinemann S., Patrick J. Protein synthesis and secretion in a myogenic cell line. Dev Biol. 1973 Jul;33(1):18–37. doi: 10.1016/0012-1606(73)90161-9. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Simonson D. C., Ferrannini E., Bevilacqua S., Smith D., Barrett E., Carlson R., DeFronzo R. A. Mechanism of improvement in glucose metabolism after chronic glyburide therapy. Diabetes. 1984 Sep;33(9):838–845. doi: 10.2337/diab.33.9.838. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Larson S., Stred S. E., Durschlag R. P. Regulation of glutamine synthetase and glutaminase activities in cultured skeletal muscle cells. J Cell Physiol. 1984 Aug;120(2):197–203. doi: 10.1002/jcp.1041200213. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Holloszy J. O. Activation of glucose transport in diabetic muscle: responses to contraction and insulin. Am J Physiol. 1985 Sep;249(3 Pt 1):C233–C237. doi: 10.1152/ajpcell.1985.249.3.C233. [DOI] [PubMed] [Google Scholar]
- Wang P. H., Beguinot F., Smith R. J. Augmentation of the effects of insulin and insulin-like growth factors I and II on glucose uptake in cultured rat skeletal muscle cells by sulfonylureas. Diabetologia. 1987 Oct;30(10):797–803. doi: 10.1007/BF00275746. [DOI] [PubMed] [Google Scholar]