Abstract
An entire mammary epithelial outgrowth, capable of full secretory differentiation, may comprise the progeny of a single cellular antecedent, i.e., may be generated from a single mammary epithelial stem cell. Early studies showed that any portion of an intact murine mammary gland containing epithelium could recapitulate an entire mammary epithelial tree on transplantation into an epithelium-free mammary fat pad. More recent studies have shown that a hierarchy of mammary stem/progenitor cells exists among the mammary epithelium and that their behavior and maintenance is dependent on signals generated both locally and systemically. In this review, we have attempted to develop the scientific saga surrounding the discovery and characterization of the murine mammary stem/progenitor cell hierarchy and to suggest further approaches that will enhance our knowledge and understanding of these cells and their role in both normal development and neoplasia.
A single mammary epithelial stem cell is capable of generating an entire mammary gland. In vivo, systemic estrogen and locally generated paracrine factors such as amphiregulin regulate stem cell cycling and differentiation.
Before the 1980s there was little if any thought that the epithelium in murine mammary glands might be engendered by or supported by a mammary epithelial specific stem cell. In 1980, Rudland et al. wrote a review entitled “Stem cells in rat mammary development and cancer: A review” and noted that dimethylbenz [α] anthracene (DMBA)-induced rat carcinomas contained all three main types of epithelium found in the normal rat gland, those lining the ductal lumina, those lining the alveolar lumina, and myoepithelial cells (Rudland et al. 1980). They suggested, based on the two types of morphologically distinct epithelial (luminal and myoepithelial) cancer cells in the clonally derived Rama 25 cell line, that a single cell might give rise to both types and this also held true when these cells were inoculated into hosts and produced tumors. Williams and Daniel (Williams and Daniel 1983) suggested that the cap cells at the tip of the growing ducts in the mouse could give rise to both luminal and myoepithelial cells during ductal morphogenesis. However, no direct evidence that a single cell could produce both epithelial cell types in vivo was available. Nevertheless, in retrospect there was evidence that full regenerative activity for mammary epithelial existed in every part of the adult mammary epithelial tree.
The experiments that originally showed the potential existence of stem cells in the mouse mammary gland were the pioneering studies of DeOme and his students, Les Faulkin and Charles Daniel. The approach they developed and optimized was serial transplantation of normal mammary gland into the cleared mammary fat pad of syngeneic mice (Deome et al. 1959; Faulkin and Deome 1960). The cleared mammary fat pad allowed the transplantation and growth of normal mammary cells into their normal anatomical site and under the influence of a normal physiological environment. Using this method, DeOme and coworkers showed that all portions of the normal mammary gland contains cells that will grow and fill the fat pad with a normal ductal mammary tree and respond to hormones with a normal differentiation program (Daniel 1975; Daniel et al. 1975). The progeny of the transplanted cells could be serially transplanted into the appropriate recipients for multiple times; however, unlike preneoplastic or neoplastic cells, the normal cells always senesced after multiple serial transplants, generally five to eight transplant generations (Daniel 1975). This was interpreted as indicating mammary stem cells possessed a finite proliferative activity (i.e., life span). This finite life span was a fundamental difference between normal and preneoplastic/neoplastic mammary cells. Cells with an indefinite in vivo life span (i.e., immortalized) have been identified in numerous mammary model systems, including MMTV-induced alveolar hyperplasia's (Callahan and Smith 2000), chemical carcinogen-induced ductal and alveolar hyperplasia's (Smith et al. 1978, 1980), hormonally induced alveolar hyperplasia, spontaneously immortalized ductal hyperplasia's (Medina 2000, 2002), and cells containing specific genetic alterations (i.e., p53 deletion, Polyoma mT antigen) (Maglione et al. 2001; Medina et al. 2002).
Subsequent studies showed that stem cells were located along the entire mammary tree and represented in all the different developmental states of the mammary gland. These stages included primary and tertiary ducts from 6- and 16-wk virgin glands, uniparous and multiparous regressed gland, 15-d pregnant and 10-d lactating glands (Smith and Medina 1988). Host age and reproductive history had little influence on the frequency of stem cells as measured by percent successful takes and life span assay (Young et al. 1971; Smith and Medina 1988). Mammary cells taken from 26-mo-old virgin mice had the same transplant potential as cells taken from 3-wk-old mice. Cell populations, from both, senesced after five transplant generations. Similarly, mammary cells in 12-mo-old multiparous mice had the same serial transplant potential as cells from 3-wk-old virgin mice (Young et al. 1971). Finally, continuous hormone stimulation did not induce additional loss of ductal growth potential. These results have important implications for understanding the role of mammary stem cells in normal mammary development because they emphasize that the mammary stem cell is a relatively quiescent cell that is only activated under conditions of gland repopulation (i.e., fetal growth stage and pubertal growth phase).
MORPHOLOGIC EVIDENCE OF STEM CELLS IN MAMMARY EPITHELIUM
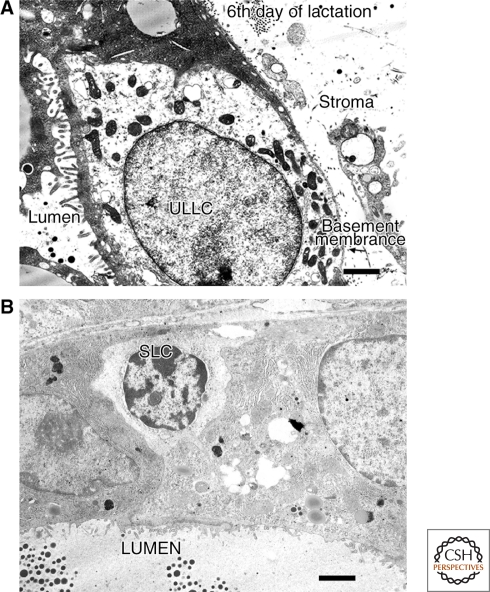
One cellular feature that held promise for distinguishing mammary stem cells from their neighbors was their ultrastructural appearance (Smith and Medina 1988). Undifferentiated (pale) cells (Fig. 1A,B) were found that showed the expected behavior of stem cells in mammary explants induced in vitro, to differentiate toward secretory cell fates. It was discovered that mouse mammary explants, like mammary epithelium in situ, contained pale or light-staining cells, and that it was only these cells that entered mitosis when mammary explants were cultured. Chepko and Smith (1997) analyzed pale-staining cells in mouse and rat mammary glands in the electron microscope using their ultrastructural features to distinguish them from other mammary epithelial cells.
Figure 1.
Electron micrographs of a ULLC and SLC in the mouse mammary gland. (A) Electron micrograph of an undifferentiated large light cell (ULLC) taken from an ultrathin section of a mammary acinus on the 6th day of lactation. The ULLC may or may not contact the lumen but rests on a basement membrane (arrow), which is in direct contact with the surrounding stroma. Bar =5.0 µm. (B) Electron micrograph of a small light cell (SLC) in a lactating acinus. It does not contact the secretory lumen but lies on the basement membrane. Bar = 5 µm.
Cell and developmental biologists who have examined growing and regenerating tissue by transmission electron microscopy have postulated that the undifferentiated cells observed within these diverse tissues represent tissue-specific stem or progenitor cells. The rate of aging is not uniform throughout the transplanted population and complete growth quiescence for all portions of a given outgrowth is reached subsequent to the 6th transplant generation. Mammary epithelial cells bearing the morphological characteristics of undifferentiated stem cells (i.e., SLC and ULLC) likewise disappear from senescent populations simultaneous with growth cessation (Smith et al. 2002). In premalignant mammary epithelial populations, which show indefinitely prolonged growth potential, both of these cell types (SLC and ULLC) are maintained. This observation provides further support for the conclusion that these ultrastructurally distinct mammary cells may represent the undifferentiated mammary stem/progenitor cell population. Mammary epithelial cells with similar stem cell properties have been shown in human breast (Gudjonsson et al. 2002). It should be noted that these cells are present by electron microscopy in immortalized mouse mammary epithelial populations such as hyperplastic alveolar nodules and their eventual outgrowths, but not in growth senescent mammary epithelial populations (Smith et al. 2002). To date, other than the correlative studies mentioned previously, no direct evidence for regenerative capacity has been shown for these pale undifferentiated cells (SLC and ULLC). The eventual identification of stem cells in situ using specific cell surface markers (see the following) may allow the relationship between these cells (SLC and ULLC) and repopulating cells to be defined.
PROSPECTIVE ISOLATION OF MOUSE MAMMARY STEM CELLS
Over recent years, several groups in the mammary gland biology field have turned their efforts toward the identification and physical isolation of mammary stem cells using an adaptation of methodologies used in the purification of stem cells from the hematopoietic compartment. Stem cells have been prospectively isolated from the mouse mammary gland and shown to display the hallmark features of multi-lineage differentiation and self-renewal in vivo (Shackleton et al. 2006; Stingl et al. 2006). Single-cell suspensions of freshly dissociated mammary tissue were fractionated using specific combinations of antibodies against cell surface proteins together with flow cytometry. The empirically derived subpopulations were then assayed for repopulating ability by transplantation of limiting numbers of cells into the cleared mammary fat pads of 3-wk-old recipients. Genetically tagged cells derived from Rosa-26 mice were also employed to prove donor origin of the mammary outgrowths. A MaSC-enriched population was identified on the basis of high expression of either CD29 (β1-integrin) or CD49f (α6-integrin) and moderate levels of CD24 (heat stable antigen). Based on these markers, the estimated frequency of MaSCs in the basal population is less than 1 in 100 cells. Using CD24 as a single marker after lineage depletion, Sleeman et al. (2006) showed that the CD24mod subset was enriched for repopulating activity. Later, it was shown that as few as 100 unsorted mammary cells were capable of producing outgrowths when inoculated in matrigel (Moraes et al. 2007), a matrix that has been shown to improve cell transplantability (Quintana et al. 2008). Estimates of MaSC numbers range between 1000–14,000 cells per young virgin female gland (Kordon and Smith 1998; Stingl et al. 2006). This large variability reflects the efficiency of dissociation, efficacy of antibody labeling and use of stringent controls for setting robust gates, as established long ago by experimental hematologists and immunologists. In addition, the figures are likely to be an underestimate as there is inevitable cell loss during the dissociation and sorting procedures. Cells from the MaSC-enriched subpopulation could generate extensive ductal outgrowths on implantation and, moreover, were serially transplantable at a clonal level. MaSCs were estimated to execute at least 10 symmetrical self-renewing divisions, thus fulfilling the stem cell requirement of extensive self-renewing capability (Stingl et al. 2006).
Evidence that MaSCs can contribute to oncogenesis comes from analysis of preneoplastic mammary tissue of MMTV-wnt-1 mice, which was found to harbor a significantly increased number of functional MaSCs (Shackleton et al. 2006; Vaillant et al. 2008). It seems likely that ectopic expression of wnt-1 in epithelial cells directly enhances the self-renewal of MaSCs, leading to an expanded target population for further oncogenic hits. Progenitor populations may also be influenced by activation of Wnt signaling, as indicated by transplantation studies using preneoplastic tissue from MMTV-wnt-1 transgenic mice (Vaillant et al. 2008) and their tumors (Li et al. 2003; Liu et al. 2004). There may be a direct relationship between normal MaSCs and the cancer stem cell-enriched populations identified in MMTV-wnt-1 tumors, displaying either a Thy1+CD24+ or CD61+CD24+ phenotype (Cho et al. 2008; Vaillant et al. 2008). In the p53 models of mammary tumorigenesis, MaSCs may also contribute to the genesis of cancer stem cells (Vaillant et al. 2008; Zhang et al. 2008; Cicalese et al. 2009).
A single microscopically visualized mammary epithelial cell has the potential to reconstitute an entire functional mammary gland (6/102 cells). Individual stem cells showed full developmental capacity during pregnancy, with the emergence of structures replete with alveolar units. Moreover, the ductal epithelial tree contained daughter stem cells with the same in vivo repopulating activity as the original stem cell (Shackleton et al. 2006; Stingl et al. 2006). To definitively confirm the self-renewing capacity of the repopulating cell, primary outgrowths of single-cell origin were subjected to secondary transplantation and analyzed at parturition. Although no supporting cells were necessary for reconstitution by the stem cell, it is likely that the MaSC creates its own epithelial niche through asymmetric cell divisions, yielding progeny that coordinate the development of a branching ductal tree. Mixing experiments using equal but limited numbers (equivalent to one mammary repopulating unit) of wild-type and genetically tagged cells from the MaSC-enriched population in transplantation assays confirmed that two or more mammary stem cells are not essential to generate an outgrowth (Shackleton et al. 2006; Stingl et al. 2006). These data further suggested that mammary stromal-derived factors have a significant role in regulating MaSC function during the initiation of an outgrowth. Nevertheless, the formation of chimeric mammary epithelial outgrowths following transplantation of large numbers of cells (Smith 1996; Brisken et al. 1998; Boulanger et al. 2005) provides evidence that cooperation between stem and progenitor cells is an important feature of mammary morphogenesis.
The emerging phenotype of the MaSC thus far is CD29hiCD49fhiCD24+/modSca-1− (Shackleton et al. 2006; Sleeman et al. 2006; Stingl et al. 2006). Although slightly different CD24 nomenclature has been used, it is recognized that the differing levels of fluorescence reflect the use of different anti-CD24 antibodies (Sleeman et al. 2007) and that luminal cells express higher levels of CD24 than basal cells. Although Sca-1 was initially considered to represent a potential marker of MaSC/progenitor cells, it has become evident that culturing mammary epithelial cells induces high levels of Sca-1 expression (Shackleton et al. 2006; Stingl et al. 2006), thereby emphasizing the importance of studying freshly dissociated cells. In addition, the side-population (SP) defined by Hoechst 33342 dye efflux, originally thought to be enriched in repopulating cells (Welm et al. 2002; Alvi et al. 2003), has been shown to be depleted of CD29hiCD49fhiCD24+ (MaSC-enriched) cells (Shackleton et al. 2006; Stingl et al. 2006). Rather, the SP fraction is highly enriched for luminal progenitor cells (Asselin-Labat et al. 2008). The expression of intracellular cytokeratins by different subpopulations has also not proven useful in distinguishing functionally distinct cell types. In the mouse mammary gland, CK14 is expressed by both the basal and luminal populations, albeit at different levels, whereas in human breast tissue, there is overlapping expression of cytokeratins in the basal and luminal cell layers (Gusterson 2009). It has proven difficult to segregate myoepithelial and stem cells as they show a common cell surface phenotype and gene expression profile (Stingl et al. 2006). This subpopulation therefore remains a heterogeneous subset of MaSCs, mature myoepithelial cells and presumptive basal progenitor cells.
Do the cell surface antigens that characterize MaSCs simply serve as markers or do they play a functional role in conveying stemness? Emerging data for β1 integrin argue that this is not a surrogate marker. Targeted deletion of β1-integrin in basal cells of the mammary gland has revealed an essential role for this integrin in maintaining MaSCs and in regulating the orientation of the basal cell division axis (Taddei et al. 2008). It is not yet clear how the other markers such as α6 integrin and CD24 contribute to MaSC function but CD24 expression has been implicated in regulating breast tumor cell proliferation and invasion (Baumann et al. 2005). The localization of MaSCs in the mammary gland is presently not clear and will only be resolved through the use of more specific markers together with lineage tracing. It is likely, however, that they enriched in the cap cell region of the terminal endbud (Kenney et al. 2001) and distributed at specific points along the ductal network (Daniel 1975).
Committed luminal progenitor cells have recently been prospectively identified. These were isolated on the basis of either CD61 (β3 integrin) expression or lack of CD133 (prominin-1) and Sca1 expression (Asselin-Labat et al. 2007; Sleeman et al. 2007). The CD61+ progenitor cells are restricted to a luminal cell fate and are devoid of regenerative capacity in vivo. The relationships between the populations defined by CD61 expression and lack of CD133 are unclear but they do not mark identical subsets and the CD61+CD29loCD24+ subset may lie within the CD133−CD24+Sca1− population (Kendrick et al. 2008). There are likely to be other committed luminal progenitor cells including an alveolar-restricted precursor cell that drives expansion of the alveoli during pregnancy.
NATURE OF THE MAMMARY EPITHELIAL CELL HIERARCHY
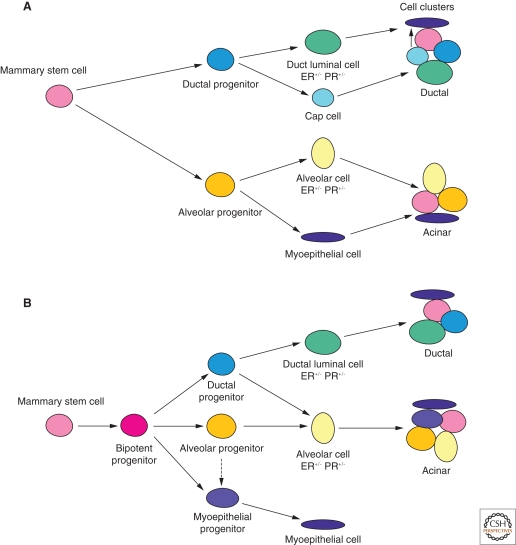
There are at least two potential hierarchical models for mammary stem cells giving rise to differentiated epithelial cells in the mammary gland. In one model, the stem cell yields bipotential progenitors for the ductal and alveolar lineages (Fig. 2A). Evidence for lobule-limited and duct-limited mammary epithelial cell activities has been established for both rats and mice by transplantation of limiting dilutions of dispersed mammary epithelial cells into hosts that were subsequently impregnated and/or treated with hormone combinations to produce alveologenesis (Kim and Clifton 1993; Kim et al. 1993; Smith 1996; Kamiya et al. 1998; Kordon and Smith 1998). These limited structures contain both luminal epithelial and myoepithelial cells and PR and ERα-positive luminal epithelial cells. Studies with retroviral-marked mammary populations showed that both of these lineage-limited activities were present within the clonal populations though repeated transplant generations indicating their derivation from a single pluripotent antecedent (Kordon and Smith 1998; Smith and Boulanger 2002). An important conclusion from this study is the demonstration that outgrowths generated from individual mammary fragments are nearly always clonal or quasi-clonal. In addition, serial passage of the retroviral-marked mammary epithelial clones in pregnant hosts showed that the capacity of individual outgrowths to produce lobulogenesis or ductal elongation were independently lost during the acquisition of growth senescence among individual transplants (Smith and Boulanger 2002). This observation was important because it showed that lobular and ductal morphogenesis can be mediated through independent pathways and confirmed the earlier conclusions drawn from limiting dilution transplants of dispersed cells (Smith 1996). The distinction between these two progenitor-mediated activities in regenerating mouse mammary tissue is that the lobule-limited progenitor is unable to produce cap cells, which are required for the penetration of the mammary fat pad at the tips of the growing terminal end buds. On the other hand, duct-limited progenitors fail to produce progeny capable of sustaining alveolar development and growth during pregnancy (Fig. 2A,B).
Figure 2.
Schematic models of the mammary epithelial hierarchy. (A) Model in which commitment to either a ductal or alveolar cell fate occurs before commitment along either the luminal or myoepithelial lineage. The cap cell, a potential myoepithelial precursor cell, resides in the outer layer of the tip of the terminal end bud in the pubertal mammary gland. Ductal and acinar structures typify the virgin and pregnant states, respectively. (B) Model in which a common progenitor commits to either a luminal or myoepithelial cell fate. The luminal lineage can be further subdivided into ductal and alveolar-restricted progenitor subtypes. During pregnancy, the alveolar cell may display bipotential differentiation capacity.
In the second model (Fig. 2B), the two distinct lineages (luminal and myoepithelial) that occur in the mammary gland remain as separate arms, analogous to that in the hematopoietic system. The luminal arm can be further subdivided into the ductal and alveolar sublineages. There may be a common luminal progenitor cell (CD61+) which gives rise to both ductal and alveolar luminal cells dependent on the hormonal status. The alveolar progenitor may also show bipotential lineage differentiation during pregnancy, compatible with the generation of lobule-restricted structures in vivo (Smith 1996). The myoepithelial progenitor is yet to be isolated but this cell likely cofractionates with MaSCs in the CD29hiCD49fhiCD24+ subpopulation. Notably, the presence of myoepithelial cell-only colonies in cultures of human breast epithelial cells (Stingl et al. 1998) provides support for their existence. The definition of a more pure MaSC population and the prospective isolation of functionally distinct progenitor populations will be required to delineate linear relationships between epithelial cell types and distinguish between the proposed models. Furthermore, it will be important to use freshly isolated cells (not cultured) in transplantation and clonogenic cellular assays.
HORMONAL REGULATION OF THE HIERARCHY
Interestingly, the MaSC-enriched population CD29hiCD49fhiCD24+/mod does not express estrogen receptor (ERα) or progesterone receptor (PR) (Asselin-Labat et al. 2006). The CD24mod population described by Sleeman et al. (2007) also lacked expression of these receptors. The luminal progenitor cell is the first cell type along the hierarchy with detectable expression of ERα, with 6%–10% of cells expressing this receptor. It is conceivable, however, that a minute fraction of MaSCs express low but physiologically relevant levels of these receptors. The identity of the cell types that respond to estrogen and progesterone signaling are of immense interest, given the importance of these hormones to breast carcinogenesis (Hankinson et al. 2004). Despite their steroid hormone receptor negative status, MaSCs have recently been shown to be highly sensitive to these hormones during mammary ontogeny, presumably responding to signals emanating from other epithelial subtypes (Asselin-Labat et al. 2010). Paracrine signals from mammary epithelial cells that express ERα are indeed required for ductal development (Mallepell et al. 2006). One of the paracrine factors that mediates these signals is amphiregulin (Ciarloni et al. 2007). Conversely, PR-expression in the mammary epithelium has been shown to be required for full secretory alveolar development, and is predominantly mediated by Wnt4 (Brisken et al. 2000). It is not yet known whether these paracrine signaling events act directly on MaSCs or downstream mammary progenitor cells. Interestingly though, the MaSC pool itself increases under the hormonal environment imposed by pregnancy, with a greater than 10-fold increase in mid-pregnancy. By late pregnancy, the number of stem cells returns to near basal levels observed in prepregnant mice (Tiede et al. 2009; Asselin-Labat et al. 2010).
PARITY-IDENTIFIED MAMMARY EPITHELIAL CELLS
With the development of the WAP-Cre model used in combination with the Rosa26LacZ reporter mice (R26R), evidence for a LacZ-marked lobular-limited progenitor observable in parous mouse mammary epithelium surfaced (Wagner et al. 2002). These LacZ-positive, parity-identified mammary cells (PI-MEC) were found to be pluripotent, self-renewing and capable of maintaining their lobule-limited progenitor activities following serial transplantation in epithelium-free mammary fat pads when the hosts were subsequently impregnated (Boulanger et al. 2005). During pregnancy in these hosts, the PI-MEC proliferated and gave rise to LacZ+ luminal progeny that were PR or ERα-positive and luminal progeny that were bereft of these steroid receptors. Further in the developing secretory acini, they contributed not only secretory progeny but also, LacZ-positive myoepithelial cells. PI-MECs have also been studied by the expression of GFP in WAP-Cre/Chicken actin gene promoter (CAG)-flox-stop-flox-GFP parous females. In these studies, GFP+ PI-MECs were sorted and found to be present in the CD49fhi population (Matulka et al. 2007). This population was shown earlier to possess essentially all of the mammary repopulating activity (Stingl et al. 2006).
Originally, it was proposed that LacZ+ PI-MECs arose from dedifferentiated secretory epithelial cells that had survived involution and remodeling of the mammary tissue however, further study indicated that these cells were present in the mammary tissue of nulliparous females (Booth et al. 2007). These cells were shown to possess all the properties of PI-MECs including self-renewal and pluripotency. It has been shown previously (Smith et al. 1984; Kordon et al. 1995; Robinson et al. 1995) that milk proteins, including WAP, are synthesized and secreted in the glands of nulliparous cycling females during estrus. Therefore, mammary progenitors for secretory luminal cells are active in intact nulliparous mice. In nulliparous glands, WAP expression may occur transiently in MaSCs, given that promiscuous expression of lineage-associated markers has been shown to occur in HSCs occurs before lineage commitment (Orkin 2003). The cycling status of MaSCs (Stingl et al. 2006) may be associated with a slowly fluctuating transcriptome that governs “priming” for cell fate commitment as recently described (Chang et al. 2008).
THE ROLE OF SELECTIVE SEGREGATION OF TEMPLATE DNA IN MAMMARY STEM CELL BIOLOGY
Long DNA label retention has repeatedly been ascribed as a property of stem cells because of their supposed absence of mitotic activity during tissue homeostasis. Recent studies have indicated that long-term label retaining cells (LREC) in a variety of tissues actually cycle and retain their original labeled DNA template strands. This has been shown in the intact mouse mammary gland and in outgrowths from transplants of mammary epithelium into cleared mammary fat pads (Smith 2005). In both instances, LREC following prolonged chase periods were labeled by a second DNA analogue and were shown to transmit the second label (associated with newly synthesized DNA strands) to their immediate progeny. This property was shown for ERα-positive, PR-positive mammary epithelium as well as those not expressing these receptors (Booth and Smith 2006). In addition, lobule-limited alveolar stem/progenitors (PI-MECs) were shown by Smith (2005) to adopt this method of asymmetric division (i.e., selective template DNA segregation) in growing transplants in nonpregnant hosts. Recently, we reported that selective segregation of template DNA persists in mammary epithelial cells during pregnancy (Booth et al. 2008a). These studies also show that lobular cells capable of selective segregation of their template DNA are newly formed during the expansion of the alveolar epithelium. These observations indicate that the property of selective segregation of DNA strands by asymmetrically dividing cells is not only a property of stem cells but also of lineage-limited stem/progenitors and perhaps specific transit amplifying committed epithelial cells as well. More studies of this important asymmetric mitotic event are required and necessary to establish both the mechanism for this selective segregation and to understand its role in tissue development, differentiation, repair and maintenance.
Although the majority of sorted MaSCs were shown to be cycling (Stingl et al. 2006), the CD29hiCD24+/mod population is enriched for BrdU-label retaining cells in vivo, suggesting that a small pool of resting stem cells resides in the mammary gland (Shackleton et al. 2006). It will be of interest to understand the relationship between these cells and the label-retaining cells shown to be distributed throughout large ducts in the mouse mammary gland (Fernandez-Gonzalez et al. 2009), as well as the SLC/ULLC described earlier. The observation of label-retaining mammary epithelial cells in the mouse mammary gland that are ERα and PR positive (Booth and Smith 2006) suggests that a hierarchy of stem cells reside within mammary tissue and that an immediate descendent of the MaSC could express low levels of ERα and PR.
NONMAMMARY STEM/PROGENITOR CELLS OBEY SIGNALS FROM THE MAMMARY MICROENVIRONMENT
It has been postulated that stem cell maintenance and function are regulated by signals emanating from its surrounding environment, commonly referred to as “the stem cell niche” (Xie and Li 2007). Using the WAPCre/Rosa26R model as an experimental tool, cells from organs other than the mammary glands of mice were tested in vivo to determine whether they might be redirected to a mammary epithelial cell fate by interaction with the mammary microenvironment. There were several advantages in this approach. First, the mammary epithelium-specific promoter, whey acidic protein (WAP), tightly regulated in its expression by mammotropic hormones was used. Second, expression from the WAP promoter is the greatest during late pregnancy. Thus nonmammary cells and their progeny comingled with wild-type epithelium would not express the recombined reporter gene (lacZ) unless pregnancy had ensued. Third, following the extensive remodeling of the lactating gland during postlactation involution, nonmammary cell progeny would only be evident (lacZ+) if they had adopted the cellular attributes of PI-MECs (lobule-limited progenitors). This provided a quick and definite positive answer regarding the successful reprogramming of nonmammary cells and their progeny during mammary regeneration and functional differentiation. The results indicated that cells (Boulanger et al. 2007) from adult male seminiferous tubules as well as neural stem cells (NSC) from both embryonic and adult brain were able to adopt mammary epithelial cell traits characteristic of PI-MECs (Booth et al. 2008b), but only following interaction with wild-type mammary epithelial cells in the context of the mammary fat pad. No mammary growth was observed when only seminiferous tubule cells or NSC were transplanted into the mammary fat pad, a result that supports an essential role of the mammary epithelial cells in the reprogramming event.
The nature of the cellular components in the presumptive mammary stem cell niche is yet to be elucidated but potential cell types include epithelial cells, macrophages, fibroblasts, and eosinophils. Stem cell repopulation assays in conjunction with selective depletion of macrophages from the mammary gland have recently implicated macrophages in playing a critical role in supporting MaSC function (Gyorki et al. 2009). In the absence of macrophages, the repopulating ability and outgrowth potential of MaSCs were severely compromised. Macrophages may therefore constitute part of the mammary stem cell niche, consistent with the findings that macrophages have a critical role in ductal morphogenesis (Van Nguyen and Pollard 2002). The roles of other heterotypic cell types in the niche are yet to be determined.
MOLECULAR REGULATORS OF MAMMARY STEM AND PROGENITOR CELLS
Over the past few years, the physiologic function of a number of transcription factors and other regulators has been evaluated in the context of the hierarchy. Strategies that have been used to address this question include the separation of mammary epithelial subpopulations from knockout mice or retroviral-mediated transduction of specific epithelial subtypes in either gain-of-function or loss-of-function experiments (Bouras et al. 2008; Welm et al. 2008). Knockdown of Cbf-1/Rbp-Jκ, a canonical effector in the Notch pathway, leads to increased stem cell repopulating activity in vivo, accompanied by aberrant terminal end buds and excessive ductal branching. These data provide evidence that the Notch pathway normally plays a role in restricting expansion of MaSCs (Bouras et al. 2008). Hedgehog signaling (Hg) also appears to play a negative regulatory role in MaSCs, given that constitutive activation of the smoothened receptor leads to diminished MaSC activity (Moraes et al. 2007). Recently, a novel role for p53 in regulating the self-renewal of MaSCs was discovered, with loss of p53 favoring symmetric cell division (Cicalese et al. 2009). In contrast, the polycomb group protein Bmi-1 is important for maintaining the function of MaSCs as well as downstream progenitor cells in the mammary gland (Pietersen et al. 2008). Moreover, Bmi-1 promotes the formation of human mammospheres in culture (Liu et al. 2006). The Wnt signaling receptor Lrp5 has also been shown to play an essential role in maintaining MaSCs and the basal cell lineage (Badders et al. 2009), suggesting that the Wnt pathway may directly control the self-renewal of MaSCs, analogous to its role in other systems. Cell cyclins such as cyclin D1 are also likely to be required for controlling mammary stem cell proliferation and activity (Jeselsohn et al. 2010).
The Notch pathway also plays a role in luminal cell fate determination. In both mouse and human mammary tissue, Notch activity promotes commitment of MaSCs to the luminal cell lineage at the expense of the myoepithelial lineage (Bouras et al. 2008; Raouf et al. 2008). Moreover, Notch activity is important for maintaining luminal cells in the alveolar units that arise during pregnancy (Buono et al. 2006). Overall, Notch activity appears to be important for establishing the luminal progenitor subset, with uncontrolled expansion of this population occurring in the presence of constitutive Notch activation (Bouras et al. 2008). Interestingly, Stat5a was recently shown to be important for establishment or maintenance of luminal progenitor cells. Therefore, the impaired alveologenesis and lactation that occurs in mice with targeted disruption of the Stat5a/5b locus in mice appears to primarily reflect a defect in progenitor cell numbers and not their differentiative ability (Yamaji et al. 2009).
Regulators of luminal cell differentiation have also been examined in the context of discrete mammary epithelial subtypes. Gata-3 is a critical transcription factor for instructing differentiation along the ductal and alveolar luminal lineages, with the accumulation of luminal progenitor cells in Gata-3-deficient glands (Asselin-Labat et al. 2007). The absolute level of Gata-3 is important because defects are evident in the mammary glands of Gata-3 heterozygous mice. The higher level of Gata-3 observed in the luminal subtypes of breast cancer may promote differentiation of tumor cells, albeit aberrant, thus leading to an improved prognosis. Gata-3 has been shown to influence different processes during tumor progression. This transcription factor has been identified as a direct repressor of the CDK inhibitor p18INK4C, which in turn plays an important role in suppressing the development of ERα-positive luminal tumors in the mammary gland by restraining luminal progenitor proliferation (Pei et al. 2009). Furthermore, Gata-3 can suppress the metastasis of luminal-like tumor cells (Kouros-Mehr et al. 2008). Like Gata-3, Elf-5 has emerged as a key regulator of alveologenesis and lactogenesis (Zhou et al. 2005; Oakes et al. 2008). A pronounced defect in alveolar development is evident in the absence of a single Elf-5 allele, accompanied by the expansion of the pool of luminal progenitor cells. However, these two transcription factors also show distinct functions, because only Gata-3 affects ductal morphogenesis (Asselin-Labat et al. 2007).
CONCLUDING REMARKS
Although much progress has been made in identifying cell types in the mouse mammary gland, further delineation of the relationships between stem, progenitor, and mature cells is required to understand the complexity of the mammary epithelial hierarchy. Markers that allow better purification of the MaSC and its descendent progenitors are needed to unambiguously identify the location of individual cells. It is presumed that there is a small pool of quiescent stem cells within the mammary gland but the signals that control their activation and decision to execute asymmetric versus symmetric cell division are unknown. What is the relationship between the small pale cell and the ULLC described by electron microscopy and do they have the same developmental potential? The role of estrogen and progesterone in regulating stem cell function is also unclear but almost certainly involves multiple paracrine factors that are yet to be identified. The earliest progenitor cell known to date to express ER and PR is the CD61+ luminal cell but another upstream progenitor that expresses physiologically relevant levels of these receptors may exist.
Although recent studies have shown that the mammary gland environment plays an instructive role in determining development of the ductal tree from mammary stem cells, the constituents of the putative niche are not yet known. Other questions relating to the niche are: is senescence a function of stem cell loss or loss of the niche, and is there a role for the mammary stroma in the stem cell niche? The niche is likely to play an important role in reprogramming cell differentiation and could lead to the appearance of neoplastic cells through dysregulation of cell-cell contacts or paracrine factors.
ACKNOWLEDGMENTS
We are grateful to D. Medina for critical reading of the manuscript.
Footnotes
Editors: Mina J. Bissell, Kornelia Polyak, and Jeffrey Rosen
Additional Perspectives on The Mammary Gland as an Experimental Model available at www.cshperspectives.org
REFERENCES
- Alvi AJ, Clayton H, Joshi C, Enver T, Ashworth A, Vivanco MM, Dale TC, Smalley MJ 2003. Functional and molecular characterisation of mammary side population cells. Breast Cancer Res 5: R1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. 2007. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9: 201–209 [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ 2006. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst 98: 1011–1014 [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Shackleton M, Bouras T, Lindeman GJ, Visvader JE 2008. Delineating the epithelial hierarchy in the mouse mammary gland. Cold Spring Harb Symp Quant Biol 73: 469–478 [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. 2010. Control of mammary stem cell function by steroid hormone signalling. Nature doi: 10.1038/nature09027 [DOI] [PubMed] [Google Scholar]
- Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO, Alexander CM 2009. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One 4: e6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, Sleeman JP 2005. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res 65: 10783–10793 [DOI] [PubMed] [Google Scholar]
- Booth BW, Smith GH 2006. Estrogen receptor-α and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res 8: R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth BW, Boulanger CA, Smith GH 2007. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol 212: 729–736 [DOI] [PubMed] [Google Scholar]
- Booth BW, Boulanger CA, Smith GH 2008a. Selective segregation of DNA strands persists in long-label-retaining mammary cells during pregnancy. Breast Cancer Res 10: R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH 2008b. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci 105: 14891–14896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Mack DL, Booth BW, Smith GH 2007. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci U S A 104: 3871–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Wagner KU, Smith GH 2005. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-β1 expression. Oncogene 24: 552–560 [DOI] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE 2008. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 3: 429–441 [DOI] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA 2000. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14: 650–654 [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA 1998. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci 95: 5076–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, Honjo T, Hennighausen L 2006. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol 293: 565–580 [DOI] [PubMed] [Google Scholar]
- Callahan R, Smith GH 2000. MMTV-induced mammary tumorigenesis: Gene discovery, progression to malignancy and cellular pathways. Oncogene 19: 992–1001 [DOI] [PubMed] [Google Scholar]
- Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S 2008. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature 453: 544–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepko G, Smith GH 1997. Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29: 239–253 [DOI] [PubMed] [Google Scholar]
- Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, Gurney A, Lewicki J, Clarke MF 2008. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells 26: 364–371 [DOI] [PubMed] [Google Scholar]
- Ciarloni L, Mallepell S, Brisken C 2007. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc Natl Acad Sci 104: 5455–5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP, Pelicci PG 2009. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell 138: 1083–1095 [DOI] [PubMed] [Google Scholar]
- Daniel CW 1975. Regulation of cell division in aging mouse mammary epithelium. Adv Exp Med Biol 61: 1–19 [DOI] [PubMed] [Google Scholar]
- Daniel CW, Aidells BD, Medina D, Faulkin LJ Jr 1975. Unlimited division potential of precancerous mouse mammary cells after spontaneous or carcinogen-induced transformation. Fed Proc 34: 64–67 [PubMed] [Google Scholar]
- Deome KB, Faulkin LJ Jr, Bern HA, Blair PB 1959. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res 19: 515–520 [PubMed] [Google Scholar]
- Faulkin LJ Jr, Deome KB 1960. Regulation of growth and spacing of gland elements in the mammary fat pad of the C3H mouse. J Natl Cancer Inst 24: 953–969 [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Illa-Bochaca I, Welm BE, Fleisch MC, Werb Z, Ortiz-de-Solarzano C, Barcellos-Hoff MH 2009. Mapping mammary gland architecture using multi-scale in situ analysis. Integ Biol 1: 80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW 2002. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev 16: 693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson B 2009. Do ‘basal-like’ breast cancers really exist? Nat Rev Cancer 9: 128–134 [DOI] [PubMed] [Google Scholar]
- Gyorki DE, Asselin-Labat ML, van Rooijen N, Lindeman GJ, Visvader JE 2009. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res 11: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson SE, Colditz GA, Willett WC 2004. Towards an integrated model for breast cancer etiology: The lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res 6: 213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C, Hinds PW 2010. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell 17: 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Gould MN, Clifton KH 1998. Quantitative studies of ductal versus alveolar differentiation from rat mammary clonogens. Proc Soc Exp Biol Med 219: 217–225 [DOI] [PubMed] [Google Scholar]
- Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M, Smalley MJ 2008. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics 9: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney NJ, Smith GH, Lawrence E, Barrett JC, Salomon DS 2001. Identification of stem cell units in the terminal end bud and duct of the mouse mammary gland. J Biomed Biotechnol 1: 133–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ND, Clifton KH 1993. Characterization of rat mammary epithelial cell subpopulations by peanut lectin and anti-Thy-1.1 antibody and study of flow-sorted cells in vivo. Exp Cell Res 207: 74–85 [DOI] [PubMed] [Google Scholar]
- Kim ND, Oberley TD, Clifton K 1993. Primary culture of flow cytometry-sorted rat mammary epithelial cell (RMEC) subpopulations in a reconstituted basement membrane, Matrigel. Exp Cell Res 209: 6–20 [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH 1998. An entire functional mammary gland may comprise the progeny from a single cell. Development 125: 1921–1930 [DOI] [PubMed] [Google Scholar]
- Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH 1995. Ectopic TGF β 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol 168: 47–61 [DOI] [PubMed] [Google Scholar]
- Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z 2008. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 13: 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. 2003. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci 100: 15853–15858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS 2006. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 66: 6063–6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BY, McDermott SP, Khwaja SS, Alexander CM 2004. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci 101: 4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, Nicholson B, Cardiff RD, MacLeod CL 2001. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res 61: 8298–8305 [PubMed] [Google Scholar]
- Mallepell S, Krust A, Chambon P, Brisken C 2006. Paracrine signaling through the epithelial estrogen receptor α is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci 103: 2196–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulka LA, Triplett AA, Wagner KU 2007. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol 303: 29–44 [DOI] [PubMed] [Google Scholar]
- Medina D 2000. The preneoplastic phenotype in murine mammary tumorigenesis. J Mammary Gland Biol Neoplasia 5: 393–407 [DOI] [PubMed] [Google Scholar]
- Medina D 2002. Biological and molecular characteristics of the premalignant mouse mammary gland. Biochim Biophys Acta 1603: 1–9 [DOI] [PubMed] [Google Scholar]
- Medina D, Kittrell FS, Shepard A, Stephens LC, Jiang C, Lu J, Allred DC, McCarthy M, Ullrich RL 2002. Biological and genetic properties of the p53 null preneoplastic mammary epithelium. FASEB J 16: 881–883 [DOI] [PubMed] [Google Scholar]
- Moraes RC, Zhang X, Harrington N, Fung JY, Wu MF, Hilsenbeck SG, Allred DC, Lewis MT 2007. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development 134: 1231–1242 [DOI] [PubMed] [Google Scholar]
- Oakes SR, Naylor MJ, Asselin-Labat ML, Blazek KD, Gardiner-Garden M, Hilton HN, Kazlauskas M, Pritchard MA, Chodosh LA, Pfeffer PL, et al. 2008. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes Dev 22: 581–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin SH 2003. Priming the hematopoietic pump. Immunity 19: 633–634 [DOI] [PubMed] [Google Scholar]
- Pei XH, Bai F, Smith MD, Usary J, Fan C, Pai SY, Ho IC, Perou CM, Xiong Y 2009. CDK inhibitor p18INK4c is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell 15: 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen AM, Evers B, Prasad AA, Tanger E, Cornelissen-Steijger P, Jonkers J, van Lohuizen M 2008. Bmi1 regulates stem cells and proliferation and differentiation of committed cells in mammary epithelium. Curr Biol 18: 1094–1099 [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ 2008. Efficient tumour formation by single human melanoma cells. Nature 456: 593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raouf A, Zhao Y, To K, Stingl J, Delaney A, Barbara M, Iscove N, Jones S, McKinney S, Emerman J, et al. 2008. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell 3: 109–118 [DOI] [PubMed] [Google Scholar]
- Robinson GW, McKnight RA, Smith GH, Hennighausen L 1995. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development 121: 2079–2090 [DOI] [PubMed] [Google Scholar]
- Rudland PS, Ormerod EJ, Paterson FC 1980. Stem cells in rat mammary development and cancer: A review. J R Soc Med 73: 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE 2006. Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88 [DOI] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ 2006. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res 8: R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ 2007. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol 176: 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GH 1996. Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat 39: 21–31 [DOI] [PubMed] [Google Scholar]
- Smith GH 2005. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development 132: 681–687 [DOI] [PubMed] [Google Scholar]
- Smith GH, Arthur LA, Medina D 1980. Evidence of separate pathways for viral and chemical carcinogenesis in C3H/StWi mouse mammary glands. Int J Cancer 26: 373–379 [DOI] [PubMed] [Google Scholar]
- Smith GH, Boulanger CA 2002. Mammary stem cell repertoire: New insights in aging epithelial populations. Mech Ageing Dev 123: 1505–1519 [DOI] [PubMed] [Google Scholar]
- Smith GH, Medina D 1988. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci 90: 173–183 [DOI] [PubMed] [Google Scholar]
- Smith GH, Strickland P, Daniel CW 2002. Putative epithelial stem cell loss corresponds with mammary growth senescence. Cell Tissue Res 310: 313–320 [DOI] [PubMed] [Google Scholar]
- Smith GH, Pauley RJ, Socher SH, Medina D 1978. Chemical carcinogenesis in C3H/StWi mice, a worthwhile experimental model for breast cancer. Cancer Res 38: 4504–4509 [PubMed] [Google Scholar]
- Smith GH, Vonderhaar BK, Graham DE, Medina D 1984. Expression of pregnancy-specific genes in preneoplastic mouse mammary tissues from virgin mice. Cancer Res 44: 3426–3437 [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Kuusk U, Emerman JT 1998. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63: 201–213 [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ 2006. Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997 [DOI] [PubMed] [Google Scholar]
- Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA 2008. β1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol 10: 716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiede BJ, Owens LA, Li F, DeCoste C, Kang Y 2009. A novel mouse model for non-invasive single marker tracking of mammary stem cells in vivo reveals stem cell dynamics throughout pregnancy. PLoS One 4: e8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE 2008. The mammary progenitor marker CD61/β3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res 68: 7711–7717 [DOI] [PubMed] [Google Scholar]
- Van Nguyen A, Pollard JW 2002. Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth. Dev Biol 247: 11–25 [DOI] [PubMed] [Google Scholar]
- Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH 2002. An adjunct mammary epithelial cell population in parous females: Its role in functional adaptation and tissue renewal. Development 129: 1377–1386 [DOI] [PubMed] [Google Scholar]
- Welm BE, Dijkgraaf GJP, Bledau AS, Welm AL, Werb Z 2008. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell 2: 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA 2002. Sca-1pos cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol 245: 42–56 [DOI] [PubMed] [Google Scholar]
- Williams JM, Daniel CW 1983. Mammary ductal elongation: Differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol 97: 274–290 [DOI] [PubMed] [Google Scholar]
- Xie T, Li L 2007. Stem cells and their niche: An inseparable relationship. Development 134: 2001–2006 [DOI] [PubMed] [Google Scholar]
- Yamaji D, Robinson GW, Na R, Feuermann Y, Pechhold S, Henninghausen L 2009. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev 23: 2382–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Medina D, DeOme KB, Daniel CW 1971. The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Exp Gerontol 6: 49–56 [DOI] [PubMed] [Google Scholar]
- Zhang M, Behbod F, Atkinson RL, Landis MD, Kittrell F, Edwards D, Medina D, Tsimelzon A, Hilsenbeck S, Green JE, et al. 2008. Identification of tumor-initiating cells in a p53 null mouse model of breast cancer. Cancer Res 68: 4674–4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Chehab R, Tkalcevic J, Naylor MJ, Harris J, Wilson TJ, Tsao S, Tellis I, Zavarsek S, Xu D, et al. 2005. Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J 24: 635–644 [DOI] [PMC free article] [PubMed] [Google Scholar]