Abstract
OBJECTIVE
Detailed investigations of cortical physiology require the ability to record brain electrical activity at a submillimeter scale. Standard intracranial electrodes result in significant averaging of potentials generated by large numbers of neurons. In contrast, microelectrode arrays (MEA) allow for recording of local field potentials (LFPs) and single unit activity. We describe our initial surgical experience with the NeuroPort™ MEA in a series of patients undergoing subdural electrode implantation for epilepsy monitoring.
TECHNIQUE
Seven patients were implanted with and underwent semichronic recording from the NeuroPort™ array during standard subdural electrode monitoring for epilepsy. The electrode was placed per company specifications in putative non-eloquent epileptogenic cortex. Following the monitoring period, MEAs were removed during explantation of subdural electrodes and resection of epileptogenic tissue.
RESULTS
Successful implantation of the MEA was achieved in all patients with minor operative difficulties. Robust and durable LFPs and single-unit recordings were obtained from all implanted individuals. Implantation times ranged from 3–28 days; histological analysis of implanted tissue demonstrated no significant tissue injury or inflammatory response. There were no neurological complications or infections associated with electrode implantation or prolonged monitoring. Two patients developed post-resection issues with wound healing at the site of scalp egress, one requiring operative wound revision.
CONCLUSIONS
Our experience demonstrates that semi-chronic micro-EEG recording can be safely and effectively achieved using the NeuroPort™ microarray. Although significant tissue injury, infection or CSF leak were not encountered, the large profile of the connection pedestal resulted in suboptimal wound closure and healing in several patients. We predict that this problem will be easily addressed in second-generation devices.
Keywords: Epilepsy surgery, intracranial EEG, microarray, neuroprosthetic, subdural electrodes
INTRODUCTION
Recording of electrical potentials from the human cerebral cortex has become increasingly relevant to current medical practice, most notably for the diagnosis and anatomical localization of epilepsy. Recent investigations have also focused on the development of devices, intended for chronic intracranial implantation, that allow for early detection and interruption of seizure activity as well as the testing of brain-machine interface paradigms (3–5).
In contrast to scalp recorded EEG, which reflects signals spatially averaged over several square centimeters of cerebral cortex (2), and standard intracranial grid electrodes, which similarly average electrical activity over more confined regions of cortex, newly developed microelectrode arrays are capable of recording brain electrical signals generated in a much more confined cortical domain (at a sub-millimeter scale). One such device is the NeuroPort™ microelectrode array (MEA), first developed for use in human neuroprosthetic systems. It consists of an array of platinum microelectrodes with 400 micron inter-electrode spacing, approximating the scale of cortical macrocolumns. Designed for subpial insertion, the device is capable of recording both unit activity and local field potentials (LFP). Preliminary data regarding safety and surgical implantation technique are provided by Hochberg et al. in a small cohort of patients with spinal cord injury/disease implanted with the NeuroPort MEA™ (at that time called the BrainGate™ system) as part of a clinical trial of a neuroprosthetic application (3). We describe our initial surgical and clinical experience with the implantation of the NeuroPort™ array in a cohort of epileptic patients undergoing craniotomy for subdural electrode insertion and chronic monitoring.
MATERIALS AND METHODS
Device description
The NeuroPort™ microelectrode array (Cyberkinetics Neurotechnology Systems Inc., Boston, MA) consists of 96 platinum microelectrodes, arranged in a 10 × 10 array without electrodes at the corners, affixed to a 4 mm2 silicon base. The electrodes are 1.5 mm long, are electrically insulated (excluding 70 microns of exposed tip), and taper to 3–5 microns in diameter. The array is designed for implantation to either 1.0 or 1.5 mm subpial depth based on the choice of device impactor. The device is FDA approved for chronic implantation up to 30 days. The MEA is attached by gold-wire microcable to a “pedestal,” designed for screw fixation to the skull and passage through the scalp for external connection to a data cable. The pedestal is designed with a built-in “break-away point” in case of excessive sideways force that might otherwise cause tissue injury. As our cohort was limited to patients with relatively bland seizures without postictal agitation or psychosis, the need for pedestal break-away was never invoked in our experience. The base of the pedestal serves as the electrical ground; two reference wires are attached to the pedestal for placement in a subdural or epidural location. A specialized/calibrated pneumatic impactor “wand” is used to accomplish rapid and relatively atraumatic penetration of the pia by the electrode array; a series of adjustable joints are assembled to allow micropositioning of the impactor wand for optimal array insertion.
Patient cohort
All patients approached for inclusion were previously selected to undergo craniotomy and implantation of subdural electrodes for intracranial EEG monitoring and localization of potential epileptogenic foci. Patients were further limited to those in whom preoperative testing identified a well-defined epileptogenic region, with implantation used to refine the area targeted for resection. All surgical procedures and subsequent EEG monitoring, relevant to the NeuroPort™ array, were approved by the Columbia University Institutional Review Board. Informed consent, independent from consent for implantation of intracranial electrodes, was obtained for implantation of the NeuroPort™ MEA, post-operative testing and data recording. The MEA was placed in non-eloquent and accessible cortex likely to be subsequently resected and, where possible, within the putative epileptogenic zone (based on non-invasive testing and, in some cases, supplemented by intraoperative corticography). A total of seven patients underwent implantation and semi-chronic recording between May 2006 and October 2007. Demographic data, clinical characteristics, and ultimate operative intervention of this cohort are outlined in Table 1.
Table 1.
| Patient | Age | Sex | Pre-op localization | Implant location | Duration | iEEG localization | Surgical intervention |
|---|---|---|---|---|---|---|---|
| 1 | 41 | F | R temporal | R middle temporal gyrus | 15 | R anterior/mesial temporal | R anteromesial temporal resection |
| 2 | 31 | F | R posterior quadrant | R superior parietal lobule | 3 | Focal R parietal | R parietal resection |
| 3 | 30 | M | L frontal | L middle frontal gyrus | 7 | Diffuse L frontal | Multiple subpial transections |
| 4 | 41 | M | L superior frontal | L middle frontal gyrus | 5 | Focal L frontal | L frontal resection |
| 5 | 33 | F | L temporal | L middle temporal gyrus | 6 | L anterior/mesial temporal | L anteromesial temporal resection |
| 6 | 49 | F | L posterior temporal | L middle temporal gyrus | 7 | L posterior temporal | L posterior temporal lesionectomy |
| 7 | 18 | F | R temporoparietooccipital | R angular gyrus | 28 | Diffuse R posterior temporal | R lateral temporal resection |
| Avg | 34.7 | 10.1 days | |||||
Surgical procedure
Representative intraoperative photographs are provided in Figure 1. Prior to incision, microarray impedance and function were verified on the sterile field away from the patient. All surgical activities, including anesthesia, positioning, craniotomy, and dural opening were performed as required for the planned implantation of the standard subdural electrodes. Prophylactic intravenous antibiotics were given prior to skin incision and maintained for twenty-four hours post-operatively as per our standard procedure for patients undergoing subdural electrode monitoring. Following exposure of the brain, the MEA implantation site was chosen based on elements of cortical and vascular anatomy, location within the targeted resection area, distance from functional/eloquent cortex, and factors related to fixation of the gold microcable to the dura. The positioning system for the impactor wand was brought onto the field, secured to a Greenberg retractor system, and the wand was preliminarily fixed in the area of array insertion. The MEA was then transferred to the surgical field, positioned appropriately, and gently compressed against the pial surface with the impactor wand. The impactor was triggered, resulting in trans-pial implantation of the microelectrodes. The gold microcable was sutured to the dura and the pedestal was affixed to the skull adjacent to the craniotomy using self-tapping screws. Final positioning of the subdural electrode strips/grids was completed and the dura was closed. Reference wires, with several millimeters of exposed contact at their ends, were left coiled within the epidural space. Final hemostasis was obtained, the bone flap was replaced, and the standard subdural electrode leads were tunneled through the skin in the usual fashion. Closure of the skin around the device pedestal was accomplished within the primary incision line in several patients, while separate stab incisions were fashioned for pedestal egress in the remainder of the cohort. Tight skin closure around the pedestal was optimized using 3-O nylon purse-string sutures and, in some cases, wrapping of the pedestal base with Xeroform gauze. The remainder of the wound was closed in the typical fashion and the patient was recovered from anesthesia. Connection of the subdural electrodes and connection to the microarray pedestal was typically instituted in the epilepsy monitoring unit on post-operative day one.
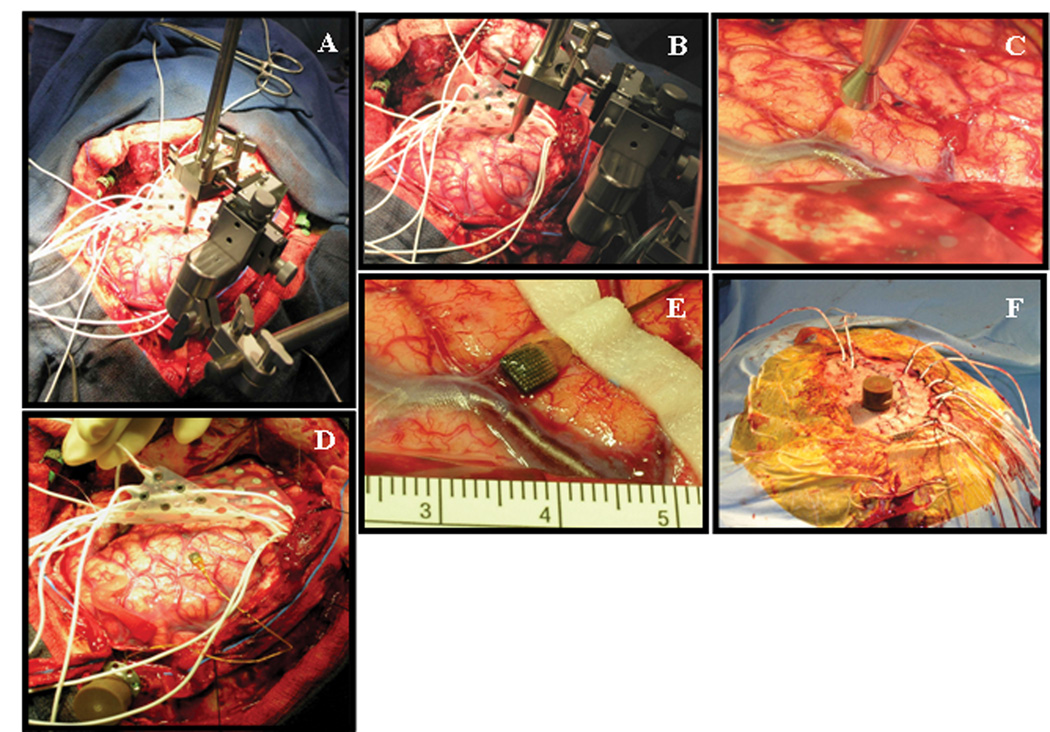
Figure 1.
Representative intraoperative photographs demonstrating elements of the microarray insertion procedure. A) Set-up of the surgical field following craniotomy, dural opening, and preliminary grid insertion. The positioning device for the impactor wand has been attached to a Greeenberg retractor system. B) Close-up view of the positioning device, demonstrating multi-dimensional capability for fine-tuning of the impactor wand. C) Close-up of the impactor wand near the cortical target, in preparation for microarray implantation. D) View of microarray following trans-pial insertion and stabilization with silk sutures to the dural edge. The device pedestal has been fixed to the skull at the edge of the craniotomy. E) Close-up of the implanted microarray, demonstrating mild sub-pial hemorrhage. F) Final appearance of the device pedestal following wound closure. In this particular patient, the pedestal was brought through the scalp flap via a separate stab incision.
RESULTS
Clinical information
A total of seven patients (five females, two males) were implanted with and underwent semi-chronic recording from the MEA. Average age of the cohort was 34.7 years. Data regarding type of epilepsy, pre-operative diagnostic workup, and location of grid implantation are provided in Table 1. There were three lateral temporal, two frontal, and two parietal implants. Implant times ranged from 3 to 28 days with an average recording period of 10.1 days. The external pedestal connection was generally well tolerated by all patients, although the short length of the cable interconnecting the pedestal on the patient’s head with the amplifier (three feet) and amplifier power cable (six feet) made sleep and daily activities challenging at times for some patients.
Throughout the recording interval, durable LFPs as well as single unit activity could be obtained from most patients. We found that the quality of recording improved over time, most notably after the first 48 hours of implantation. Referential recordings were often noisy, perhaps related to trapped air, and a “ground reference” was used on some occasions. Recording from the array became limited several days prior to explantation in one individual (described below). On return to the operating room, four patients underwent neocortical resections and two patients had anteromesial temporal resections. The remaining individual received multiple subpial transections (MST) without tissue resection. In all cases, the site of implantation was either resected or treated with MST.
Technical aspects of surgical procedure
Successful implantation was achieved in all patients who agreed to participate in the study. Overall, there was little difficulty in identifying an appropriate gyral surface for implantation. Initial positioning of the electrode was somewhat cumbersome, as the gold microcable retained significant torsional “memory” that resisted free placement of the array. We were able to overcome this technical issue by suturing the microcable to the dura prior to final positioning of the array. In two patients, immediate intraoperative reinsertion of the MEA was required: the MEA in one patient was inadvertently pulled out of the cortex due to accidental traction on the gold microcable, while in the second patient initial implantation was performed over a particularly concave gyral surface, resulting in non-flush positioning of the grid and variable penetration of the microelectrodes. In both cases re-implantation was accomplished without difficulty and excellent potentials were subsequently recorded from the MEA. The operative time associated with MEA implantation was typically less than thirty minutes.
We employed several different strategies to close the scalp around the device pedestal. In two patients the pedestal was brought through the skin in the primary incision line and watertight closure was obtained using nylon purse-string sutures around the pedestal itself. A T-limb was added to the primary incision line and closed as above for an additional two patients, to allow for pedestal egress. In the remaining three patients a separate incision was made within the scalp flap.
Data acquisition/analysis
Local field potentials and single-unit activity were successfully recorded from the MEA in all implanted patients (Figure 2). Our initial characterization of the resultant data will be discussed elsewhere (Schevon CA et al, submitted for publication). Briefly, we noted the frequent occurrence of “micro” epileptiform discharges and “microseizures” that were undetected by the standard subdural electrode arrays. The extent of these microdischarges, often isolated to focal areas of the array, suggested that the observed activity was generated within isolated sub-millimeter cortical domains. In two cases, seizure initiation was directly related to microepileptiform activity. These observations support the view that high spatial resolution EEG data, recorded with the NeuroPort MEA and similar devices, are likely to provide important insights into the process of epileptogenesis and perhaps other aspects of cortical physiology.
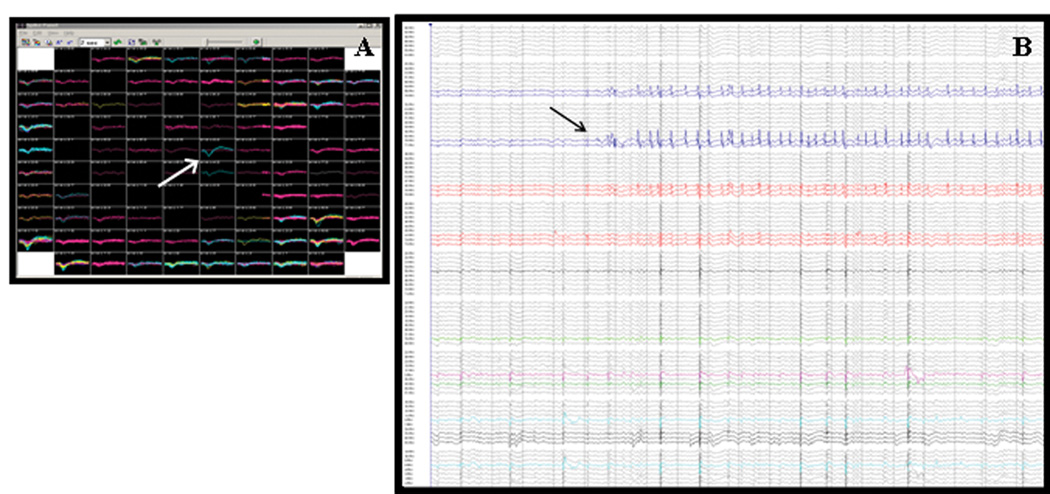
Figure 2.
A) Screenshot from the real-time data montage for a single NeuroPort™ microarray, depicting unit activity (arrow) recorded during a two-second period. Each individual box represents activity recorded from a single microelectrode. Two-dimensional relationships between electrodes are equivalent to their physical positioning within the array. B) Local field potential activity recorded during a typical 10-second period. The arrow points to a “microseizure”, restricted to only eight electrodes. Local field potentials were recorded from over 80% of the microelectrodes in this array.
Complications
There were no major complications associated with implantation, post-operative monitoring, or removal of the MEA. Although the MEA was positioned to avoid injury to visible cortical vessels during implantation, several patients had minor subpial bleeding associated with the initial impaction of the MEA. In all cases subpial bleeding resolved quickly with gentle irrigation over the implanted region. On return to the operating room following the period of monitoring, visual inspection of implanted cortex indicated no evidence for significant tissue response or deformation associated with device presence (Figure 3). Histological analysis of implanted cortex demonstrated minimal microscopic tissue response, including microglial activation and scattered microhemorrhage (data not shown). The electrode tips were identified in layers IV to V of cerebral cortex. There were no cases of obvious parenchymal hemorrhage or injury associated with device implantation, although one patient was found to have a moderate subtemporal hematoma on re-operation that included the region of MEA implantation; in this case recording of local field potentials was preserved, but recording of action potentials had been lost. We speculate that the array may have been partially lifted toward the cortical surface. The patient exhibited no associated clinical signs or symptoms during the period of implantation and remained at her neurological baseline following the subsequent resection procedure.
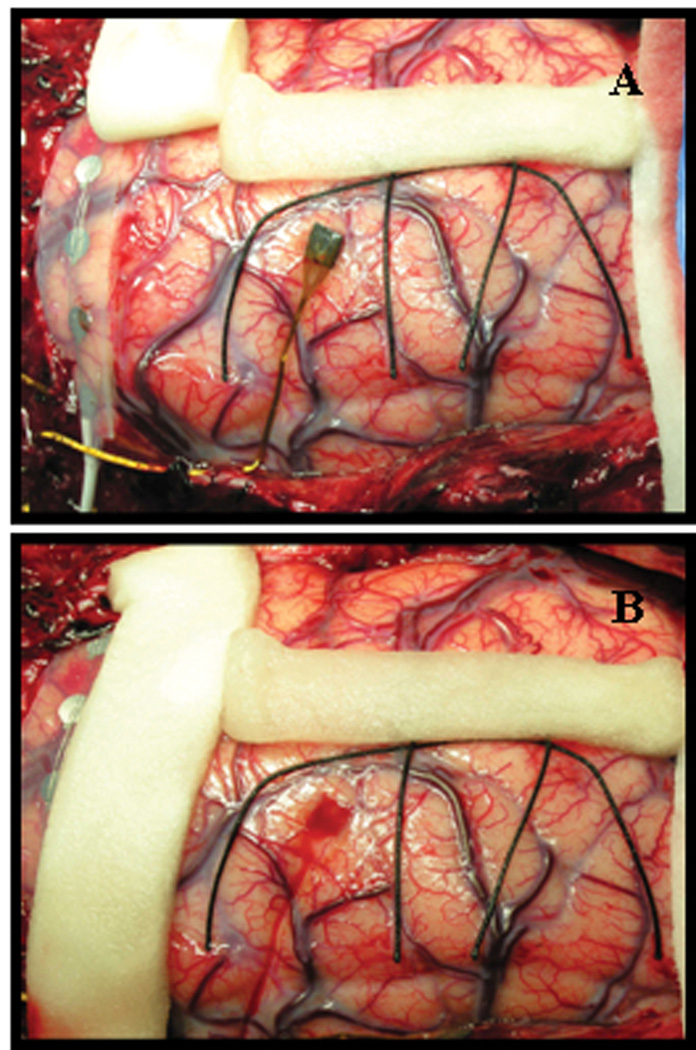
Figure 3.
Tissue response to semi-chronic microarray implantation. A) Appearance of MEA and surrounding cortex after 10 days of implantation. There is no macroscopic evidence of local tissue response/inflammation. B) Following removal of the array, mild hyperemia without evidence of tissue damage is evident in the cortex adjacent to microarray contact.
There were no infections associated with the presence of the MEA. Post-implantation CSF leak was noted in several patients, primarily associated with the site of pedestal egress through the scalp. In the first patient, recording from the MEA was interrupted several times due to CSF leakage into the electrical connection between the pedestal and data cable, each time requiring removal of dressing and attachment of a fresh cable. This problem was addressed in subsequent patients through the use of an O-ring seal at the base of the pedestal attachment to the data cable. Two patients experienced issues with delayed or poor wound healing at the site of scalp closure around the pedestal. In both of these patients, the pedestal was brought out in line with the primary incision, resulting in the need for tight nylon suturing to achieve adequate skin approximation at the pedestal base. One patient required operative wound revision, due to an area of poor healing and localized necrosis associated with the site of pedestal egress through the primary incision. There was no evidence of either superficial or deep infection and the wound subsequently healed well. The other patient, who was implanted for the longest period of time (28 days), developed a slightly widened scar at the pedestal egress site.
DISCUSSION
Prior to this study, the NeuroPort™ MEA had been chronically implanted in the human brain in quadriplegic patients with the goal of utilizing recorded brain activity to control a computer-based neuroprosthetic system (3). The current study was initiated with the goal of determining whether this MEA could be safely implanted in patients with epilepsy, undergoing implantation of standard subdural electrodes, to yield additional high-resolution recording. Our initial experience with the NeuroPort™ MEA in a series of patients undergoing intracranial EEG monitoring confirmed that safe semi-chronic recording is possible, and that high-resolution brain electrical activity, including both local field potentials and unit activity, is easily recorded.
Although there were some initial technical difficulties associated with device insertion, the surgical procedure is ultimately straightforward and does not significantly add to the operative time. It should be noted that the presence of a reasonably experienced assistant is helpful during the initial positioning, trans-pial insertion, and dural fixation due to torsional effects of the gold cable. These technical difficulties may be mitigated in the future with improved intraoperative stabilization hardware and the development of second-generation devices. We experienced issues with wound healing in several patients, secondary to the need for incision closure around the external connection pedestal. Within our cohort, it became clear that, where possible, a separate incision is helpful for pedestal egress with regard to final wound closure and healing. Although we did not experience any infections, several of our initial patients had CSF leakage at the pedestal site.
Although the currently available NeuroPort™ MEA demonstrated utility in our cohort, further development of a practical clinical MEA for chronic use in humans will require some modification of the device. First, the gold cable connecting the MEA and the skull-mounted pedestal should be modified to have little or no torsional “memory,” in order to facilitate ideal positioning of the MEA prior to impaction through the pial surface. Second, the profile of the skull-mounted pedestal must be minimized and designed for subgaleal positioning. Ideally, the pedestal would be replaced with an external connection cable that approximates the physical characteristics of leads used in standard subdural electrodes. Ultimately, wireless technology might be employed to minimize CSF leakage and infection risk.
Our experience indicates that durable LFPs and single unit activity can be recorded from patients implanted for long periods of time (up to one month in our cohort). We obtained evidence for highly focal epileptiform activity, detected by array microelectrodes, that was not detected by the standard grid electrodes. These data may provide important insights into the functional disturbances found in epileptogenic cortex. However, the potential contribution of the NeuroPort MEA to current protocols for surgical epilepsy evaluation remains as yet unclear.
It is clear that the design and construction of new generations of implantable hardware intended for long-term recording from (and potential stimulation of) the human brain will provide smaller-profile and increasingly sensitive technology. In addition to use in clinical monitoring for the localization of epileptogenic cortex, such technology will be critical in the continued evolution of devices designed for the early detection and termination of evolving seizure activity in epileptic patients, as well as in the implementation of a functional brain-machine interface. It is essential that neurological surgeons, experienced with the implantation and potential clinical complications of these technologies, remain integral contributors to the design and insertion protocols of future microelectrode arrays.
Acknowledgments
Grant support: there was no specific grant support.
Footnotes
Financial disclosures: the authors have no relevant financial interests to report.
Authorship justification:
Allen Waziri – first surgical assistant, data collection, writing of manuscript
Catherine Schevon – clinical care of patients, data analysis, project design
Joshua Cappell – project design and technical set-up, data analysis
Ronald Emerson – co-initiator of project, epilepsy project director, liason to CKI, technical set-up, clinical care of patients
Guy McKhann 2nd – attending surgeon (two cases)
Robert R. Goodman – co-initator of project, attending surgeon (five cases)
Contributor Information
A. Waziri, Department of Neurological Surgery, The Neurological Institute, Columbia University, New York, NY..
C. A. Schevon, Department of Neurology, The Neurological Institute, Columbia University, New York, NY..
J. Cappell, Department of Neurology, The Neurological Institute, Columbia University, New York, NY..
R. G. Emerson, Department of Neurology, The Neurological Institute, Columbia University, New York, NY..
G. M. McKhann, 2nd, Department of Neurological Surgery, The Neurological Institute, Columbia University, New York, NY..
R. R. Goodman, Department of Neurological Surgery, The Neurological Institute, Columbia University, New York, NY..
REFERENCES
- 1.Bartfeld E, Grinvald A. Relationships between orientation-preference pinwheels, cytochrome oxidase blobs, and ocular dominance columns in primate striate cortex. Proc Natl Acad Sci USA. 1992;89:11905–11909. doi: 10.1073/pnas.89.24.11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebersole JS. Defining epileptogenic foci: past, present, future. J Clin Neurophys. 1997;14(6):470–483. doi: 10.1097/00004691-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 4.Kossoff EH, Ritzl EK, Politsky JM, Murro AM, Smith JR, Duckrow RB, Spencer DD, Bergey GK. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45:1560–1567. doi: 10.1111/j.0013-9580.2004.26104.x. [DOI] [PubMed] [Google Scholar]
- 5.Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19:164–168. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- 6.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]