Abstract
The Dpp/BMP signaling pathway is highly conserved between vertebrates and invertebrates. The recent molecular characterization of the Drosophila crossveinless-2 (cv-2) mutation by Conley and colleagues introduced a novel regulatory step in the Dpp/BMP pathway (Development 127 (2000) 3945). The CV-2 protein is secreted and contains five cysteine-rich (CR) domains similar to those observed in the BMP antagonist Short gastrulation (Sog) of Drosophila and Chordin (Chd) of vertebrates. The mutant phenotype in Drosophila suggests that CV-2 is required for the differentiation of crossvein structures in the wing which require high Dpp levels. Here we present the mouse and human homologs of the Drosophila cv-2 protein. The mouse gene is located on chromosome 9A3 while the human locus maps on chromosome 7p14. CV-2 is expressed dynamically during mouse development, in particular in regions of high BMP signaling such as the posterior primitive streak, ventral tail bud and prevertebral cartilages. We conclude that CV-2 is an evolutionarily conserved extracellular regulator of the Dpp/BMP signaling pathway.
Keywords: Crossveinless-2, Chordin, Twisted gastrulation, Short gastrulation, BMP, Dpp, Tolloid, CR domain, von Willebrand factor type D domain, TIL domain, Extracellular matrix, Organogenesis, Somite, Sclerotome, Notochord, Neural crest, Dorsal root ganglion, Branchial arch, Cartilage, Vertebra, Intervertebral disc, Lung
1. Results
The Dpp/BMP (Decapentaplegic/Bone Morphogenetic Protein) signaling pathway is one of the best examples of conservation of mechanisms of development between vertebrates and invertebrates. In addition to the signal transduction machinery, a network of extracellular signaling regulators is conserved. Binding of BMP to the antagonist Sog/Chordin blocks Dpp/BMP activity. This inhibition is itself regulated by the co-factor Twisted gastrulation (Tsg) and by cleavage by the metalloproteinase Tolloid (reviewed by De Robertis et al., 2000). The recent molecular characterization of the crossveinless-2 (cv-2) mutation, which affects Drosophila wing patterning, introduced a novel regulator of Dpp signaling (Conley et al., 2000). The cv-2 fruitfly mutation results in the loss of the posterior crossvein and of the tip of longitudinal veins. Similar phenotypes are observed in mild dpp loss of function mutations, in mutants of the tolloid homolog tolkin, and by overexpression of the BMP antagonist Sog (de Celis, 1997; Conley et al., 2000; Yu et al., 2000). Thus, the phenotype of cv-2 suggests that this gene promotes high BMP signaling. Another Drosophila mutation with an identical phenotype in wing venation is crossveinless (cv or cv-1), which was first identified by Calvin Bridges many years ago (Bridges, 1920). Interestingly, the cv-1 mutation maps to a gene encoding a second Drosophila Twisted gastrulation homolog, dTsg-2 (L. Marsh, communication to FlyBase FBgn0000394). The CV-2 protein contains five cysteine-rich domains (CRs) with sequence similarity to the BMP-binding domains of Chordin (Conley et al., 2000).
In this study we present the cloning of the first vertebrate homolog of the Drosophila cv-2 gene. Mouse CV-2 is expressed dynamically during mouse embryogenesis, in particular at multiple sites of high BMP signaling during development.
1.1. Cloning of the mouse CV-2 homolog
We identified a mouse EST (GenBank AK014221) as a candidate for the homolog of the Drosophila crossveinless-2 gene. BLAST search of the GenBank database at NCBI (http://www.ncbi.nlm.nih.gov/blast/) uncovered another partial mouse EST (BE289531), which was used to screen a mouse embryonic day 8.5 cDNA library. We cloned a 3.8 kb full-length cDNA containing a 570 bp 5′ untranslated region (UTR), a 2 kb open reading frame and a 1.2 kb 3′ UTR. This clone sequence was deposited in GenBank as AF454954.
BLAST searches of NCBI genomic databases showed that the mouse CV-2 locus is located on chromosome 9, band 9A3 (http://www.ncbi.nlm.nih.gov/genome). Additional searches identified several human ESTs (BF997956, BF999553) and a putative human genomic homolog located on chromosome 7p14 (Contig NW_000351.1). A recent report from NCBI indicates a predicted protein XP_166592 at this locus, which was annotated as ‘similar to crossveinless-2’ but lacked the two N-terminal CR domains and the signal peptide. We could deduce the complete sequence of the putative human CV-2 transcript by aligning human genomic sequence with the mouse cDNA; the predicted human CV-2 amino acid sequence is shown in Fig. 1A.
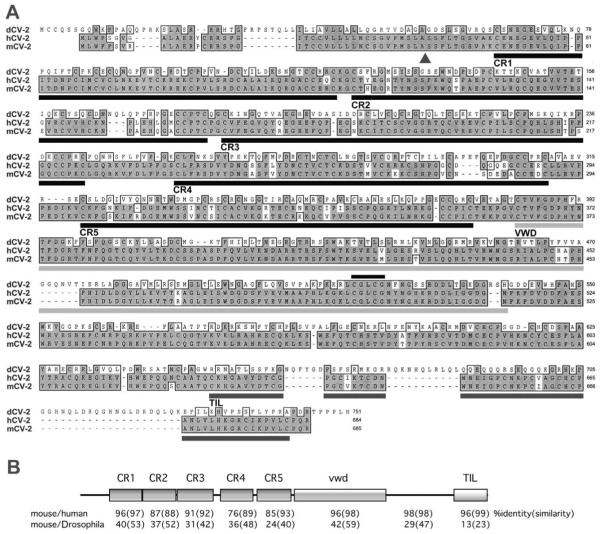
Fig. 1.
Comparison of the mouse, human and Drosophila CV-2 proteins. (A) Alignment of the protein sequences using ClustalW. Amino acid identities are indicated by a dark shading, and similarities with a lighter shade. The arrowhead indicates the cleavage site for the predicted mammalian signal peptides. Bars underneath the sequences indicate the structural domains present in the mammalian proteins: CR 1–5, Chordin-like cysteine-rich domains; VWD, von Willebrand factor type D domain; TIL, Trypsin Inhibitor-like cysteine-rich domain. The black bar above the sequences indicates the disulfide isomerase motif, CGLCG, conserved in all three proteins. (B) Structure of the mouse protein (GenBank AF454954) and comparison of its amino acid sequence to the human predicted protein and to Drosophila CV-2 (NP_524809).
1.2. Comparison of the mouse, human and Drosophila CV-2 proteins
The mouse Crossveinless-2 cDNA encodes a 685 amino acid protein with a putative signal peptide (SignalP V1.1; Nielsen et al., 1997) (Fig. 1A). The mouse CV-2 protein is secreted; it was detected in the supernatant of 293T cells transiently transfected with a construct encoding the full-length protein carrying a C-terminal epitope tag (data not shown). When synthetic mRNA encoding mouse CV-2 transcripts were injected into Xenopus embryos, secondary axes of the types seen for other regulators of BMP signaling were induced (our unpublished observations). The mouse CV-2 protein shares 30% overall identity (44% similarity by ClustalW analysis) with its fly homolog and 93% identity (96% similarity) with the predicted human CV-2 protein (Fig. 1B). The mouse protein contains five Chordin-like cysteine-rich domains and one von Willebrand factor type D domain (vwd), as described for Drosophila CV-2 (Conley et al., 2000). The CR domains of CV-2 share sequence similarities with the cysteine-rich domains (CR) of Chordin, which bind BMP (reviewed in Garcia Abreu et al., 2002). In addition, a search of the Pfam database revealed the existence of a carboxy-terminal Trypsin Inhibitor-like cysteine-rich domain (TIL; Fig. 1A) which was absent from the Drosophila CV-2 protein (http://www.sanger.ac.uk/Software/Pfam/; Bateman et al., 2002). The TIL domain and the von Willebrand factor D domain could mediate the oligomerization of CV-2 or its interaction with other proteins. A motif noted by Conley et al. (2000), the CGLCG sequence observed in disulfide isomerases could regulate the formation of disulfide bonds; it is conserved in the mouse, human and Drosophila CV-2 proteins (Fig. 1A).
1.3. Expression of the mouse CV-2 gene
CV-2 expression during mouse embryogenesis was investigated by in situ hybridization on whole mounts and sections. At mid-gastrulation, CV-2 transcripts were detected in the proximal part of the embryonic region (Fig. 2A). In histological sections, CV-2 is expressed in the mesoderm and can be detected both in the posterior primitive streak and in the precardiac mesoderm (Fig. 2B). By embryonic day 9 (E9.0), CV-2 expression was observed in the ventral tail bud (Fig. 2C,D). CV-2 was also expressed at the closing anterior neuropore and in the roof of the neural tube (Fig. 2C,D).
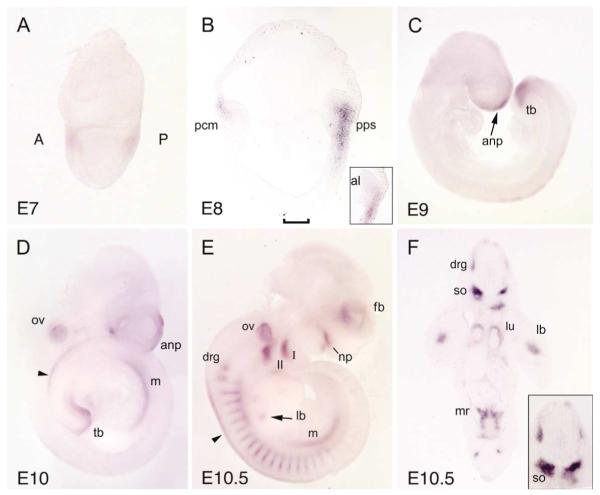
Fig. 2.
Expression of CV-2 during early mouse development. In situ hybridizations on (A) mid-gastrula, (B) sagittal sections at head-fold stage (note the lack of expression in the allantois, as shown in the inset), (C) E9 embryo, (D) E10 embryo, (E) E10.5 embryo, and (F) transverse section of E10.5 embryo (inset: higher magnification of the neural tube and somites); note that, in histological section, strong staining in the sclerotome is seen, as well as weaker expression in the mesonephric ridge, lung and limb buds. A, anterior region; al, allantois; anp, anterior neuropore; drg, dorsal root ganglion; fb, forebrain; lb, limb bud; lu, lung; m, mesonephros; mr, mesonephric ridge; np, nasal process; ov, otic vesicle; P, posterior; pcm, precardiac mesoderm; pps, posterior primitive streak; so, somite; tb, tail bud; I and II, first and second branchial arches; bracket in (B) indicates Hensen’s node; arrowheads in (D,E) indicate the neural tube roof.
CV-2 expression is dynamic and evolved rapidly between E9 and E10.5 as additional domains of expression appeared, such as the mesonephric ridge (Fig. 2E,F). In the developing head, CV-2 expression was limited to the telencephalon and several craniofacial structures: the tip of the nasal processes, the posterior part of the first branchial arch, the second branchial arch and the otic vesicle (Fig. 2E). The expression started in the central part of the anterior limb buds at E10.5 (Fig. 2E,F). In the lung, CV-2 expression was limited to the mesenchyme from the onset of its formation and was maintained through embryonic development (Figs. 2F and 3A,B). In adult mice, the lung was the tissue with the strongest expression of CV-2, as determined by northern blot (Fig. 3E).
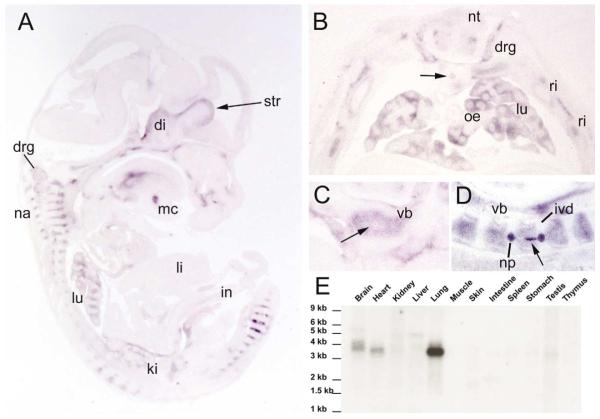
Fig. 3.
CV-2 expression during late organogenesis and in adult mouse tissues. In situ hybridization on (A) parasagittal section of E13.5 embryo, (B) transverse section of E14.5, (C) vertebral body at E14.5, (D) sagittal section through the spinal cord at E14.5; (E) Northern blot analysis of CV-2 expression in adult mouse tissues. A major transcript of 3.8 kb was detected strongly in the lung and heart; two transcripts of 3.8 and 4 kb were detected in the brain, suggesting CV-2 produces alternative transcripts. di, diencephalon; drg, dorsal root ganglion; in, intestine; ivd, intervertebral disc; ki, kidney; li, liver; lu, lung; mc, Meckel’s cartilage; na, neural arches; np, nucleus pulposus deriving from the notochord in the intervertebral disc; nt, neural tube; oe, esophagus; ri, ribs; str, striatum of the basal ganglia; vb, cartilage of the vertebral bodies; arrows indicate the notochord in (B–D).
During somitogenesis, CV-2 expression was detected in dorsal root ganglia and migrating neural crest cells (Fig. 2E,F). In histological sections at E10.5, intense CV-2 expression was detected in the somite on each side of the notochord, in the presumptive sclerotome (Fig. 2F, see inset). The notochord was negative for CV-2 expression at that stage but later, at E14.5, CV-2 expression was detected in the caudal region of the notochord and in the nucleus pulposus of the intervertebral disc (Fig. 3D). At E13 and E14.5, CV-2 expression was observed in multiple cartilages of the developing skeleton (Fig. 3A–D). In the axial skeleton, CV-2 expression in the cartilage of the developing vertebral bodies was complementary to that of Chordin, which is expressed in the intervertebral regions (Figs. 3C,D and 4). These mutually exclusive expression domains are reminiscent of those observed in Drosophila wing discs, in which cv-2 is expressed in the veins and short gastrulation, the Chordin homolog, is expressed in the intervein domains (Conley et al., 2000; Yu et al., 1996).
Fig. 4.
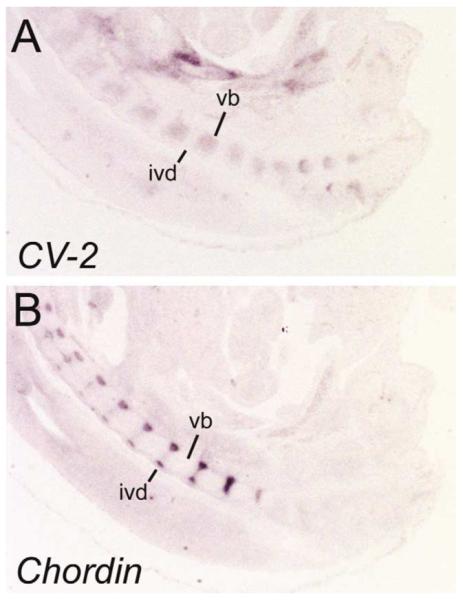
CV-2 and Chordin are expressed in a complementary pattern in the vertebral bodies and intervertebral discs at E14.5. Expression of (A) CV-2 and (B) Chordin transcripts on parasagittal sections of a E14.5 embryo. vb, cartilage of the vertebral bodies; ivd, intervertebral discs.
We conclude that CV-2 encodes a novel secreted protein containing CR domains similar to the BMP binding domains of Chordin. CV-2 is expressed in regions of high BMP signaling, such as the posterior primitive streak, the ventral tail bud, the growing branchial arches and prevertebral cartilages (Hogan, 1996). These domains correlate with the expression of several BMP genes. For example, the ventral mesoderm of the tail bud strongly expresses BMP4 (Goldman et al., 2000), BMP5 and BMP7 are expressed in the midline of the forebrain (Solloway and Robertson, 1999), and BMP6 is expressed in a similar manner to CV-2 on the dorsal side of the neural tube (Kim et al., 2001). The BMP antagonist Chordin is expressed in a complementary pattern in some of these tissues (Fig. 4). For example, Chordin is expressed in the dorsal mesoderm (notochord and Hensen’s node; Bachiller et al., 2000) and intervertebral regions whereas CV-2 is expressed in the ventral mesoderm and prevertebrae. In zebrafish, Chordin transcription is negatively regulated by BMP signaling (Schulte-Merker et al., 1997). The expression pattern of CV-2 suggests that this gene is positively regulated by BMP signals. Thus, BMP homeostasis in the embryo may be regulated by two secreted molecules, CV-2 and Chordin, that contain similar cysteine-rich BMP-binding modules and are under opposing transcriptional controls.
2. Methods
The full-length CV-2 cDNA contained in pCMV-Sport 2 was isolated from a Superscrip™ Mouse 8.5 day embryo cDNA library (Life Technologies). The probe used was a 600 bp EcoRI-XbaI fragment isolated from a partial mouse EST (GenBank BE289531) containing 400 bp of coding sequence and 200 bp of 3′ UTR. This fragment was also used as a probe to hybridize a mouse Multiple Choice™ Northern Blot (OriGene Technologies, Inc.) that contained 2 μg of polyA 1 RNA in each lane. Digoxigenin-labeled anti-sense probes were synthesized using the CV-2 EST plasmid linearized with EcoRI. For mouse Chordin, a pCS2-myc-Chordin plasmid was linearized with HindIII. Both probes were transcribed using the T7 RNA polymerase (Boehringer Mannheim). In situ hybridizations were realized as previously described (http://www.hhmi.ucla.edu/derobertis/; Belo et al., 1997; Henrique et al., 1995). Sequence alignments were produced using the ClustalW program (Mac Vector 6.5; Thompson et al., 1994).
Acknowledgements
We thank the members of our laboratory for technical help and critical comments. C.C. is a Human Frontier Science Program Organization postdoctoral fellow. This work was supported by the Howard Hughes Medical Institute.
References
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer EL. The Pfam protein families database. Nucleic Acids Res. 2002;30:276–280. doi: 10.1093/nar/30.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech. Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Bridges C. The mutant crossveinless in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1920;6:660–663. doi: 10.1073/pnas.6.11.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. doi: 10.1242/dev.127.18.3947. [DOI] [PubMed] [Google Scholar]
- de Celis JF. Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development. 1997;124:1007–1018. doi: 10.1242/dev.124.5.1007. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Larrain J, Oelgeschlager M, Wessely O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Abreu J, Coffinier C, Larrain J, Oelgeschlager M, De Robertis EM. Chordin-like CR domains and the regulation of evolutionarily conserved extracellular signaling systems. Gene. 2002;287:39–47. doi: 10.1016/s0378-1119(01)00827-7. [DOI] [PubMed] [Google Scholar]
- Goldman DC, Martin GR, Tam PPL. Fate and function of the ventral ectodermal ridge during mouse tail development. Development. 2000;127:2113–2123. doi: 10.1242/dev.127.10.2113. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev. Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126:1753–1768. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Sturtevant MA, Biehs B, Francois V, Padgett RW, Blackman RK, Bier E. The Drosophila decapentaplegic and short gastrulation genes function antagonistically during adult wing vein development. Development. 1996;122:4033–4044. doi: 10.1242/dev.122.12.4033. [DOI] [PubMed] [Google Scholar]
- Yu K, Srinivasan S, Shimmi O, Biehs B, Rashka KE, Kimelman D, O’Connor MB, Bier E. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development. 2000;127:2143–2154. doi: 10.1242/dev.127.10.2143. [DOI] [PubMed] [Google Scholar]