Abstract
Rationale
Individual differences in subjective alcohol effects have been shown to differ by risk status (e.g., family history of alcoholism) and to predict future risk for alcohol-related problems. Presumably, individual differences in both stimulant and sedative responses affect the rewarding value of drinking which, in turn, impacts future drinking behavior. Although plausible, this theoretical model is largely untested.
Objectives
The current study attempted to provide experimental evidence for the impact of subjective alcohol responses on within session drinking behavior.
Materials and methods
Using a placebo-controlled between-subjects alcohol administration paradigm, experiences and evaluations of stimulant and sedative alcohol effects (after a target dose of 0.06 g%) were assessed as predictors of ad-libitum consumption in the context of anticipatory stress.
Results
Analyses indicated that an initial dose of alcohol increased experiences of both stimulation and sedation although stimulant effects were evaluated much more positively. In addition, stimulant effects after a priming dose predicted further consumption, whereas sedative effects did not.
Conclusions
At least among moderate to heavy drinking college students, stimulant alcohol effects are more reinforcing and predict within session drinking behavior under social stress. Increased attention should be given to stimulant alcohol effects as a risk factor for excessive consumption in this population. Incorporating information about stimulant alcohol effects in prevention and intervention programs may also be important if additional research supports the current results.
Keywords: Alcohol challenge, Stimulant effects, Sedative effects, Alcohol consumption, Priming, Placebo control, Experiences, Evaluations
Introduction
Although alcohol consumption is widespread in the USA, the vast majority of drinkers do not develop alcohol-use disorders. The American Psychiatric Association (DSM-IV-TR, 2000) reports that between two thirds and 90% of American adults have consumed alcohol during their lifetime, whereas the lifetime risk for alcohol dependence is approximately 15%, with an annual rate for alcohol dependence of approximately 5%. Although heavy alcohol consumption is a risk factor, consumption of alcohol alone is not sufficient for the development of alcohol-use disorders. Thus, differences at the environmental and individual levels are clearly important in understanding the development of alcohol use disorders.
At the individual level, one of the most robust risk factors is a positive family history of alcohol use disorders. Individuals with a positive family history of alcoholism are at substantially increased risk for developing alcohol abuse and dependence (Heath et al. 1997; Merikangas et al. 1998). Furthermore, there is evidence that much of the familial transmission of alcohol use disorders is due to genetic influence (McGue 1998; Prescott and Kendler 1999). Although there are likely multiple mechanisms through which genetic risk contributes to alcohol problems (e.g., personality traits, neurotransmitter function, etc.), one important mechanism appears to be the subjective response to alcohol (Krueger et al. 2002; Slutske et al. 1998; Schuckit et al. 2001).
Individual differences in subjective response to alcohol have been linked to both a positive family history of alcohol problems and increased risk for developing drinking problems. Sons of alcoholics have been shown to have a reduced subjective response to alcohol (Schuckit et al. 1987) and less body sway at 135 min after a low dose of alcohol (Schuckit 1985). Low-level responders are thought to consume more alcohol to feel the effects of alcohol that others receive at lower doses, placing them at risk for overconsumption. Consistent with this theoretical model, individuals with a low subjective response to alcohol in early adulthood were shown to be at increased risk for the development of alcohol use disorders 15 years later (Schuckit and Smith 2000). Although this model has received considerable support, some have suggested that the low subjective response characteristic of individuals with a positive family history of alcoholism is restricted to the descending limb of the blood alcohol curve (Newlin and Thomson 1990). Further, there is evidence to suggest that a family history of alcoholism may actually be associated with a stronger response to alcohol as blood alcohol levels are rising (Kaplan et al. 1983; Nagoshi and Wilson 1987).
In an attempt to provide a more comprehensive model of subjective response to alcohol, Newlin and Thomson developed the “differentiator model” which hypothesizes that individuals at high risk for developing alcohol-related problems have reduced sensitivity to the sedative effects and increased sensitivity to the stimulant effects of alcohol (Newlin and Thomson 1990). Support for this model has been found in heavy drinking populations. Compared to lighter drinkers, heavy drinkers are more sensitive to alcohol’s stimulating effects on the rising limb of the BAC curve and less sensitive to the sedating effects on the descending limb (Holdstock et al. 2000; King et al. 2002).
Additional support for the differentiator model comes from research on alcohol expectancies, or the effects that individuals expect to receive from alcohol. Individuals with a familial risk of alcoholism and heavy drinkers expect greater stimulation and less sedation (Earleywine 1994; Holdstock et al. 2000; Dunn and Earleywine 2001). Although it is unclear the extent to which expected effects are consistent with the actual effects experienced when drinking, the convergence of evidence from studies on pharmacological and expectancy effects provides rather compelling evidence in support of the differentiator model. Individuals at high risk for alcohol problems appear to experience greater stimulation and less sedation, which presumably puts them at risk for heavy drinking because they find alcohol use more rewarding. An important assumption of this argument, however, is that the stimulant effects experienced on the ascending limb are uniformly rewarding, whereas the descending limb effects are evaluated less positively.
Research, however, has shown that significant variability exists in evaluations of the desirability of specific alcohol effects (Critchlow 1987). Outcomes often assumed to be negative, such as irresponsibility or increased motor impairment, are sometimes evaluated as positive incentive motivators (Fromme et al. 1994). With respect to the differentiator model, alcohol abusers cite both euphoria and reducing negative affect as motivation to drink (Wood et al. 1992). Drinkers who hope to reduce tension may find sedative effects more desirable and thereby evaluate effects on the descending limb more positively. The value of sedative effects may be particularly high for these individuals in the face of stressful circumstances in the environment. Although previous studies have not assessed evaluations of sedative responses after stress, there is evidence that stress influences alcohol’s subjective effects in healthy social subjects by decreasing the strength of stimulant effects and increasing the strength of sedative effects (Söderpalm and de Wit 2002; de Wit et al. 2003). Furthermore, increased societal stress has been shown to increase population levels of consumption (Linsky et al. 1987), and increased alcohol consumption has been found following a variety of different stressors including insoluble arithmetic problems, interpersonal evaluations, and stressful imagery (Higgins and Marlatt 1975; Hull and Young 1983). The effects of stress on alcohol consumption are thought to be associated with ethanol’s anxiolytic effect (Conger 1956; Sher and Levenson 1982), which predominates on the descending limb of the blood alcohol curve. Thus, it seems critical to examine both stimulant and sedative responses and evaluations of these responses as predictors of drinking behavior under stressful conditions.
Although the relative reinforcement value of alcohol can be measured in a number of ways, one of the best measures is the ability of an initial dose of alcohol to prime further consumption. Priming studies have been utilized to differentiate expectancy effects from the reinforcement value attributable to the true pharmacological effects of alcohol. Although a landmark study by Marlatt et al. (1973) suggested that priming was largely due to expectancy effects, more recent studies using higher initial doses suggest that pharmacological effects do, in fact, prime further consumption (Fillmore 2001; Fillmore and Rush 2001; Holdstock et al. 2000; Kirk and de Wit 2000). However, little is known about the specific aspects of subjective response that serve to prime further consumption. Duka et al. (1998), found that participants who experienced increased ‘alertness’ and ‘attentiveness’ after a priming dose consumed more alcohol when given free access. Duka and colleagues, however, assessed only the role of stimulant effects, characteristic of the ascending limb of the blood alcohol curve. Studies of the full range of subjective alcohol effects and evaluations of these effects are needed to improve our understanding of the rewarding properties of alcohol that drive further consumption.
The current study sought to more thoroughly evaluate the full range of subjective alcohol effects and evaluations of these effects by utilizing a placebo-controlled alcohol challenge. Following alcohol (or placebo) dosing, a social stressor was introduced and participants were given adl-ibitum access to alcohol. Inclusion of a social stressor in the protocol provided an additional potential incentive motivation to drink, and provided circumstances under which sedative effects might be positively evaluated, and therefore desirable. We assessed both the magnitude of stimulant and sedative effects and personal evaluations of how pleasurable these effects were to the participant. By analyzing both the experience of subjective effects and the reward value of these effects, we hoped to develop an improved understanding of how these effects may motivate continued drinking following a priming dose. We hypothesized that participants who received the initial alcohol dose would experience stronger subjective effects than those who received a placebo-priming dose, thus supporting the pharmacological properties of alcohol as essential to the priming phenomenon. Second, it was hypothesized that alcohol-primed individuals who favorably evaluated the subjective effects they experienced (either stimulation or sedation) would drink more when given free access to alcohol.
A sample of heavy drinking college students, without contraindications to alcohol consumption (e.g., history of treatment for alcohol use disorders, adverse reactions to alcohol) comprised the study sample. The college environment is one that poses significant risk for the development of alcohol-related problems, thus it provides an important population in which to explore factors that may result in problematic alcohol use. Although overall rates of use are similar to the general population (approximately 70% of college students consume alcohol), rates of heavy episodic drinking are quite high, exceeding 40% in national studies (Johnston et al. 2006; Wechsler et al. 2002). Of greater concern, one study found that approximately 31.6% of college students met diagnostic criteria for alcohol abuse with an additional 6.3% meeting criteria for alcohol dependence (Knight et al. 2002).
Materials and methods
Participants
Roughly equal numbers of male and female (50.3%) participants between the ages of 21 and 30 (M=22.17 years; SD=3.37), were recruited through a Southwest university newspaper and were compensated $50 for their involvement. A total of 174 participants met inclusion criteria of consuming five or more drinks (four or more for women) on at least 1 day each week, (Mean drinks per month= 61.55, SD=39.79). The racial breakdown of the sample included 69.0% European-American, 3.4% African-American, 14.4% Latina/o, 6.3% Asian-American, and 6.9% who indicated “other” or “multi-ethnic.” The participants with contraindications to the consumption of alcohol (e.g., symptoms of alcohol dependence; pregnancy) were not accepted into the study.
Measures
Manipulation check
For the purpose of assessing the credibility of the placebo manipulation, participants were asked to estimate the number of standard drinks they had consumed following beverage administration. The participants were also asked to indicate how “high” and “intoxicated” they felt using a 100-mm visual analog scale. These measures are consistent with manipulation checks used in prior placebo-controlled alcohol administration studies.
Biphasic alcohol effects scale
The biphasic alcohol effects scale (BAES; Martin et al. 1993) is a 14-item questionnaire comprising two sub-scales that assess subjective experiences of alcohol stimulation (alpha=0.94) and sedation (alpha=0.87). The stimulant subscale consists of the following descriptors: elated, energized, excited, stimulated, talkative, up, and vigorous. The sedative subscale consists of the following descriptors: down, heavy head, inactive, sedated, slow thoughts, sluggish, and difficulty concentrating. The participants rated the extent to which they experienced each effect on 11-point scales from not at all (0) to extremely (10). The BAES was modified to also assess the desirability of each effect on the same 11-point scales. For each item, a rating experience by evaluation score was created by multiplying the experience and evaluation scores. These scores were then summed to create the stimulation and sedation sub-scales. Reliability of the modified BAES was comparable to the original measure (alpha=0.95 for simulation; alpha= 0.89 for sedation).
Profile of mood states
A revised version of the profile of mood states (POMS; Gabrielli et al. 1991; Nagoshi and Wilson 1988) was used to assess changes in mood over the course of the experiment. This version of the POMS is composed of five subscales: tension and anxiety, hostility, depression, being energetic, and friendliness. Using a 5-point Likert scale, participants were asked to rate the degree to which each item “best describes how you feel right now.”
Time-line follow back interview
The time-line follow back interview (TLFB) is a structured interview that assesses past alcohol consumption (Sobell et al. 1986). Interviewers used a calendar to help participants recall how much alcohol they consumed on each day during the past month. Past research using this instrument has shown that individuals can provide accurate information about past drinking as far back as 3 months (e.g., Babor et al. 1987; Sobell et al. 1986).
Procedures
The participants completed the study in groups of two to four on weekday evenings. They were instructed not to eat after noon on the day of participation or to consume alcohol for 24 h prior. After providing informed consent, the participants completed a demographic form and the TLFB. The participants were then led into a simulated bar where two research assistants (one male and one female) served as bartenders. Each participant was given 10 min to consume each of three drinks consisting of either 80-proof vodka or placebo (decarbonated tonic water) combined with carbonated mixer (Cherry 7-up and lime juice). To reach a target blood alcohol concentration (BAC) of 0.06 g%, drink volumes were adjusted by weight and gender (to account for sex differences in total body water) of the participant. To facilitate the placebo manipulation, a small amount of 190-proof alcohol was floated on the surface of each drink and the glasses were rimmed with vodka. Participants and bartenders were blind to the contents (either vodka or placebo) of the bottle used to mix the drinks.
A 15-min absorption period followed completion of the third drink and blood alcohol concentration (BAC) was then assessed using an Intoxilyzer 5000 and a handheld Alcosensor IV. Readings from the handheld Alcosensor were taken throughout the protocol and used in all analyses. The second reading from the Intoxilyzer was taken because participants are able to see that the machine is printing the output of their BAC test. Printouts substituted by the researcher (but identical in appearance to the ones seen by participants) were then used to give visual feedback indicating a BAC of approximately 0.04 g%, regardless of beverage condition. The participants then completed the first BAES and POMS measures. During the next 30 min, the participants completed three blocks of trials on a stop signal task (Logan and Cowan 1984) each of which lasted approximately 10 min. Assessment of alcohol effects on the stop signal task was one of the goals of the study but is not directly relevant to hypothesis presented here. Following the stop signal task, breath alcohol concentrations and subjective experiences were reassessed.
Employing a standardized social stressor, the participants were then informed they would be giving a 5-min speech about what they liked and disliked about their bodies. This type of manipulation has been found to increase consumption of alcohol when ad-libitum access is provided (Higgins and Marlatt 1975). A second POMS was administered as a manipulation check on the stressor. For the next 20 min, the participants were given free ad libitum access to a variety of alcoholic and nonalcoholic beverages, including wine, beer, mixed drinks, shots, sodas, water, and juice. For their own protection, no participant was allowed to order a drink that would lead to a breath alcohol concentration greater than .12 g%. The amount of alcohol consumed by each participant (mg) was recorded by an objective observer and calculated as a dependent measure of alcohol consumption. Following the ad-lib period, participants completed the speech, which was videotaped for later content analysis (Bacon et al. unpublished data). The participants were debriefed and held until their BAC was less than .02 g%, then paid and provided with transportation to their home.
Data Analyses
All analyses were conducted using SPSS, version 13.0 (SPSS Inc. 2004). All variables to be used in the regression models were first examined for outliers and assumptions of normality. Evaluations of sedative alcohol effects and the sedative alcohol effects composite showed significant non-normality in the data as indicated by values greater than 3 for skewness and kurtosis. These variables were log transformed to normalize the data (Tabachnick and Fidell 2005). Next, subjective effect measures (stimulant and sedative composite scores) were mean centered to minimize issues of collinearity and to facilitate the interpretation of interactions with gender and limb of the blood alcohol curve. Interaction terms were created by multiplying the relevant variables (e.g., gender × stimulant response) consistent with procedures outlined by Cohen and Cohen (1983).
Before evaluating subjective responses as predictors of ad lib consumption, independent samples t tests and Chi-square analyses were conducted to test the effectiveness of the random assignment procedures in preventing baseline differences between groups. Additional preliminary analyses were conducted to assess the effectiveness of the placebo manipulation and to establish that subjective responses in the alcohol condition were not due purely to expectancy effects. Multivariate analysis of variance (MANOVA) was used to examine the impact of beverage condition and gender on subjective alcohol responses for the full sample (N=174). The remaining analyses were conducted separately for participants in the alcohol (n=91) and placebo (n=81) conditions. Analyses were conducted separately by condition because participants in the alcohol condition (mean BAC prior to ad-lib consumption=0.067) were able to consume less alcohol than placebo participants (mean BAC prior to ad-lib=0.000) before reaching the cutoff of .12 g%. Thus, direct comparisons of the amount consumed by beverage condition would be confounded by the amount of alcohol participants were allowed to consume.
Within each beverage condition, we first examined changes in mood resulting from the social stressor as predictors of ad lib consumption. The predictors comprised changes in POMS subscale scores from before to after the introduction of the social stressor. The subscales that were significantly associated with ad-lib consumption were included as covariates in the regression models testing the primary hypotheses. Changes in “tension and anxiety” subscale scores were included even when they did not significantly predict ad-lib consumption given their conceptual relevance with respect to the social stressor.
For the primary analyses, separate multiple regression models were used to assess stimulant and sedative effects as predictors of ad-lib consumption. In the alcohol condition, gender, typical monthly consumption, BAC, BAC limb, and POMS scores were entered in step one. We used the BAC measurement taken just before ad-lib consumption (45 min), and BAC limb was based on change in BAC from 15 min post-alcohol administration to 45 post-administration. The result was a dichotomous variable coded as 0 (BAC falling) and 1 (BAC rising). This variable was included given well-established differences in subjective response by BAC limb. Subjective responses (stimulation or sedation), were entered in step two, followed by the gender by subjective response and BAC limb by subjective response interactions in step three. The dependent measure of ad-lib drinking was adjusted to account for the effect of the maximum BAC limit on consumption. For participants who ordered a drink but were not allowed to consume it (due to maximum BAC) the amount ordered was added to the total amount consumed. Thus, for those who did not reach their maximum BAC, values reflected the total amount actually consumed whereas the value represented an “estimated” total consumption for those who reached their maximum BAC. The bivariate correlation between total amount consumed and the adjusted total consumption measure was 0.907. Analyses in the placebo condition were identical with the exception of the BAC and BAC limb variables, which were not relevant for this group (BAC= 0.000 g%). As a consequence, the gender by BAC limb interaction was also eliminated from the placebo models.
Results
Comparability of Groups at Baseline
Analyses were first conducted to determine if random assignment to alcohol and placebo groups was successful in preventing baseline differences between groups. Independent samples t tests were used to test for baseline differences in age and typical weekly alcohol consumption, and Chi-square analyses were used to test for gender and ethnic group differences by beverage condition. Results of the t tests indicated that the groups did not differ with respect to age, t(172)=.887, p=.376, or typical weekly drinking, t(171)=−0.640, p=0.523. Chi-square analyses also failed to identify any significant group differences [gender; χ2(1)=.005, p=.942 and race/ethnicity; χ2(5)= 6.789, p=.237]. Mean (or percentile) values are presented by beverage condition in Table 1.
Table 1.
Demographic characteristics by beverage condition
| Variable | Alcohol |
Placebo |
||
|---|---|---|---|---|
| M/% | SD | M/% | SD | |
| Gender | ||||
| Male | 50.5% | 50.00 | ||
| Female | 49.5% | 50.00 | ||
| Race/Ethnicity | ||||
| Caucasian | 69.2% | 68.7% | ||
| African-American | 4.4% | 2.4% | ||
| Hispanic/Latino | 9.9% | 19.3% | ||
| Asian-American | 8.8% | 3.6% | ||
| Multi-racial | 3.3% | 4.8% | ||
| Other Race/Ethnicity | 4.4% | 1.2% | ||
| Age | 21.96 | 4.42 | 22.41 | 1.55 |
| Typical Weekly Alcohol Consumption | 14.79 | 9.36 | 13.89 | 9.23 |
Credibility of the placebo
A MANOVA with beverage condition (alcohol versus placebo) as the independent variable and subjective response measures (perceived number of standard drinks, “high” and “intoxication” ratings) as dependent variables found a significant omnibus effect of beverage condition, F (3,167)=26.067, p<0.001, and significant univariate effects for each outcome measure (p values<0.001). Although the participants in the placebo condition reported lower values on all measures, ratings compared favorably to recent studies in other laboratories (Fillmore and Rush 2001; Kirk and de Wit 2000). Placebo participants believed they consumed a moderate dose of alcohol and reported moderate levels of intoxication. Estimated number of standard drinks and ratings of “high” and “intoxication” are presented by beverage condition in Table 2.
Table 2.
Subjective ratings of alcohol effects by beverage condition
| Variable | Alcohol |
Placebo |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Subjective High | 39.59 | 20.40 | 20.63 | 16.21 |
| Subjective Intoxication | 35.80 | 20.61 | 13.47 | 12.38 |
| Perception of Number of Standard Drinks Consumed | 3.08 | 0.94 | 2.28 | 0.94 |
M, mean; SD, standard deviation
Effects of beverage condition on subjective responses
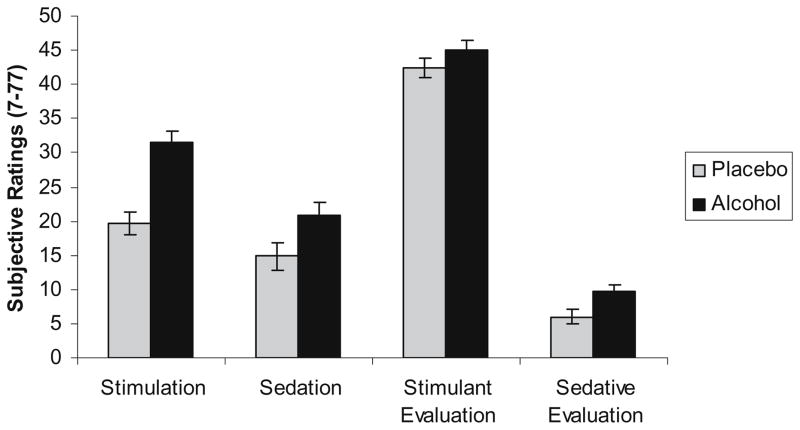
A 2 (beverage condition) by 2 (gender) multivariate analysis of variance (MANOVA) was used to evaluate differences in subjective experiences and evaluations of stimulation and sedation immediately before the ad-lib period. Individual measures of stimulant and sedative experiences and evaluations were used as the dependent measures in this analysis for ease of interpretation, although results were very similar when the analyses were conducted using the composite scores. Consistent with study hypotheses, subjective alcohol effects differed significantly by beverage condition, F(4, 150)=9.419, p<0.001, with participants in the alcohol condition reporting greater stimulation, F(1, 153)=26.636, p<0.001, and sedation, F (1, 153)=9.603, p=0.002. Although evaluations of stimulant effects did not differ by condition, participants in the alcohol condition evaluated sedative effects more favorably than did placebo participants, F(1, 153)=4.639, p=0.033. See Fig. 1 for subjective responses by beverage condition. An overall effect of gender also emerged, F(4, 150)=4.33, p=0.002, with men reporting greater stimulation, F(1, 153)= 6.206, p=0.014, and more positive evaluations of sedative effects, F(1, 153)=5.705, p=0.018. When the covariate of typical weekly drinking was added to the model to control for gender differences in alcohol use, the gender difference for evaluations of sedative effects remained significant, F(1, 151)=5.164, p=0.024, whereas the gender difference in stimulant response was only marginally significant, F(1, 151)=2.847, p=0.094.
Fig. 1.
Subjective experiences and evaluations of stimulation and sedation by beverage condition
Subjective alcohol effects, stress, and ad-libitum consumption in the alcohol condition
Analyses were first conducted within the alcohol condition, in which the mean number of drinks consumed during the ad-lib period was 1.32 (SD=0.72). The adjusted number of drinks consumed (accounting for participants who were not allowed to consume the last drink they ordered) was 1.65 (SD=0.95). An initial regression model was conducted to examine changes in mood related to the social stressor as predictors of ad-lib consumption. Change scores (before and after introduction of the social stressor) for the five POMS subscales were entered as simultaneous predictors in this model. The only POMS subscale significantly associated with ad-lib consumption was the “being energetic” subscale. The participants who reported greater increases in energy after the introduction of the social stressor consumed more alcohol during the ad-lib period.
Next, stimulant effects were assessed as a predictor of ad-lib drinking. Variables entered in the first step of this model (gender, typical weekly drinking, pre-ad-lib BAC, BAC limb, and POMS “tension and anxiety” and “being energetic” scores) accounted for a significant proportion of the variance in ad lib drinking, although the only significant beta coefficients were for typical weekly alcohol use and changes in “being energetic” associated with the social stressor. Heavier typical drinking and greater increases in energy following the introduction of the social stressor were associated with greater ad-lib consumption. At step 2, the stimulant effects composite was significantly and positively associated with ad-lib drinking. The interaction terms entered in step three did not account for significant variance in ad-lib drinking. See Table 3 for all β and p values in the regression model. Because the majority of participants were on the ascending limb of the blood alcohol curve before ad-lib consumption, the power to detect a limb by subjective response interaction was relatively low. For this reason, we looked at the standardized coefficients for those on the ascending (n=55) and descending (n=17) limbs, respectively, and found a stronger effect of stimulation on ad-lib drinking for those on the ascending limb (β=0.370), relative to those on the descending limb (β=0.212).
Table 3.
Stimulant effects in alcohol condition predicting ad-lib consumption
| Entry Step | Variable | ΔR2 | B |
|---|---|---|---|
| Step 1 | 0.221* | ||
| Gender | 0.112 | ||
| Typical weekly drinking | 0.320* | ||
| Pre-ad-lib BAC | −0.056 | ||
| BAC limb | 0.042 | ||
| POMS tension change | 0.075 | ||
| POMS energy change | 0.261 | ||
| Step 2 | 0.070* | ||
| Stimulant effects composite | 0.286* | ||
| Step 3 | 0.015 | ||
| Gender × stimulation | 0.065 | ||
| BAC Limb × stimulation | 0.185 |
p<.05
In the sedative effects regression model, the same pattern of results emerged in step 1 of the model, but the sedative effects composite entered in step 2 was not significantly associated with ad-lib consumption, β=0.103, p=0.356. In addition, the interaction terms entered in step three did not account for significant variance in ad-lib drinking, F(2, 66)= 1.588, p=0.212. For the reasons stated previously, we looked at the standardized coefficients for those on the ascending and descending limbs, respectively. The size of the effect for those on the ascending limb (β=0.148) was larger than for those on the descending limb (β=0.048).
Subjective alcohol effects, stress, and ad-libitum consumption in the placebo condition
Within the placebo group, the mean number of drinks consumed during the ad-lib period was 1.66 (SD=1.34). The adjusted number of drinks consumed (accounting for participants who were not allowed to consume the last drink they ordered) was 1.69 (SD=1.39). Once again, an initial regression model was conducted to examine changes in mood related to the social stressor as predictors of ad-lib consumption. In the placebo group, changes in POMS scores were not associated with ad libitum consumption for any of the subscales (all p values>0.15). Thus, only changes in “tension and anxiety” scores were included in the primary regression models.
In the stimulant effects regression model, variables entered in the first step (gender, typical weekly drinking, and POMS “tension and anxiety” scores) accounted for a significant proportion of the variance in ad lib drinking, although none of the individual regression coefficients were statistically significant. In contrast to the results in the alcohol condition, the stimulant effects composite entered in step 2 failed to predict ad-lib drinking. The interaction term (gender by stimulation) entered in step three also failed to account for significant variance in ad-lib drinking. All β and p values are provided in Table 4 to allow comparison to the results for the alcohol condition (Table 3).
Table 4.
Stimulant effects in placebo condition predicting ad-lib consumption
| Entry Step | Variable | ΔR2 | B |
|---|---|---|---|
| Step 1 | 0.161* | ||
| Gender | 0.215 | ||
| Typical Weekly Drinking | 0.229 | ||
| POMS Tension Change | −0.151 | ||
| Step 2 | 0.021 | ||
| Stimulant Effects Composite | −0.152 | ||
| Step 3 | 0.001 | ||
| Gender × Stimulation | −0.043 |
In the sedative effects regression model, the same pattern of results emerged in step 1 of the model with significant variance accounted for, F(3, 60)=3.682, p=0.017, but no significant regression coefficients. Consistent with the sedative effects model in the alcohol condition, the sedative effects composite entered in step 2 was not significantly associated with ad-lib consumption, β=0.001, p=0.993. The gender by sedative effects interaction term also failed to account for significant variance in ad-lib drinking, β= 0.235, p=0.143.
Discussion
The current study examined the experience and evaluation of stimulant and sedative alcohol effects as predictors of ad-libitum consumption following a priming dose of alcohol (target BAC of 0.06 g %) or placebo in the context of anticipatory stress associated with an upcoming speech. The alcohol and placebo primed groups differed with respect to their subjective experiences, with alcohol primed participants reporting stronger stimulant and sedative effects. The participants in the alcohol condition who reported greater stimulant effects also consumed significantly more alcohol when given ad-libitum access to alcohol. Sedative effects, on the other hand, were not predictive of ad-lib consumption, suggesting that such effects may not be as rewarding. Consistent with this possibility, both men and women reported stronger stimulant relative to sedative alcohol effects and evaluated stimulant effects more positively. In addition, men, who are consistently at higher risk for alcohol-related problems, reported greater stimulation than did women. It is important to note, that the increased stimulant response in men was at least partly due to their heavier typical drinking. When typical weekly consumption was accounted for the gender difference in stimulant response was only marginally significant.
In contrast to results in the alcohol condition, stimulant effects experienced by placebo participants were unrelated to ad-libitum alcohol consumption. Although not statistically significant, stimulant effects were inversely associated with ad-lib drinking for placebo participants. These results lend confidence to the assertion that stimulant effects that were predictive of drinking among participants in the alcohol condition were true pharmacological effects. In other words, the expectation of stimulation, in the absence of true stimulant alcohol effects, was not sufficient to prime alcohol consumption. Sedative effects following placebo also failed to predict ad-lib drinking, suggesting that neither expected or experienced sedation primed further drinking in the context of anticipatory stress.
The lack of association between sedative effects and ad-lib consumption did not support the tension reduction (TR) model, which suggests that individuals learn to use alcohol for its tension-reducing effects, leading to increased consumption and eventual alcohol abuse and dependence (Bales 1946; Conger 1956; Cappell and Herman 1972; Wechsler and Wilson 1982). However, it is important to recognize that sedative response, as assessed by the measure used in the current study (BAES), fails to adequately assess more positive sedative effects (e.g., anxiolysis) that are most relevant to the TR model. Thus, additional studies that better capture these effects are necessary to adequately test the TR model. Nonetheless, the lack of support for the TR model in the current study is consistent with a number of other retrospective and prospective studies (for a review, see Pohorecky 1991). The lack of consistent support for the TR model may be due, at least in part to the fact that only a subset of individuals drink primarily in response to stress, such as those with comorbid anxiety disorders (Hussong et al. 2001). Thus, the population of moderate to heavy drinking college students in the current study may have included a very small number of individuals who drink primarily to reduce stress. Future studies of sedative effects as a predictor of within session drinking should be extended to populations for whom such effects may pose the greatest risk.
The results of the current study suggest that a comprehensive model of alcohol abuse must incorporate the full range of alcohol effects an individual might find rewarding (Holdstock et al. 2000; King et al. 2002; Martin et al. 1993; Martin et al. 1991). In addition to identifying specific risk factors for individual populations, such studies have the potential to improve our understanding of how different subjective effects may be important at different stages in the development of alcohol-related problems. Neurobiological research has suggested that a shift in the rewarding effects of a substance from positive to negative reinforcement may be associated with dependence. According to this model, the development of alcohol dependence results from the emergence of negative emotional states associated with a decrease in activity of the brain reward system, increases in activity of the brain stress systems, and dysregulation of the brain anti-stress systems resulting from heavy use (Koob 2006). Again, the population of heavy drinking college students in the current study may not have experienced a motivational shift away from stimulant effects towards sedative effects. Thus, additional research is needed to explore whether subjective effects predictive of within session drinking change as heavy drinking evolves into dependence.
Although sedative effects may emerge as an important predictor of within session drinking with time, the current study indicates that stimulant effects are an important influence among individuals who consume alcohol heavily. Stimulant effects were an important predictor of ad lib consumption even under social stress which might reduce the reward value of these effects. Thus, it is important to assess the full range of subjective effects, as the reward value of stimulant effects may be an early marker of risk that could be used to identify individuals at risk for developing later problems. In addition, interventions developed to address heavy drinking may benefit from increasing the focus on the rewarding aspects of stimulant effects as a drinking motive, rather than solely focusing on the sedative effects used to self-medicate.
Despite the important implications of these findings, there were several limitations of the current study that might be addressed in future studies. First, absolute levels of ad-lib consumption could not be compared across study conditions because the participants in the placebo condition were able to consume more alcohol before reaching the cutoff BAC of .12 g%. Although this methodology is not ideal, allowing participants to self-administer alcohol at higher levels would raise serious ethical concerns. Second, because ad-lib consumption was assessed in the context of anticipatory stress, we can say with confidence only that stimulant effects lead to further consumption under conditions of social stress. Additionally, all participants were subjected to a social stressor making it difficult to identify what, if any, effects were due to stress. Furthermore, although the subjective value of the stimulant and sedative effects were assessed, there was no direct evaluation of possible reasons why these effects motivated further drinking in this context. For example, participants with a strong stimulant response may have consumed alcohol to facilitate speech performance, whereas those with more of a sedative response may have avoided further alcohol for the same reason. To further understand the impact of social stress on how stimulant and sedative effects prime drinking behavior, future studies would benefit from direct assessment of motivations to achieve stimulant or sedative effects within the context in which the drinking occurs. Additional studies in other contexts are also needed to establish the generalizability of these findings to contexts in which participants do not experience an acute stressor.
Another limitation to the current study was the exclusive reliance on self-report measures of stimulation and sedation, as individuals could experience the same physiological sensations but label them differently. The inclusion of physiological measures of stimulation and sedation, such as heart rate, blood pressure, and skin conductance, would strengthen the current findings. Although there is often a low correlation between self-reported emotional states and physiological indices (Lang 1994) this has not been thoroughly investigated for the subjective effects of alcohol. Previous research has demonstrated, for example, that the stimulant properties of alcohol involve both physiological and subjective elements (Conrod et al. 2001; Davidson et al. 2002; King et al. 2002). Additional research showing that physiological stimulation leads to further drinking would provide a stronger foundation for the argument that individual differences in subjective experiences of stimulation are based upon pharmacology rather than expectancy.
Finally, due to the timing of the ad-lib period in the current study, most participants were on the ascending limb of the blood alcohol curve before the ad-lib period. Because sedative effects are more prominent on the descending limb, the predictive value of sedative effects may have been reduced by assessing these effects primarily on the ascending limb. Although we cannot rule out this possibility, examination of the regression coefficients separately for those on the ascending and descending limb provided no evidence in support of this possibility. The positive relation between sedative effects and ad-lib consumption was actually stronger among those assessed on the ascending limb. Nonetheless, studies of sedative effects on the descending limb with larger sample sizes are needed.
Despite the limitations of the study, the results have important implications for future research and practice in the area of addictive behaviors. Consistent with the idea that there are multiple pathways to addiction (Zucker et al. 1995), stimulant effects may dominate for some, whereas sedative effects may be more central for others. Strong experiences of stimulation or sedation, for example, may provide a useful marker for alcoholism risk, assisting in the identification of at-risk individuals. Second, treatment for alcohol problems often focuses on effectively coping with stress and negative emotional states. Although this is undoubtedly an important aspect of treatment, additional treatment components may be needed to address the positive reinforcing role that alcohol plays for many drinkers. Additionally, examining both sides of the drinking curve over the evolution of drinking experience may provide important information about how transitions in the reward values of stimulant and sedative effects may accompany the transition from heavy use to dependence.
Acknowledgments
The authors extend their gratitude to the important contributions of Youngsuk Kim, M.A., Bryan Hartzler, Ph.D., Marc Kruse, M.A., Jason A. Roth, B.S. and a number of dedicated undergraduate research assistants. The experiment presented in this manuscript complied with all current US laws. The authors have no financial relationship with any of the agencies providing funding for the research presented in this manuscript.
This research has been supported by grants from the Texas Commission of Alcohol and Drug Abuse (517-9-8444), the National Institute on Alcohol Abuse and Alcoholism (5T32-AA07471; RO1-AA11683) the Co-operative Society at The University of Texas at Austin, and the Waggoner Center for Alcohol and Addiction Research.
Contributor Information
William R. Corbin, Email: william.corbin@yale.edu, Department of Psychology, Yale University, 2 Hillhouse Ave, New Haven, CT 06520–8205, USA
Ashley Gearhardt, Department of Psychology, Yale University, 2 Hillhouse Ave, New Haven, CT 06520–8205, USA.
Kim Fromme, Department of Psychology, The University of Texas at Austin, Austin, TX 78712, USA.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Babor TF, Stephens RS, Marlatt GA. Verbal report methods in clinical research on alcoholism: response bias and its minimization. J Stud Alc. 1987;48:410–424. doi: 10.15288/jsa.1987.48.410. [DOI] [PubMed] [Google Scholar]
- Bales RF. Cultural differences in rates of alcoholism. Q J Stud Alcohol. 1946;6:480–499. [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2. Erlbaum Associates; Hillsdale: L: 1983. [Google Scholar]
- Cappell H, Herman CP. Alcohol and tension reduction; a review. Q J Stud Alcohol. 1972;33:33–64. [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: theory, problem, and challenge. II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Critchlow B. A utility analysis of drinking. Addict Behav. 1987;12:269–273. doi: 10.1016/0306-4603(87)90038-4. [DOI] [PubMed] [Google Scholar]
- de Wit H, Söderpalm AH, Nikolayev L, Young E. Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol Clin Exp Res. 2003;27:1270–1277. doi: 10.1097/01.ALC.0000081617.37539.D6. [DOI] [PubMed] [Google Scholar]
- Davidson D, Hutchinson K, Dagon C, Swift R. Assessing the stimulant effects of alcohol in humans. Pharmacol Biochem Behav. 2002;72:151–156. doi: 10.1016/s0091-3057(01)00758-4. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, Stephens DN. Alcohol choice and outcome expectancies in social drinkers. Behav Pharmacol. 1998;9:643–653. doi: 10.1097/00008877-199811000-00019. [DOI] [PubMed] [Google Scholar]
- Dunn ME, Earleywine M. Activation of alcohol expectancies in memory in relation to limb of the blood alcohol curve. Psychol Addict Behav. 2001;15:18–24. doi: 10.1037/0893-164x.15.1.18. [DOI] [PubMed] [Google Scholar]
- Earleywine M. Anticipated biphasic effects of alcohol vary with risk for alcoholism: a preliminary report. Alcohol Clin Exp Res. 1994;18:711–714. doi: 10.1111/j.1530-0277.1994.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Cognitive preoccupation with alcohol and binge drinking in college students: alcohol-induced priming of the motivation to drink. Psychol Addict Behav. 2001;15:325–332. [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Alcohol effects on inhibitory and activational response strategies in the acquisition of alcohol and other reinforcers: priming the motivation to drink. J Stud Alc. 2001;62:646–656. doi: 10.15288/jsa.2001.62.646. [DOI] [PubMed] [Google Scholar]
- Fromme K, Marlatt GA, Baer JS, Kivlahan DR. The Alcohol Skills Training Program: a group intervention for young adult drinkers. J Subst Abuse Treat. 1994;11:143–154. doi: 10.1016/0740-5472(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Gabrielli WF, Nagoshi CT, Rhea SA, Wilson JR. Anticipated and subjective sensitivities to alcohol. J Stud Alc. 1991;52:205–214. doi: 10.15288/jsa.1991.52.205. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Higgins RL, Marlatt GA. Fear of interpersonal evaluation as a determinant of alcohol consumption in male social drinkers. J Abnorm Psychol. 1975;84:644–651. doi: 10.1037//0021-843x.84.6.644. [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Hull JD, Young RD. Self-consciousness, self-esteem, and success-failure as determinants of alcohol consumption in male social drinkers. J Pers Soc Psychol. 1983;44:1097–1109. doi: 10.1037//0022-3514.44.6.1097. [DOI] [PubMed] [Google Scholar]
- Hussong AM, Hicks RE, Levy SA, Curran PJ. Specifying the relations between affect and heavy alcohol use among young adults. J Abnorm Psychol. 2001;110:449–461. doi: 10.1037//0021-843x.110.3.449. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2005. Volume II: College students and adults ages 19–45 (NIH Publication No. 06–5884) Bethesda, MD: National Institute on Drug Abuse; 2006. [Google Scholar]
- Kaplan RF, Meyer RE, Stroebel CF. Alcohol dependence and responsivity to an ethanol stimulus as predictors of alcohol consumption. Brit J Addict. 1983;78:259–267. doi: 10.1111/j.1360-0443.1983.tb02510.x. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Individual differences in the priming effect of ethanol in social drinkers. J Stud Alc. 2000;61:64–71. doi: 10.15288/jsa.2000.61.64. [DOI] [PubMed] [Google Scholar]
- Knight JR, Wechsler H, Kuo M, Seibring M, Weitzman ER, Schuckit MA. Alcohol abuse and dependence among U.S. college students. J Stud Alcohol. 2002;63:263–270. doi: 10.15288/jsa.2002.63.263. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: A Hedonic Calvinist view. In: Miller WR, William R, Carroll KM, editors. Rethinking substance abuse: what the science shows, and what we should do about it. Guilford Press; New York: 2006. pp. 25–45. [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Lang PJ. The varieties of emotional experience: A meditation on James-Lange Theory. Psychol Rev. 1994;101:211–221. doi: 10.1037/0033-295x.101.2.211. [DOI] [PubMed] [Google Scholar]
- Linsky AS, Colby JP, Straus MA. Social stress, normative constraints and alcohol problems in American States. Soc Sci Med. 1987;10:875–883. doi: 10.1016/0277-9536(87)90189-4. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of act and control. Psychol Rev. 1984;91:295–327. [Google Scholar]
- Marlatt GA, Demming B, Reid JB. Loss of control drinking in alcoholics: An experimental analogue. J Abnorm Psychol. 1973;81:233–241. doi: 10.1037/h0034532. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alc Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Rose RJ, Obremski KM. Estimation of blood alcohol concentrations in young male drinkers. Alc Clin Exp Res. 1991;15:494–499. doi: 10.1111/j.1530-0277.1991.tb00549.x. [DOI] [PubMed] [Google Scholar]
- McGue M. A behavioral-genetic perspective on children of alcoholics. Alcohol Health Res World. 1998;21:210–217. [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Mehta RL, Molnar BE, Walters EE, Swendsen JD, Auilar-Gaziola S, Bijl R, Borges G, Caraveo-Anduaga JJ, Dewit DJ, Kolody B, Vega W, Wittchen HU, Kessler RC. Comorbidity of substance use disorders with mood and anxiety disorders: Results of the international consortium in psychiatric epidemiology. Addict Behav. 1998;23:893–908. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR. Influence of family alcoholism history on alcohol metabolism, sensitivity, and tolerance. Alcohol Clin Exp Res. 1987;11:392–398. doi: 10.1111/j.1530-0277.1987.tb01330.x. [DOI] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR. One-month repeatability of emotional responses to alcohol. Alcohol Clin Exp Res. 1988;12:691–697. doi: 10.1111/j.1530-0277.1988.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alc Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk of alcoholism: A noncausal association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Am J of Psychiatry. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987;44:942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J Stud Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–329. [PubMed] [Google Scholar]
- Sher KJ, Levenson RW. Risk for alcoholism and individual differences in the stress-response-dampening effect of alcohol. J Abnorm Psychol. 1982;91:350–367. doi: 10.1037//0021-843x.91.5.350. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PAF, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Söderpalm AHV, de Wit H. Effects of stress and alcohol on subjective state in humans. Alcohol Clin Exp Res. 2002;26:818–826. doi: 10.1097/00000374-200206000-00011. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. SPSS Base 13.0 for Windows User’s Guide. SPSS Inc; Chicago IL: 2004. [Google Scholar]
- Tabachinick BG, Fidell LS. Using multivariate statistics. 5. Boston: Allyn & Bacon; 2005. [Google Scholar]
- Wechsler HT, Wilson GT. Self-control of emotional behavior. New York: Plenum Press; 1982. Alcohol and anxiety. Recent evidence on the tension reduction theory of alcohol use and abuse; pp. 343–353. [Google Scholar]
- Wechsler HT, Lee JE, Kuo M, Seibring M, Nelson TF, Lee H. Trends in college binge drinking during a period of increased prevention efforts. J Am Coll Health. 2002;50:203–217. doi: 10.1080/07448480209595713. [DOI] [PubMed] [Google Scholar]
- Wood MD, Nagoshi CT, Dennis DA. Alcohol norms and expectations as predictors of alcohol use and problems in a college student sample. Am J Drug Alcohol Abuse. 1992;18:461–476. doi: 10.3109/00952999209051042. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Fitzgerald HE, Moses HD. Emergence of alcohol problems and the several alcoholisms: A developmental perspective on etiologic theory and life course trajectory. In: Cicchetti D, Cohen DJ, editors. Manual of Development and Psychopathology. Volume 2: Risk, disorder, and adaptation. Wiley; New York: 1995. pp. 677–711. [Google Scholar]