Abstract
We studied the incidence and progression of coronary artery calcification in people with early chronic kidney disease. We used a cohort of 562 adult patients with chronic kidney disease who had an estimated glomerular filtration rate of <60 ml/min/1.73 m2, in a community-based study of people without clinical cardiovascular disease, the Multi-Ethnic Study of Atherosclerosis. The majority had stage 3 disease. Coronary artery calcification was measured at baseline and again approximately 1.6 or 3.2 years later. The prevalence of coronary artery calcification at baseline was 66%, and its adjusted prevalence was 24% lower in African Americans as compared to Caucasians. The incidence of coronary artery calcification was 6.1% per year in women and 14.8% in men. Coronary artery calcification progressed in approximately 17% of subjects per year across all subgroups, and diabetes was associated with a 65% greater adjusted risk of progression. Male gender and diabetes were the only factors associated with adjusted coronary artery calcification incidence and progression, respectively. Our study shows that coronary artery calcification is common in people with stage 3 disease, progresses rapidly, and may contribute to cardiovascular risk.
Keywords: chronic kidney disease, coronary calcification, vascular calcification
Vascular and soft tissue calcification is common among individuals with chronic kid ney disease (CKD) and may represent an important mechanism linking kidney dysfunction with cardiovascular risk.1–4 Among individuals with end-stage renal disease, coronary artery calcification (CAC) is highly prevalent, progresses rapidly, and is associated with an increased risk of death.1,2,5,6 Even young dialysis patients with few traditional cardiovascular risk factors have extensive CAC.7
Among nondialyzed individuals with CKD, the rates of incidence and progression of CAC are not comprehensively described. Previous case series describing calcification in non-dialysis CKD were largely cross-sectional, spanned a wide range of kidney function, and included relatively few subjects.3,4,8–12 Therefore, prevalence estimates for coronary calcification in CKD vary considerably. Previous studies also included individuals with prevalent cardiovascular disease who may have already had extensive calcification.
In this descriptive cohort study, we examined the natural history of CAC in a multiethnic population with predominantly stage III CKD. Study participants were free of known clinical cardiovascular disease at baseline, providing an opportunity to describe the calcification process when it may be first developing.
RESULTS
Study population
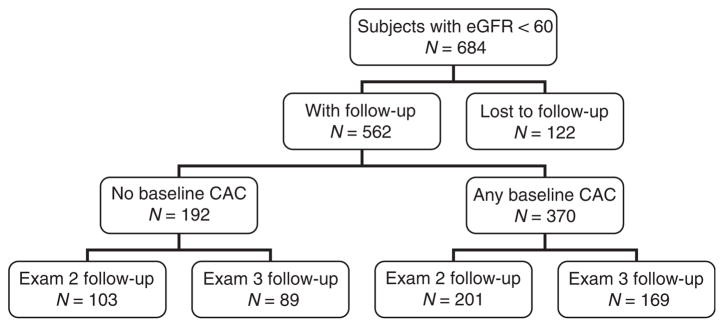
There were 684 Multi-Ethnic Study of Atherosclerosis (MESA) participants with an estimated glomerular filtration rate (GFR) of <60 ml/min per 1.73 m2 at baseline examination. Among this group, 562 (82%) participants returned for a follow-up computed tomography (CT) scan (Figure 1). Compared with returning participants, those who did not complete follow-up CT scanning were more likely to be diabetic (27 versus 15%), were African American (29 versus 18%), and had a modestly lower baseline estimated GFR (mean 49 versus 52 ml/min per 1.73 m2). Nonreturning participants also had higher baseline CAC scores (median 76 versus 28 Agatston units) compared with those who completed follow-up. Among the 122 nonreturning participants, 19 (16%) died during the follow-up period.
Figure 1. Flow chart of study participation.
CAC, coronary artery calcification; eGFR, estimated glomerular filtration rate.
Subsequent analyses were conducted among the 562 participants who completed follow-up CT scans. Among this study population, 98% had stage III CKD at baseline (median estimated GFR: 55.4 ml/min per 1.73 m2; interquartile range: 50.1, 57.3) and 19% had microalbuminuria.
Prevalence of CAC
CAC was present at baseline in 66% of the MESA CKD study population. Compared with CKD participants without baseline CAC, CKD participants with prevalent CAC were older, more likely to be male, had lower HDL-cholesterol levels, lower estimated GFR, and a modestly greater urine albumin to creatinine ratio, (Table 1). African-American participants were significantly less likely to have prevalent CAC. After adjustment for demographics, diabetes, smoking, hypertension, body mass index, serum cholesterol levels, C-reactive protein, cystatin C, and urine albumin-to-creatinine ratio, African-American race remained statistically associated with a 24% lower prevalence of CAC (95% CI: 10–36% lower; P = 0.002 for comparison with Caucasians). In contrast, the adjusted CAC prevalence among Chinese-American, Hispanic, and Caucasian participants with CKD was statistically indistinguishable. Older age, male sex, hypertension, and low-density lipoprotein levels were also statistically associated with a higher prevalence of CAC in the multivariable model.
Table 1.
Baseline characteristics according to prevalent coronary calcification status
| All participants (n = 562) | Stratified by presence of calcification |
|||
|---|---|---|---|---|
| No CAC (n = 192) | Any CAC (n = 370) | P-value* | ||
| Age (years) | 68.7 (8.7) | 63.9 (8.4) | 71.2 (7.9) | <0.001* |
| Male gender | 222 (40) | 55 (29) | 167 (45) | <0.001* |
| Race/ethnicity | ||||
| Caucasian | 306 (54) | 100 (52) | 206 (56) | |
| Chinese | 63 (11) | 22 (11) | 41 (11) | |
| African American | 100 (18) | 42 (22) | 58 (16) | |
| Hispanic | 93 (17) | 28 (15) | 65 (18) | 0.29 |
| BMI (kg/m2) | 28.4 (5.3) | 28.6 (5.4) | 28.3 (5.2) | 0.56 |
| Pre-hypertension | 72 (13) | 29 (15) | 43 (12) | |
| Hypertension | 400 (71) | 110 (57) | 291 (78) | <0.001* |
| Family history of MI | 240 (47) | 78 (44) | 162 (48) | 0.45 |
| LDL cholesterol (mg per 100 ml) | 117.7 (32.7) | 114.6 (32.5) | 119.4 (32.7) | 0.10 |
| HDL cholesterol (mg per 100 ml) | 51.8 (15.1) | 54.1 (16.4) | 50.6 (14.3) | 0.01 |
| Impaired fasting glucose | 83 (15) | 23 (12) | 60 (16) | |
| Diabetes | 85 (15) | 28 (15) | 57 (15) | 0.36 |
| Smoking | ||||
| Never | 304 (55) | 114 (61) | 190 (51) | |
| Ever | 207 (37) | 58 (31) | 149 (40) | |
| Current | 45 (8) | 16 (9) | 29 (8) | 0.08 |
| Cystatin C (mg/l) | 1.19 (0.42) | 1.11 (0.34) | 1.24 (0.45) | <0.001* |
| eGFR (ml/min per 1.73 m2) | 52.2 (7.9) | 53.2 (6.8) | 51.7 (8.4) | 0.02 |
| C-reactive protein (mg/l)a | 2.2 (1.0, 4.5) | 2.3 (1.1, 5.0) | 2.2 (0.9, 4.4) | 0.22 |
| Albumin/creatinine (mg/g)a | 6.6 (3.6, 17.8) | 4.9 (3.1, 10.8) | 7.8 (4.0, 23.4) | 0.001* |
| Microalbuminuria | 106 (19) | 26 (14) | 80 (22) | 0.02 |
| Serum phosphate (mg per 100 ml) | 3.53 (0.53) | 3.48 (0.57) | 3.56 (0.51) | 0.17 |
| Serum 25-hydroxyvitamin D (ng/ml) | 24.1 (13.9) | 22.5 (11.2) | 25.0 (15.2) | 0.07 |
BMI, body mass index; CAC, coronary artery calcification; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction.
Median (interquartile range). P-value based on comparison of log-transformed values.
Statistically significant after Bonferroni’s correction for multiple comparisons.
We questioned whether the lower prevalence of CAC in African Americans with CKD might have been due to selection bias caused by the explicit use of race to calculate Modification of Diet in Renal Disease (MDRD) GFR. To address this concern, we created a second CKD study population, defined by an estimated GFR of <60 ml/min per 1.73 m2 using the equation eGFRcystatin = 76.7 × (cystatin C)−1.19, which does not include race. In this cystatin C-based CKD population, African-American race was associated with a 27% lower (95% CI: 11–39%; P = 0.001) adjusted prevalence of CAC.
Extent of baseline CAC
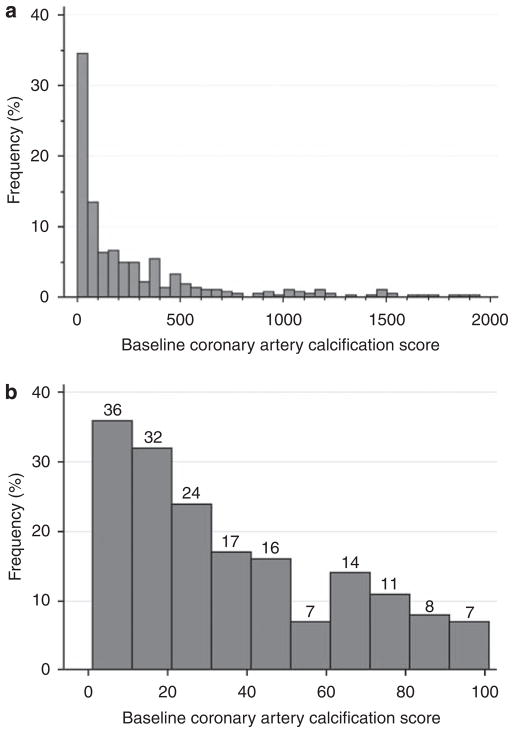
Among participants with prevalent calcification at baseline, the median CAC score was 120 Agatston units (interquartile range: 31, 377 units; Figure 2). There were 91 participants with CAC scores between 1 and 30 Agatston units. In the fully adjusted multivariate model, older age, male sex, and higher C-reactive protein levels were statistically associated with a greater amount of baseline CAC among participants with prevalent CAC, whereas African-American race was associated with a lower amount of baseline CAC.
Figure 2. Distribution of non-zero coronary artery calcium scores.
Distribution of (a) all nonzero coronary artery calcium scores and (b) of coronary artery calcium scores between 1 and 100. Eight participants with coronary artery calcification scores > 2000 not shown in panel a.
Incidence of CAC
The median follow-up time between CT scans was 2.0 years (interquartile range: 1.5, 3.1). Incident CAC developed in 20% of CKD study participants without baseline CAC (N = 39; estimated incidence rate: 8.5% per year). Incident CAC was more than twice as common in men than in women (14.8% per year in men and 6.1% per year in women; Table 2). In contrast, there were no detectable differences in incident CAC rates by race/ethnicity. After full adjustment, male sex was the only characteristic that was associated with a greater incidence of CAC (Table 2).
Table 2.
Risk factors for incident coronary artery calcification
| Variable | Unadjusted IRR (95% CI) | Adjusteda IRR (95% CI) | Adjustedb IRR (95% CI) | P-valuec |
|---|---|---|---|---|
| Age (1 year older) | 1.02 (0.99, 1.05) | 1.01 (0.98, 1.04) | 1.01 (0.98, 1.05) | 0.38 |
| Race/ethnicity | ||||
| Caucasian | Reference | Reference | Reference | |
| Chinese | 1.95 (0.92, 4.14) | 2.13 (0.82, 5.50) | 2.09 (0.73, 5.98) | 0.17 |
| African American | 1.45 (0.76, 2.77) | 1.05 (0.54, 2.03) | 0.84 (0.40, 1.76) | 0.64 |
| Hispanic | 0.84 (0.31, 2.32) | 0.87 (0.31, 2.46) | 0.68 (0.24, 1.92) | 0.47 |
| Male gender | 2.43 (1.43, 4.12) | 2.26 (1.32, 3.88) | 2.27 (1.26, 4.09) | 0.006 |
| Diabetes | 1.82 (0.93, 3.55) | 1.44 (0.71, 2.92) | 1.08 (0.55, 2.14) | 0.82 |
| Current smoking | 2.03 (1.00, 4.12) | 2.05 (1.10, 3.83) | 1.66 (0.90, 3.07) | 0.11 |
| Hypertension | 1.49 (0.84, 2.66) | 1.32 (0.70, 2.51) | 1.12 (0.56, 2.26) | 0.74 |
| Body mass index (1 unit greater) | 1.03 (0.98, 1.09) | 1.07 (1.00, 1.13) | 1.06 (0.99, 1.13) | 0.10 |
| LDL cholesterol (10 mg per 100 ml greater) | 1.01 (0.93, 1.10) | 1.01 (0.93, 1.10) | 1.02 (0.93, 1.11) | 0.71 |
| HDL cholesterol (10 mg per 100 ml greater) | 0.87 (0.71, 1.07) | 0.98 (0.81, 1.19) | 1.03 (0.84, 1.26) | 0.76 |
| C-reactive protein (1 mg/l greater) | 1.00 (0.95, 1.06) | 1.01 (0.97, 1.06) | 1.00 (0.95, 1.05) | 0.95 |
| Cystatin C (0.2 mg/l greater) | 1.10 (0.98, 1.24) | 1.07 (0.93, 1.23) | 1.02 (0.87, 1.19) | 0.85 |
| Log albumin-to-creatinine ratio | 1.22 (1.04, 1.42) | 1.16 (0.98, 1.37) | 1.11 (0.93, 1.32) | 0.24 |
CI, confidence interval; HDL, high-density lipoprotein; IRR, incidence rate ratio; LDL, low-density lipoprotein.
Adjusted for age, race, gender, and scanner pair.
Adjusted for all of the factors in the table plus scanner pair.
P-value for fully adjusted model.
N = 39 participants with incident coronary calcification.
Progression of CAC
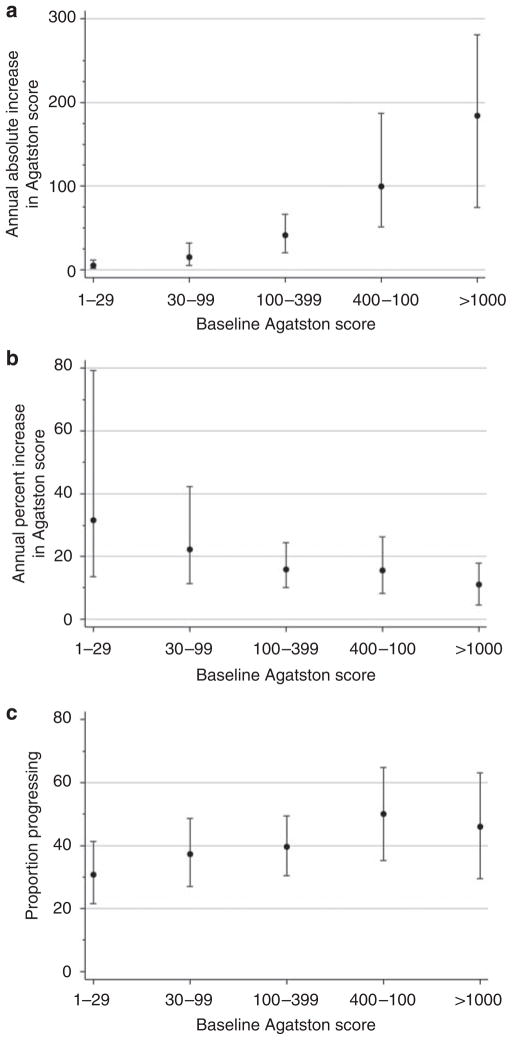
Among participants with prevalent CAC at baseline, the median absolute increase in CAC scores was + 24 Agatston units per year (intraquartile range: + 6, + 66). The extent of CAC progression was strongly dependent on the baseline score; higher baseline Agatston scores were associated with higher absolute and lesser relative changes in CAC during follow-up (Figure 3). The dichotomous MESA definition of CAC progression was less dependent on the baseline score than was either absolute or relative change.
Figure 3. Definitions of CAC progression according to baseline score.
(a) Annual absolute increase in coronary calcium score as a function of baseline score. (b) Annual percentage change in coronary calcium score as a function of baseline score. (c) Proportion progressing as a function of baseline score. Proportion progressing defined by a change in coronary calcium score that exceeds the 95% reproducibility limits of the scanner, as described in methods. All panels restricted to participants with prevalent coronary calcium at baseline. CAC, coronary artery calcification.
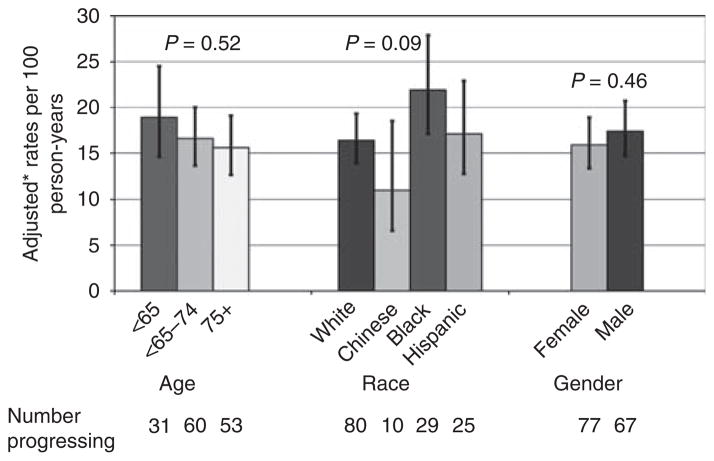
On the basis of the MESA definition of CAC progression, 144 (39%) participants with prevalent CAC progressed during follow-up, corresponding to an estimated progression rate of 16.8% per year. CAC progression was not statistically associated with age, race, or sex (Figure 4). After adjustment, diabetic participants were ~65% more likely to progress compared with nondiabetic participants (Table 3; 95% CI: 25–118% more likely). Estimated kidney function as defined by cystatin C levels was not associated with CAC progression. Substituting serum creatinine level or MDRD estimated GFR for cystatin C in the multivariate model for CAC progression did not yield significant results for either variable (P = 0.58 for serum creatinine; P = 0.60 for MDRD estimated GFR).
Figure 4. Progression of coronary artery calcification by age, race, and sex categories.
*Rates adjusted for age, race, sex, body mass index, estimated glomerular filtration rate, time between scans, and scanner pair.
Table 3.
Risk factors for progression of coronary artery calcification
| Variable | Unadjusted IRR (95% CI) | Adjusteda IRR (95% CI) | Adjustedb IRR (95% CI) | P-valuec |
|---|---|---|---|---|
| Age (1 year older) | 1.00 (0.98, 1.01) | 1.00 (0.98, 1.01) | 1.00 (0.98, 1.02) | 0.79 |
| Race/ethnicity | ||||
| Caucasian | Reference | Reference | Reference | |
| Chinese | 0.67 (0.39, 1.14) | 0.82 (0.46, 1.47) | 0.74 (0.41, 1.34) | 0.33 |
| African American | 1.36 (1.03, 1.81) | 1.27 (0.94, 1.73) | 1.04 (0.75, 1.43) | 0.81 |
| Hispanic | 1.07 (0.77, 1.48) | 1.04 (0.74, 1.47) | 0.95 (0.66, 1.38) | 0.79 |
| Male gender | 1.10 (0.87, 1.39) | 1.06 (0.84, 1.35) | 1.10 (0.84, 1.42) | 0.50 |
| Diabetes | 1.77 (1.40, 2.24) | 1.69 (1.31, 2.18) | 1.65 (1.25, 2.18) | <0.001 |
| Current smoking | 1.38 (0.99, 1.90) | 1.36 (0.96, 1.92) | 1.40 (0.95, 2.04) | 0.09 |
| Hypertension | 1.11 (0.82, 1.50) | 1.08 (0.80, 1.47) | 0.95 (0.69, 1.33) | 0.78 |
| Body mass index (1 unit greater) | 1.01 (0.99, 1.04) | 1.00 (0.98, 1.03) | 1.00 (0.97, 1.02) | 0.78 |
| LDL cholesterol (10 mg per 100 ml greater) | 1.01 (0.98, 1.05) | 1.01 (0.98, 1.05) | 1.01 (0.98, 1.05) | 0.39 |
| HDL cholesterol (10 mg per 100 ml greater) | 0.97 (0.90, 1.06) | 1.00 (0.92, 1.09) | 1.02 (0.94, 1.12) | 0.60 |
| C-reactive protein (1 mg/l greater) | 1.02 (1.00, 1.04) | 1.01 (0.99, 1.03) | 1.01 (0.98, 1.03) | 0.60 |
| Cystatin C (0.2 mg/l greater) | 1.07 (1.04, 1.09) | 1.05 (1.03, 1.08) | 1.05 (0.99, 1.12) | 0.10 |
| Log albumin-to-creatinine ratio | 1.09 (1.02, 1.17) | 1.08 (1.01, 1.15) | 1.03 (0.95, 1.12) | 0.46 |
CI, confidence interval; BMI, body mass index; HDL, high-density lipoprotein; IRR, incidence rate ratio; LDL, low-density lipoprotein.
Adjusted for age, race, gender, and scanner pair.
Adjusted for all of the factors in the table plus scanner pair.
P-value for fully adjusted model.
N = 144 participants with progression.
DISCUSSION
In a multiethnic cohort with predominantly stage III CKD and no clinically apparent cardiovascular disease, 66% of participants had prevalent CAC, confirming a high prevalence of subclinical coronary atherosclerosis and/or dystrophic calcification in this population. Compared with Caucasians with CKD, African Americans with CKD had a 24% lower adjusted prevalence of CAC. Incident CAC developed at a rate of 14.8% per year in men and 6.1% per year in women. Progression of existing CAC was statistically similar by race/ethnicity and sex, and was strongly associated with the presence of diabetes. These data provide population-based estimates of CAC rates among individuals with CKD and extend previous findings describing chronic kidney disease in the MESA population.8,13–15
In previous case series, prevalence estimates for CAC in CKD vary from 40 to 73%.3,4,9,11,12 For example, Russo et al.4 observed a 40% prevalence of CAC among 85 patients (mean age: 54 years) with stage III–V CKD who had no known cardiovascular disease or diabetes. Tomiyama et. al.12 reported a 64% prevalence of CAC among 96 patients (mean age: 55 years) with an estimated creatinine clearance of 15–90 ml/min per 1.73 m2 from an outpatient nephrology clinic population in Brazil. Qunibi et al.3 reported prevalence rates of 38 and 73% among 55 Hispanic Americans with diabetic nephropathy and stages I–II and IV–V CKD, respectively (mean ages: 52 and 56 years, respectively). Our prevalence estimate from this somewhat older, relatively large, multiethnic sample without cardiovascular disease is within the range of previously reported rates.
Estimates of the prevalence and extent of coronary calcification observed among patients with an estimated GFR of <60 ml/min per 1.73 m2 in this study were similar to estimates reported among patients with microalbuminuria in the same MESA population. Kramer et al.16 found an ~66% prevalence of coronary calcification among MESA participants with microalbuminuria, defined using sex-specific cutoff points. The distributions of nonzero coronary calcium scores were also similar comparing microalbuminuria and estimated GFR of <60 ml/min per 1.73 m2 sub-populations in MESA.
Some previous studies in the general population and in chronic dialysis patients have also reported a lower prevalence of CAC among African Americans.17,18 On the other hand, a recent genetic admixture study conducted in young adults found no association of African ancestry with CAC score.19 Possible reasons for ethnic differences in CAC are unclear, given that CAC could represent atherosclerosis and/or dystrophic mineralization. Some studies have shown that compared with Caucasians, African Americans tend to have higher bone mineral density,20,21 which is inversely correlated with CAC.22 Given the relatively small number of African-American participants with CKD in our study, it is possible that observed differences in the presence and extent of CAC were due to sampling variation. It is also possible that African Americans in this study had a shorter duration of CKD because of a more rapid kidney function decline.
In the general population, the presence and extent of CAC predicts future cardiovascular events. For example, among the full 6814-member MESA cohort, CAC scores of 1–100, 101–300, and >300 were associated with 3.9-, 7.1-, and 6.8-fold higher risks of incident cardiovascular events, respectively, compared with a CAC score of 0.23 Although CAC may be less prevalent among African Americans, its existence more strongly predicts future cardiovascular risk in this race group. In a general cohort study of 14,812 adults, the association of CAC score with mortality was more than twofold higher among African Americans compared with the non-Hispanic Caucasian reference cohort.24
Coronary calcification in the setting of early CKD may represent intimal atherosclerosis, medial vessel calcification, or both. Histological studies of epigastric arteries removed from chronic dialysis patients have shown evidence of medial arterial calcification.25 Nontraditional risk factors, such as higher serum phosphate levels and greater intake of calcium containing oral phosphorous binders, are associated with coronary calcification scores in chronic dialysis patients,7,26 suggesting that at least some CAC might represent medial calcification in advanced kidney failure.27 In stage III CKD, clinically detectable disturbances in mineral metabolism are subtle. For example, serum parathyroid hormone levels tend to be only modestly elevated and serum phosphorous levels are generally preserved.28 On the other hand, nontraditionally measured mineral metabolism markers, such as serum fibroblast growth factor 23 and urinary phosphorous excretion, are disturbed early during the course of CKD.29 Whether such factors might contribute to vascular calcification in early stages of CKD remains to be tested.
Consistent with previous studies, the baseline CAC score was the strongest determinant of future calcification.2 Incident CAC developed in only 8.5% of participants annually, and the amount of incident CAC that was detected was low. Absolute and relative changes in existing CAC were highly dependent on the extent of baseline CAC, whereas this dependence was less pronounced using the MESA definition of CAC progression. Nearly 17% of individuals with preexisting CAC progressed annually, when progression was defined as a change that exceeds the 95% reproducibility limits of the scanner. Individuals with CKD, who remain persistently noncalcified, represent an interesting future research opportunity to extend the understanding of the intrinsic mechanisms that trigger calcification.
These data have some limitations. Follow-up time was limited to a median of only 2 years; therefore, results describe relatively short-term changes in calcification, and are limited to detecting risk factor associations that exceed the short-term variability of the scanner. At present, no imaging method can reliably distinguish intimal calcification, which represents calcified atherosclerotic plaque, from medial calcification, which represents osseous transformation of vascular smooth muscle cells that is more specific to individuals with kidney disease and diabetes. The distinction between atherosclerosis and medial calcification is important because therapies for these conditions may differ. The range of kidney function in this community-based cohort was restricted to stage III CKD, and findings may differ among individuals with more advanced CKD. Furthermore, estimating equations lose precision when estimated GFR values are close to 60 ml/min per 1.73 m2.30 Some survival bias due to loss to follow-up is expected in this longitudinal study of CAC measurements. Fortunately, mortality between scans was low. Finally, power to detect separate risk factors for incidence and progression is limited by the relatively small number of incident events; however, the initiation and extension of vascular calcification may represent different pathophysiological processes, motivating separate analyses.
The strengths of this study include the community-based, multiethnic population, the focus on individuals without clinical cardiovascular disease to evaluate coronary calcification when it first develops, and the uniform and precise data collection methods that were used in MESA. In summary, we describe the natural history of CAC in stage III CKD. Future areas for study include longer term assessment of CAC, further scrutiny of persistently noncalcified individuals with CKD, and distinction between atherosclerosis and medial calcification pathways in nondialysis CKD.
MATERIALS AND METHODS
Study population
We studied participants from the MESA, a multicenter, community-based study of subclinical cardiovascular disease. Details of the MESA study design and sampling procedures are described in detail elsewhere.31 Briefly, MESA investigators recruited 6814 men and women, 45–84 years old, who identified their race/ethnicity as White/ Caucasian, Black/African American, Chinese, or Spanish/Hispanic/ Latino from six US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St Paul, MN) between July 2000 and August 2002. Individuals were not eligible for MESA if they had a previous diagnosis of cardiovascular disease (physician-diagnosed heart attack, angina, stroke, transient ischemic attack, heart failure, atrial fibrillation, taking nitroglycerin, or having undergone angioplasty, coronary artery bypass graft, valve replacement, pacemaker or defibrillator implantation, or any surgery on the heart or arteries). MESA investigators recruited participants according to prespecified race/ethnicity categories. Each participant provided informed consent and the Institutional Review Board at each site approved the study protocol.
For the present analysis, we selected MESA participants who had CKD at the time of their baseline examination. We defined CKD by an estimated GFR of <60 ml/min per 1.73 m2 using the four-variable MDRD equation.32 MESA laboratory personnel calibrated serum creatinine levels to the Cleveland Clinic laboratory.33 We excluded participants who did not complete a follow-up CT measurement of coronary calcium (n = 122).
Measurement of CAC
MESA field centers measured CAC using electron beam CT or multidetector row helical CT, as described previously.34 An expert committee developed protocols to standardize scan acquisition across the two technologies,34 and data quality was equivalent between CT scan techniques.35 MESA investigators scanned each participant twice over phantoms of known physical calcium concentration, averaged the two scan readings, and scored them using the Agatston method.36 All scans were read centrally at the Harbor-UCLA (University of California, Los Angeles) reading center. Intra-reader variability was 2.8% and inter-reader variability was 4.1%.34 All MESA participants underwent CT scans for CAC at their baseline visit. Half of the participants were selected at random to undergo a repeat scan during the second MESA examination; the other half underwent repeat scanning during the third MESA examination. Examinations were conducted an average of 1.6 and 3.2 years after baseline exam and used identical protocols as the baseline examination.
Measurement of other study data
MESA participants completed self-administered questionnaires and provided fasting blood and spot urine samples. Trained MESA research staff conducted participant interviews and study examinations. We defined diabetes by the use of any diabetes medication, or a fasting blood glucose level of ≥126 mg/dl,37 and defined impaired fasting glucose by a fasting glucose level of 100–125 mg/dl in the absence of diabetes. We defined microalbuminuria by a urinary albumin-to-creatinine ratio of ≥30 mg/g.33 MESA research staff obtained three seated blood pressure measurements 5 min apart using an automated sphygmomanometer, and averaged the last two measurements for analysis. The Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT, USA) measured serum cystatin C levels using a BNII nephelometer (Dade Behring Inc., Deerfield, IL, USA).
Statistical analysis
We defined prevalent CAC by a baseline Agatston score >0. This cutoff point has been used in previous general population studies of coronary calcification and is relevant to incident cardiovascular events in MESA.18,38 We defined incident CAC by a follow-up Agatston score >0 among participants without baseline CAC. A score of 0 indicates two consecutive negative scans, because calcification scores in MESA were calculated as the mean of two scans obtained 5 min apart.
We examined progression of CAC scores as the absolute and relative annualized change among participants with a positive baseline CAC score. We also utilized a dichotomous definition of CAC progression (yes versus no) that required an absolute change in CAC that exceeded the 95% repeatability limits of the scanner (absolute increase in CAC score greater than the expected change because of measurement error alone). Re-scan limits for CAC were determined earlier in a repeatability study of the full 6742-member MESA cohort with repeat scans as follows:39
For hypothetical participants with baseline CAC scores of 10, 50, 100, and 400 Agatston units, the minimum absolute changes in CAC required to meet this definition of progression would be 19, 45, 68, and 169 Agatston units, respectively.
We used Poisson regression with robust variance estimation to model dichotomous outcomes (CAC prevalence, incidence, and progression) as a function of predictor covariates. For analyzing the change in CAC score, we included an offset term for time between scans and a term for scanner pair to account for equipment upgrades that occurred between some visits. We used linear regression to model continuous outcomes such as the extent of log-transformed baseline Agatston score, the mean absolute change, and the relative change in the Agatston score. We included a linear term for time between CT scans in linear regression models. We adjusted multiple regression models for demographics and traditional cardiovascular risk factors such as age, race/ethnicity, sex, diabetes, hypertension, lipids, smoking, body mass index, C-reactive protein, log urine albumin-to-creatinine ratio, and serum cystatin C. We conducted statistical analyses using STATA version 9.0 (STATA, College Station, TX, USA).
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95166, N01-HC-95169, R01-HL-071739, and R01-HL-072403 from the National Heart, Lung, and Blood Institute and a National Institutes of Health Career Development Award, K23 DK63274-01. The authors thank other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
DISCLOSURE
BRK reports receiving consulting fees from Shire, Abbott, and Genzyme, and also reports receiving a clinical research grant from Amgen.
References
- 1.Blacher J, Guerin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 3.Qunibi WY, Abouzahr F, Mizani MR, et al. Cardiovascular calcification in Hispanic Americans (HA) with chronic kidney disease (CKD) due to type 2 diabetes. Kidney Int. 2005;68:271–277. doi: 10.1111/j.1523-1755.2005.00402.x. [DOI] [PubMed] [Google Scholar]
- 4.Russo D, Palmiero G, De Blasio AP, et al. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–1030. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Raggi P, Bellasi A, et al. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62 :245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 7.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 8.Ix JH, Katz R, Kestenbaum B, et al. Association of mild to moderate kidney dysfunction and coronary calcification. J Am Soc Nephrol. 2008;19:579–585. doi: 10.1681/ASN.2007070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer H, Toto R, Peshock R, et al. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol. 2005;16:507–513. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 10.Mikami S, Hamano T, Fujii N, et al. Serum osteoprotegerin as a screening tool for coronary artery calcification score in diabetic pre-dialysis patients. Hypertens Res. 2008;31:1163–1170. doi: 10.1291/hypres.31.1163. [DOI] [PubMed] [Google Scholar]
- 11.Dellegrottaglie S, Saran R, Gillespie B, et al. Prevalence and predictors of cardiovascular calcium in chronic kidney disease (from the Prospective Longitudinal RRI-CKD Study) Am J Cardiol. 2006;98:571–576. doi: 10.1016/j.amjcard.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Tomiyama C, Higa A, Dalboni MA, et al. The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrol Dial Transplant. 2006;21:2464–2471. doi: 10.1093/ndt/gfl291. [DOI] [PubMed] [Google Scholar]
- 13.Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ix JH, De Boer IH, Peralta CA, et al. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol. 2009;4:609–615. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer H, Palmas W, Kestenbaum B, et al. Chronic kidney disease prevalence estimates among racial/ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Clin J Am Soc Nephrol. 2008;3:1391–1397. doi: 10.2215/CJN.04160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer H, Jacobs DR, Jr, Bild D, Post W, et al. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 17.Aiyer AN, Kip KE, Marroquin OC, et al. Racial differences in coronary artery calcification are not attributed to differences in lipoprotein particle sizes: the Heart Strategies Concentrating on Risk Evaluation (Heart SCORE) Study. Am Heart J. 2007;153:328–334. doi: 10.1016/j.ahj.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Loria CM, Liu K, Lewis CE, et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49:2013–2020. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Reiner AP, Carlson CS, Ziv E, et al. Genetic ancestry, population substructure, and cardiovascular disease-related traits among African-American participants in the CARDIA Study. Hum Genet. 2007;121:565–575. doi: 10.1007/s00439-007-0350-2. [DOI] [PubMed] [Google Scholar]
- 20.Luckey MM, Meier DE, Mandeli JP, et al. Radial and vertebral bone density in white and black women: evidence for racial differences in premenopausal bone homeostasis. J Clin Endocrinol Metab. 1989;69:762–770. doi: 10.1210/jcem-69-4-762. [DOI] [PubMed] [Google Scholar]
- 21.Nelson DA, Jacobsen G, Barondess DA, et al. Ethnic differences in regional bone density, hip axis length, and lifestyle variables among healthy black and white men. J Bone Miner Res. 1995;10:782–787. doi: 10.1002/jbmr.5650100515. [DOI] [PubMed] [Google Scholar]
- 22.Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, et al. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 23.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 24.Nasir K, Shaw LJ, Liu ST, et al. Ethnic differences in the prognostic value of coronary artery calcification for all-cause mortality. J Am Coll Cardiol. 2007;50 :953–960. doi: 10.1016/j.jacc.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 25.Moe SM, O’Neill KD, Duan D, et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 26.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 27.Gross ML, Meyer HP, Ziebart H, et al. Calcification of coronary intima and media: immunohistochemistry, backscatter imaging, and x-ray analysis in renal and nonrenal patients. Clin J Am Soc Nephrol. 2007;2:121–134. doi: 10.2215/CJN.01760506. [DOI] [PubMed] [Google Scholar]
- 28.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 30.Rule AD, Bergstralh EJ, Slezak JM, et al. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69:399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 31.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 33.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 34.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 35.Detrano RC, Anderson M, Nelson J, et al. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility—MESA study. Radiology. 2005;236:477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 36.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 37.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 38.Cheng YJ, Church TS, Kimball TE, et al. Comparison of coronary artery calcium detected by electron beam tomography in patients with to those without symptomatic coronary heart disease. Am J Cardiol. 2003;92:498–503. doi: 10.1016/s0002-9149(03)00714-8. [DOI] [PubMed] [Google Scholar]
- 39.Chung H, McClelland RL, Katz R, et al. Repeatability limits for measurement of coronary artery calcified plaque with cardiac CT in the Multi-Ethnic Study of Atherosclerosis. AJR Am J Roentgenol. 2008;190:W87–W92. doi: 10.2214/AJR.07.2726. [DOI] [PubMed] [Google Scholar]