Abstract
Background
Little is known regarding the associations between high-molecular-weight (HMW-) adiponectin, leptin and soluble leptin receptor (sOB-R) and metabolic syndrome (MetS) in Chinese. Also few studies elucidate the effects of inflammation and body fat mass on the relations.
Methods
Plasma HMW-adiponectin, leptin and sOB-R were measured among 1055 Chinese men and women (35∼54 yrs). Whole body and trunk fat mass were determined by Dual-energy X-ray absorptiometry. MetS was defined by the updated NCEP/ATPIII criterion for Asian-Americans.
Results
HMW-adiponectin was inversely associated with MetS in multivariate model including fat mass index (FMI), inflammatory markers, leptin and sOB-R (OR in the highest quartile = 0.30, 95%CI 0.18∼0.50, P<.0001). Plasma sOB-R was also inversely associated with MetS independent of body fatness and inflammatory markers, whereas the association was somewhat attenuated after adjusting HMW-adiponectin (OR for the highest quartile = 0.78, 95%CI 0.47∼1.32, P = 0.15). In contrast, leptin was associated with increased odds of MetS independent of inflammatory markers, HMW-adiponectin, and sOB-R (OR for the highest quartile = 2.64, 95%CI 1.35∼5.18, P = 0.006), although further adjustment for FMI abolished this association.
Conclusions
HMW-adiponectin exhibited strong inverse associations with MetS independent of body composition, inflammation, leptin and sOB-R; while the associations of leptin and sOB-R were largely explained by fat mass or HMW-adiponectin, respectively.
Introduction
Adipose tissue is an endocrine organ playing a pivotal role in the pathogenesis of metabolic diseases. Several adipose-derived adipokines have been demonstrated as mediators linking obesity to insulin resistance, dyslipidemia and inflammation [1]. Adiponectin, one of the most abundant adipokines in circulation, exhibits insulin-sensitizing, fat-burning and anti-inflammatory properties [2], [3]. Hypoadiponectinmia frequently appeared in Individuals with obesity, metabolic syndrome (MetS) and type 2 diabetes [4], [5]. However, there are at least 3 isomers of adiponectin, i.e., trimer, hexamer and high-molecular-weight (HMW-) multimer [2]. Accumulating evidence suggests that HMW-adiponectin is the most physiologically active form related to glucose tolerance [6], insulin sensitivity [7], central fat distribution and multiple metabolic disorders [8]. Previous studies also indicated that HMW-adiponectin could be a useful marker to evaluate risk of MetS or type 2 diabetes in elderly Japanese [9], Japanese-Americans [10] or Caucasian women [11].
Another major and well studied adipokine in blood stream is leptin which could suppress food intake and stimulate energy expenditure [12]. However, rather than leptin deficient, obese persons often have hyperleptinemia specified as “selective leptin resistance” associated with MetS, type 2 diabetes and cardiovascular disease (CVD) [13], [14]. Circulating leptin is either in a free form which could trigger downstream signaling or in a binding form with its soluble receptor (sOB-R). Recently, a large prospective cohort reported a strong inverse association between sOB-R and diabetes, independent of BMI, leptin and adiponectin [15]. However, data regarding the associations of leptin and sOB-R and MetS, the important risk factor of diabetes and CVD, is rather limited and inconsistent [14]–[19].
Adipokines dysregulation has been considered as one of the major mechanisms mediating adverse effects of excess body fat on metabolic abnormalities. However, most studies so far have used BMI to evaluate adiposity and it remains unclear how fat mass or fat distribution per se influences the associations between the adipokines and metabolic disorders. Moreover, body composition may also vary according to ethnical background. Compared to Caucasians, Asians are more likely to have abdominal obesity under “normal BMI” [20] and prone to type 2 diabetes at lower BMI [21]. Meanwhile, accompanying rapid diet and lifestyle transition, epidemic trend of metabolic diseases has become a major public health problem in China [22], which is estimated to have 92.4 million adults with diabetes and another 148.2 million people with prediabetes [23]. Obviously, understanding the roles of these adipokines and also their modifying factors could be critical for metabolic disease control and prevention in countries like China.
Therefore, our primary aim was to examine the associations of plasma HMW-adiponectin, leptin and sOB-R with risk of MetS and its components. Meanwhile, we also evaluated how these associations were modified by BMI, inflammatory markers, body fat mass or trunk fat percentage and other established risk factors in 1055 middle-aged Chinese men and women.
Methods
Study design and subjects
The study population was non-institutionalized residents from The Gut Microbiota and Obesity Study, a case-control study of normal weight (18≤ BMI <24.0 kg/m2) and overweight/obesity (BMI ≥24.0 kg/m2) participants [22] aged from 35 to 54 years living in Shanghai for at least 10 years. Detailed study design and inclusion/exclusion criteria were described elsewhere [24]. Four men with missing values for adipokines were excluded. The final analytical sample comprised 557 overweight/obese and 498 normal-weight subjects. The protocol was approved by the institutional review board of the Institute for nutritional sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Written informed consents were obtained from all participants.
Information of demographics, health status, diet and lifestyles was collected using a standardized questionnaire during home interview. Dietary intake was assessed with a modified food frequency questionnaire used in the National Survey on the Status of Nutrition and Health of the Chinese People in 2002 [25]. Smoking and drinking were defined as “yes” or “no”. Family history of chronic diseases was defined as one of the parents or siblings having CVD, stroke or type 2 diabetes. Educational attainment was categorized according to self-reported school years. Levels of physical activity were calculated as a sum of metabolic equivalent (MET)-minute/week score [26] and then classified as low, moderate and high. Sleeping was assessed by average daily sleep time.
All participants had a physical examination after overnight fasting. Body weight, height, waist circumference, blood pressure were measured according to a standardized protocol described elsewhere [27]. A dual-energy X-ray absorptiometry scan (DEXA, QDR-4500, Hologic, Waltham, MA, USA) was conducted in 956 (90.6%) individuals and no significant difference in characteristics was found between those with and without DEXA. Values for total fat mass, trunk fat mass and trunk mass (kg) were obtained by using software built into the scanner (version 11.2.1) and daily quality control was performed using phantom #12447 the same as previous study [28].
Laboratory measurements
The blood processing procedure and assays for fasting plasma glucose, insulin, triglycerides, HDL cholesterol (HDL-C), high-sensitive C-reactive protein (hsCRP) and IL-6 were described in a previous study [29].
HMW-adiponectin was assessed using an ELISA kit (Millipore, St. Charles, MO, USA). Leptin and sOB-R were determined also by ELISA (R&D Systems Inc., Minneapolis, MN, USA). The average intra-assay and inter-assay coefficients of variation (CVs) were <10%.
Ascertainment of metabolic syndrome
Metabolic syndrome was defined according to the updated National Cholesterol Education Program Adult Treatment Panel III criteria for Asian-Americans [30], which includes at least three of the following components: 1) waist circumferences ≥90 cm in men or ≥80 cm in women; 2) triglycerides ≥1.7 mmol/L; 3) HDL-C <1.03 mmol/L in men or <1.30 mmol/L in women; 4) blood pressure ≥130/85 mmHg, or current use of anti-hypertensive medications; 5) fasting plasma glucose ≥5.6 mmol/L.
Statistical analysis
Fat mass index (FMI) was calculated based upon DEXA data as total fat mass (kg)/height (m)2 [28]. Trunk fat percentage was the ratio of trunk fat mass (kg) to total trunk mass (kg). Homeostasis model assessment of insulin resistance (HOMA-IR) was computed using updated homeostasis model assessment methods (http://www.dtu.ox.ac.uk/).
All continuous variables were log-transformed to improve normality. General linear model was applied for the comparisons between non-MetS and MetS (Table S1). Spearman partial correlation coefficients were estimated after adjustment for age, sex and other covariates (Table 1). Because of gender differences in the distributions of adipokines, sex-specific quartile cut-points were used. Multivariate logistic regression models were used to estimate the odds ratios, adjusting for age (continuous), sex, newly diagnosed diabetes (fasting plasma glucose ≥7.0 mmol/L or 2-h post load plasma glucose ≥11.1 mmol/L), smoke (past/current or not), current alcohol use (yes or not), family history of chronic diseases (yes or not), education (0∼9, 10∼12 or >12 years), physical activity (low, moderate or high), sleep (<7, 7∼9 or ≥9 h/d), total energy intake (kcal/d), BMI or FMI or trunk fat percentage, hsCRP, IL-6 and adipokines (log-transformed, continuous). All statistical analyses were performed using Stata 9.2 (Stata, College Station, TX, USA). Two-sided P<0.05 was considered statistically significant.
Table 1. Spearman partial correlation coefficients with metabolic parameters 1.
| HMW-adiponectin(µg/mL) | Leptin(ng/mL) | sOB-R(ng/mL) | |
| BMI (kg/m2) 2, 3 | −0.31 | 0.66 | −0.31 |
| FMI (kg/m2) 2 | −0.27 | 0.75 | −0.31 |
| Trunk fat percentage (%) | −0.18 | −0.04ns | −0.05ns |
| Waist circumference (cm) | −0.19 | −0.02ns | −0.13 |
| Systolic blood pressure (mm Hg) | −0.06ns | −0.03ns | 0.09 |
| Diastolic blood pressure (mm Hg) | −0.02ns | −0.006ns | 0.06ns |
| Fasting glucose (mmol/L) | −0.03ns | 0.04ns | 0.04ns |
| Insulin (µU) | −0.22 | 0.13 | −0.16 |
| HOMA-IR | −0.22 | 0.13 | −0.15 |
| Triglycerides (mmol/L) | −0.28 | 0.09 | −0.09 |
| HDL cholesterol (mmol/L) | 0.29 | 0.01ns | 0.17 |
| hsCRP (mg/L) | −0.05ns | 0.03ns | −0.009ns |
| IL-6 (pg/mL) | −0.08 | 0.008ns | −0.11 |
| HMW-Adiponectin (µg/mL) | 1 | - | - |
| Leptin (ng/mL) | 0.02ns | 1 | - |
| sOB-R (ng/mL) | 0.17 | −0.002ns | 1 |
Analyses were conducted in 956 participants. All coefficients were significant unless indicated “ns”. Adjusted for age, sex, diabetes, smoke, alcohol, family history of chronic diseases, education, physical activity, sleep, total energy intake and fat mass index (FMI).
Adjusted all abovementioned variables except FMI.
Analyses were conducted in 1055 participants.
Results
Individuals with MetS were older, and had higher BMI, waist circumference, blood pressure, fasting glucose, insulin, HOMA-IR, triglycerides, inflammatory markers (hsCRP and IL-6), fat mass index and trunk fat percentage, but lower HDL-C. Meanwhile, they also exhibited lower concentrations of HMW-adiponectin and sOB-R, but greater leptin compared with those without MetS (Table S1).
HMW-adiponectin was inversely associated with BMI (Table 1, r = −0.31) and FMI (r = −0.27). Controlling for FMI, HMW-adiponectin was still associated negatively with trunk fat percentage (r = −0.18), waist circumference (r = −0.19), insulin (r = −0.22), HOMA-IR (r = −0.22), triglycerides (r = −0.28) and IL-6 (r = −0.08), while positively with HDL-C (r = 0.29) and sOB-R (r = 0.17). In comparison, sOB-R showed somewhat weaker associations: r = −0.13 with waist circumference, r = −0.09 with triglycerides, r = 0.17 with HDL-C and no relation to trunk fat percentage (r = −0.05).
In contrast, leptin was strongly correlated with BMI (r = 0.66) and FMI (r = 0.75). After adjustment for FMI, only the correlations with insulin (r = 0.13), HOMA-IR (r = 0.13), triglycerides (r = 0.09), remained statistically significant.
Risk of metabolic syndrome
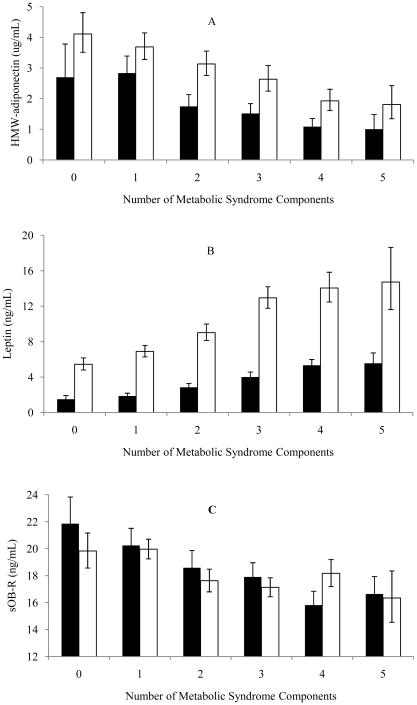
HMW-adiponectin and sOB-R decreased (Figure 1, A and C) while leptin increased (Figure 1B) with an increased number of MetS components in both men and women (all P<.0001).
Figure 1. Geometric means (95%CI) of concentrations of HMW-adiponectin (A), leptin (B) and soluble leptin receptor (sOB-R, C) in individuals with 0 to 5 components of metabolic syndrome.
Black bars = Men; white bars = Women. All P values were <.0001.
In multivariate logistic regression analyses, both HMW-adiponectin and sOB-R were negatively, whereas leptin was positively, associated with the risk of MetS independent of BMI and inflammatory markers (Table 2, Model 1 and 2). The odds ratios (ORs) comparing the highest with the lowest quartile were 0.34 (95%CI 0.20∼0.58, P trend<.0001) for HMW-adiponectin, 0.68 (95%CI 0.41∼1.13, P trend = 0.05) for sOB-R and 2.60 (95%CI 1.34∼5.05, P trend = .005) for leptin. Further adjustments of leptin and sOB-R showed little impact on the association between HMW-adiponectin and MetS. Similarly, adjustment for sOB-R and HMW-adiponectin did not affect the association between leptin and MetS. However, the significant association between sOB-R and MetS disappeared after HMW-adiponectin was included in the Model 3 (P trend = 0.15). Replacing BMI with FMI (Model 4) did not substantially change the significant associations for both HMW-adiponectin and sOB-R (ORs in the highest quartile were 0.30, 95%CI 0.18∼0.50, P trend<.0001 and 0.61, 95%CI 0.36∼1.02, P trend = 0.02, accordingly); but abolished the significance between leptin and the risk of MetS (P trend = 0.23).
Table 2. Odds ratio (95%CI) of metabolic syndrome according to sex-specific quartile of HMW-adiponectin, leptin and sOB-R.
| Adipokines | Q1 | Q2 | Q3 | Q4 | P for trend |
| HMW-adiponectin 1 | |||||
| Model 1 2 | 1 | 0.66 (0.41, 1.04) | 0.46 (0.29, 0.74) | 0.34 (0.20, 0.56) | <.0001 |
| Model 2 2 | 1 | 0.65 (0.41, 1.04) | 0.46 (0.29, 0.74) | 0.34 (0.20, 0.58) | <.0001 |
| Model 3 2 | 1 | 0.64 (0.40, 1.02) | 0.46 (0.29, 0.74) | 0.35 (0.21, 0.59) | <.0001 |
| Model 4 3 | 1 | 0.60 (0.37, 0.96) | 0.49 (0.30, 0.79) | 0.30 (0.18, 0.50) | <.0001 |
| Leptin 1 | |||||
| Model 1 2 | 1 | 1.89 (1.07, 3.34) | 2.76 (1.51, 5.01) | 3.02 (1.57, 5.78) | 0.0009 |
| Model 2 2 | 1 | 1.84 (1.03, 3.28) | 2.60 (1.41, 4.78) | 2.60 (1.34, 5.05) | 0.005 |
| Model 3 2 | 1 | 1.86 (1.03, 3.36) | 2.57 (1.39, 4.75) | 2.64 (1.35, 5.18) | 0.006 |
| Model 4 3 | 1 | 1.44 (0.80, 2.59) | 1.70 (0.92, 3.17) | 1.59 (0.78, 3.24) | 0.23 |
| sOB-R 1 | |||||
| Model 1 2 | 1 | 0.92 (0.58, 1.44) | 0.61 (0.38, 0.98) | 0.59 (0.36, 0.97) | 0.01 |
| Model 2 2 | 1 | 0.99 (0.62, 1.57) | 0.64 (0.40, 1.04) | 0.68 (0.41, 1.13) | 0.05 |
| Model 3 2 | 1 | 1.07 (0.67, 1.71) | 0.70 (0.43, 1.13) | 0.78 (0.47, 1.32) | 0.15 |
| Model 4 3 | 1 | 0.82 (0.52, 1.32) | 0.56 (0.34, 0.91) | 0.61 (0.36, 1.02) | 0.02 |
Model 1: adjusted for age, sex, diabetes, smoke, alcohol, family history of chronic diseases, education, physical activity, sleep, total energy intake and log-transformed BMI.
Model 2: model 1+ inflammatory factors (hsCRP and IL-6).
Model 3: model 2+ other adipokines (leptin and sOB-R or HMW-adiponectin).
Model 4: adjusted for log-transformed FMI instead of BMI in Model 1.
Quartiles of HMW-adiponectin (µg/mL) were <1.08, 1.08–1.84, 1.84–3.16, >3.16 for men and <1.86, 1.86–3.15, 3.15–5.33, >5.33 for women. Quartiles of leptin (ng/mL) were <1.95, 1.95–3.22, 3.22–5.58, >5.58 for men and <5.92, 5.92–9.64, 9.64–15.21, >15.21 for women. Quartiles of sOB-R (ng/mL) were <15.16, 15.16–18.19, 18.19–22.15, >22.15 for men and <15.54, 15.54–18.45, 18.45–21.82, >21.82 for women.
Data were available for 956 participants.
With respect to individual components of MetS (Table 3), HMW-adiponectin was strongly associated with a decreased risk of hypertriglyceridemia (OR in the highest quartile = 0.26, 95%CI 0.16∼0.42, P trend<.0001) and low HDL-C (OR in the highest quartile = 0.22, 95%CI 0.14∼0.35, P trend<.0001), while marginally associated with central obesity (OR in the highest quartile = 0.49, 95%CI 0.25∼0.97, P trend = 0.06) in the FMI-adjusted model. Furthermore, trunk fat percentage adjustment did not affect these associations substantially (data not shown). Meanwhile, sOB-R was negatively associated with abdominal fat (OR in the highest quartile = 0.36, 95%CI 0.18∼0.73, P trend = 0.002), high triglycerides (OR in the highest quartile = 0.64, 95%CI 0.40∼1.01, P trend = 0.02) and low HDL-C (OR in the highest quartile = 0.47, 95%CI 0.31∼0.73, P trend<.0001). In contrast, leptin was only positively associated with hypertriglyceridemia after adjustment for FMI (OR in the highest quartile = 2.90, 95%CI 1.46∼5.77, P trend = 0.006).
Table 3. Odds ratio (95%CI) of individual metabolic syndrome component according to sex-specific quartile of HMW-adiponectin, leptin and sOB-R 1.
| Adipokines | Q1 | Q2 | Q3 | Q4 | P for Trend 2 |
| HMW-adiponectin | |||||
| Central obesity | 1 | 0.65 (0.35, 1.21) | 0.68 (0.35, 1.31) | 0.49 (0.25, 0.97) | 0.06 |
| Hyperglycemia | 1 | 0.93 (0.61, 1.41) | 0.80 (0.53, 1.22) | 0.88 (0.58, 1.35) | 0.45 |
| Elevated blood pressure | 1 | 0.69 (0.46, 1.05) | 0.99 (0.65, 1.50) | 0.88 (0.57, 1.35) | 0.96 |
| Hypertriglyceridemia | 1 | 0.68 (0.45, 1.03) | 0.39 (0.25, 0.60) | 0.26 (0.16, 0.42) | <.0001 |
| Reduced HDL-C | 1 | 0.78 (0.53, 1.13) | 0.54 (0.36, 0.79) | 0.22 (0.14, 0.35) | <.0001 |
| Leptin | |||||
| Central obesity | 1 | 0.60 (0.30, 1.23) | 0.84 (0.40, 1.76) | 0.50 (0.20, 1.30) | 0.44 |
| Hyperglycemia | 1 | 1.48 (0.97, 2.25) | 1.74 (1.06, 2.86) | 1.50 (0.83, 2.73) | 0.15 |
| Elevated blood pressure | 1 | 0.97 (0.61, 1.53) | 0.76 (0.45, 1.27) | 0.83 (0.45, 1.53) | 0.43 |
| Hypertriglyceridemia | 1 | 2.12 (1.21, 3.70) | 2.76 (1.51, 5.05) | 2.90 (1.46, 5.77) | 0.006 |
| Reduced HDL-C | 1 | 0.96 (0.61, 1.50) | 0.88 (0.53, 1.46) | 0.90 (0.50, 1.61) | 0.69 |
| sOB-R | |||||
| Central obesity | 1 | 0.56 (0.29, 1.09) | 0.34 (0.17, 0.66) | 0.36 (0.18, 0.73) | 0.002 |
| Hyperglycemia | 1 | 1.24 (0.82, 1.87) | 0.88 (0.58, 1.33) | 1.32 (0.86, 2.03) | 0.47 |
| Elevated blood pressure | 1 | 0.75 (0.50, 1.14) | 1.02 (0.67, 1.55) | 1.05 (0.68, 1.62) | 0.54 |
| Hypertriglyceridemia | 1 | 0.87 (0.57, 1.33) | 0.65 (0.42, 1.01) | 0.64 (0.40, 1.01) | 0.02 |
| Reduced HDL-C | 1 | 0.88 (0.60, 1.28) | 0.57 (0.38, 0.84) | 0.47 (0.31, 0.73) | <.0001 |
Number of cases: central obesity (496), hyperglycemia (616), elevated blood pressure (395), hypertriglyceridemia (305), reduced HDL-C (331).
Adjusted for the same variables as Model 4 in Table 2, including FMI. Analyses were conducted in 956 participants.
Stratified analyses
Considering the obesity case-control design of this study and to explore how obesity, total fat mass and trunk fat percentage influenced the observed relationships, we conducted BMI-stratified analyses. In both normal-weight and overweight group, HMW-adiponectin showed strong inverse associations with modified MetS, regardless whether BMI, FMI or trunk fat percentage was adjusted (Table S2, Model 1 to 3). However, leptin was not significantly associated with modified MetS under control of total or abdominal adiposity. A negative association between sOB-R and modified MetS was observed in normal weight individuals only (Model 1), and the significant association disappeared following adjustments of FMI or trunk fat percentage (Model 2 and 3). The results remained essentially the same and no significant interaction was found in consequent subgroup analyses according to gender, FMI, hsCRP and HOMA-IR (Table S3).
Discussion
Among 1055 middle-aged Chinese men and women, we observed that reduced plasma HMW-adiponectin and sOB-R, and elevated leptin level were significantly associated with an increased risk of MetS and some of its components independent of multiple confounders including BMI and inflammatory markers. However, body fat mass or adipokines showed different modifying effects on these associations. Unlike the association between HMW-adiponectin and MetS which was unaffected by adjusting for fat depots or other adipokines, the positive associations between leptin and MetS were mainly explained by total fat mass, while the associations between sOB-R and MetS were largely influenced by HMW-adiponectin.
HMW-adiponectin and metabolic syndrome
Our study indicated that low plasma HMW-adiponectin was a strong and independent risk factor related to MetS in Chinese when diet, lifestyles, adiposity, inflammatory factors leptin and sOB-R were extensively controlled. The inverse associations of adiponectin with metabolic diseases and type 2 diabetes have been well established [4], [5]. However, most studies only measured total adiponectin rather than its high-molecular-weight multimer (12–18mer), which may be more biologically active than trimer and hexamer [6], [7], [8]. Evidence regarding the associations between HMW-adiponectin and MetS was sparse and most of them were limited by small sample size and residual confounding. In one relatively large-scale study, Tabar et al. [9] reported inverse associations between HMW-adiponectin and MetS and its components, except high blood pressure in middle-aged to elderly Japanese. Likewise, our study also supported such associations in Chinese population. On the other hand, we found that low HMW-adiponectin concentration was only significantly associated with high triglyceride and low HDL-C, but not with elevated glucose and central obesity. This minor discrepancy could be due to the different definitions for MetS between two studies. It is also plausible that more confounders were controlled in our analyses.
One interesting observation in current study is that associations between HMW-adiponectin and MetS were independent of adiposity measured by BMI, FMI, or trunk fat percentage. In the Nurses' Health Study, Heidemann et al. reported that higher HMW-adiponectin was associated with lower insulin and diabetes risk independent of BMI or waist circumference [11]. While our data demonstrated that significant negative correlations between HMW-adiponectin and insulin or HOMA-IR (both r = −0.22) were independent of FMI. Moreover, adjustment for IL-6 and hsCRP had little effect on the association between HMW-adiponectin and MetS (Table 2). The mechanism underlining adiponectin regulation and signaling pathway is rather complex and non-adipose factors might also be involved. Besides fat cells, adiponectin could be secreted by skeletal muscle, cardiac myocytes and endothelial cells as well [2]. Moreover, two receptors, namely AdipoR1 and AdipoR2, operate closely with AMPK or PPAR-α pathway to enhance glucose uptake and utilization in muscle, promote lipid oxidation in liver, and improve systemic insulin sensitivity [2], [31]. In addition, insulin resistance and inflammatory factors, hallmarks of MetS, are proposed to down regulate adiponectin production [2]. Previous small-scale infusion studies suggested that correlations of adiponectin with insulin sensitivity were independent of BMI [32], total adiposity measured by DEXA, visceral fat measured by magnetic resonance imaging and intramyocellular lipid measured by 1H-magnetic resonance spectroscopy [33]. Indeed, both enlarged size and increased number of subcutaneous adipocytes could lead to accumulation of fat in non-adipose tissue, such as skeletal muscle, liver and heart. These ectopic fats are closely related to hypoadiponectinemia and might modify its link with insulin resistance [34]. Due to the limitation of DEXA method in current study, it is not possible to exclude the effects of visceral adiposity, intramyocellular or intrahepatic lipid content or adipocytes size by adjustment of FMI, although BMI were already partitioned by fat mass and fat free mass,respectively [35]. Collectively, data from our study and others supported that HMW-adiponectin was an important biomarker for MetS in addition to many established risk factors.
Leptin, soluble leptin receptor and metabolic syndrome
In this study, we also found strong associations between leptin and risk of MetS with adjusting BMI, inflammation and several known confounders (Table 2, Model 1 to 3). Interestingly, in contrast to the case of HMW-adiponectin, these associations could be diminished by adjusting FMI (Table 2, Model 4 and Table 3). Previously, data from a cohort study in Caucasian population suggested that leptin could predict future MetS independent of baseline BMI [14]. While leptin was associated with MetS risk in older Chinese women, but not in men after adjustment for BMI [36]. However, it was noteworthy that most existing studies utilized BMI to evaluate adiposity status, containing both fat and fat-free mass, which have different influences on metabolic disorders [28]. As an adipose-derived hormone, leptin plays a critical role in regulating energy homeostasis [13]. However, it is still not clear whether leptin resistance is one of the causes or the consequences of obesity, or the “vicious cycle” of them might be the culprit of metabolic disorders. In this study, we provide direct evidence highlighting the key role of fat mass in hyperleptinemia associated metabolic abnormalities. Our finding might be particularly important for Chinese who are characterized to have more body fat under given BMI [20], [21].
In addition to hyperleptinemia, our data revealed that reduced soluble leptin receptor (sOB-R), another component of leptin resistance, was significantly associated with an increased MetS risk independent of fat mass. sOB-R, formed by cleaving the ectodomain of membrane-anchored leptin receptors, represents the major leptin binding fraction in blood [37]. It regulates the available leptin pool and downstream signaling [38]. Few epidemiological studies have simultaneously investigated the associations of both plasma leptin and sOB-R with MetS. In a study from Framingham third generation participants (n = 362), leptin and free leptin index (molar ratio of leptin and sOB-R) were positively associated with severity of MetS after adjustment for age and sex [16]. With larger sample size, we further adjusted for diet, lifestyle, BMI/FMI, inflammatory markers and adipokines. However, our data indicated that leptin and sOB-R had unique properties and might reflect the different aspects of MetS. For instance, sOB-R was negatively associated with central obesity, elevated triglyceride and reduced HDL-C, whereas leptin was only associated with hypertriglyceridemia in multivariate regression including FMI (Table 3). Given the wide distribution in tissues containing membrane leptin receptors, it is reasonable to speculate that non-adipose mechanism(s) such as tissue specific effect might also play a role in sOB-R regulation. On the other hand, leptin was almost exclusively secreted by adipocytes, particularly subcutaneous fat. Hypertriglyceridemia might result from the limited capacity of adipose tissue to store excessive fat or up-regulated hepatic triglyceride secretion and down-regulated plasma triglyceride degradation [39], [40]. However, adjustment for HMW-adiponectin could abolish significance between sOB-R and MetS but not that between leptin and MetS (Table 2, Model 3). Indeed, findings from the Nurses' Health Study showed an inverse association between sOB-R and diabetes independent of BMI and HMW-adiponectin [15]. Kim et al. found if leptin-deficient mice have over-expressed adiponectin would result in improvements of glucose and lipid metabolism and inflammatory profile [41]. Obviously, further studies are warranted to clarify whether there are functional and/or signaling crosstalk among adiponectin, leptin and its receptor (s).
Strengths and Limitations
This is the first study that systematically investigates the associations of HMW-adiponectin, leptin, sOB-R, body fat mass measured by DEXA, along with a wide range of inflammatory and metabolic parameters with MetS and its features in a relatively large population with both sexes. Meanwhile, we acknowledge some limitations. Because of the cross-sectional nature, no causal relationship could be established. Also, despite of the originally obesity case-control design, we adapted a cross-sectional approach in data analyses in order to enhance statistical power. Nevertheless, similar associations of HMW-adiponectin, leptin with MetS were observed in both normal and overweight/obese individuals as well as in further subgroup analyses. Obviously, our findings need to be confirmed prospectively in different populations.
In conclusion, we found strong inverse associations between HMW-adiponectin and MetS independent of adiposity, inflammatory statuses, leptin and sOB-R. Similar associations between sOB-R and MetS were also evidenced, but were weak and attenuated by HMW-adiponectin adjustment. In contrast, leptin showed strong positive associations with the risk of MetS which could be mainly explained by body fat mass. Overall, current study provides more mechanistic insights into the linkage of adipose tissue, adipokines and inflammation to metabolic syndrome and also more evidences for detection of decreased HMW-adiponectin and leptin resistance in clinical settings to prevent metabolic disorders and future diabetic and cardiovascular outcomes.
Supporting Information
Characteristics of participants 1. Abbreviations: MetS = metabolic syndrome, hsCRP = high sensitive C-reactive protein, HMW-adiponectin = high-molecular-weight adiponectin, sOB-R = soluble leptin receptor. 1 Data were Mean ± SD or Median (IQR) or Number (percentage). 2 Adjusted for age and sex. 3 Data were available for 956 participants.
(DOC)
Odds ratio (95% CI) of modified metabolic syndrome according to sex-specific tertile of HMW-adiponectin, leptin and sOB-R in BMI-stratified analyses 1. 1 Definition of metabolic syndrome was modified: having 2 or more components of metabolic syndrome without central obesity. Tertiles were based on sex-specific levels in BMI-stratified subgroup. NO. of cases/control were 160/338 in normal weight group and 414/143 in overweight group. 2 Adjusted for the same variables in Table 2. Model 1: including BMI; Model 2: including FMI; Model 3: adjustment for log-transformed trunk fat percentage. 3 Data were available for 956 participants. NO. of cases/control were 147/307 in normal weight group and 369/133 in overweight group.
(DOC)
Odds ratio (95% CI) of metabolic syndrome according to sex-specific tertile of HMW-adiponectin, leptin and sOB-R in subgroup analyses 1. 1 Tertiles were based on sex-specific levels in each subgroup. Adjusted for the same variables as Model 4 in Table 2, including FMI. Data were available for 956 participants. 2 Median concentration of FMI was 5.87 for men and 7.79 for women. Definition of metabolic syndrome was modified: having 2 or more components of metabolic syndrome without central obesity. No adjustment for FMI. 3 Median concentration of hsCRP was 0.87 mg/L. 4 Median level of HOMA-IR was 1.08.
(DOC)
Acknowledgments
We express our sincere appreciation to all participants of the Gut Microbiota and Obesity Study, the healthcare professionals from the Centers for Disease Control and Prevention of Zhabei and Luwan districts in Shanghai and our group members (Xingwang Ye, Lihua Chen, An Pan, Ying Wu, Jing Wang, Qibin Qi, Geng Zong, Wei Gan, Shaojie Ma, He Zheng and Qianlu Jin).
Footnotes
Competing Interests: Dr. Qi Sun was supported by a postdoctoral fellowship from the Unilever Corporate Research before September 2010. This does not alter our adherence to all the PLoS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Funding: This study was supported by grant from the Chinese Academy of Sciences (KSCX2-YW-R-116, KSCX2-EW-R-10); the Ministry of Science and Technology of China (2008DFA31960, 863 Program 2009AA022704, 973 program 2011CB504002); the National Natural Science Foundation of China (30930081, 81021002); the Chief Scientist Program of Shanghai Institutes and (SIBS2008006). Dr. Qi Sun was supported by a postdoctoral fellowship from the Unilever Corporate Research before September 2010. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 2.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 3.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Li H, Franco OH, Yu Z, Liu Y, et al. Adiponectin and metabolic syndrome in middle-aged and elderly Chinese. Obesity (Silver Spring) 2008;16:172–178. doi: 10.1038/oby.2007.42. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 6.Fisher M, Trujillo M, Hanif W, Barnett AH, McTernan PG, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 7.Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007;56:2174–2177. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 8.Lara-Castro C, Luo NL, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 9.Tabara Y, Osawa H, Kawamoto R, Tachibana-Iimori R, Yamamoto M, et al. Reduced high-molecular-weight adiponectin and elevated high-sensitivity C-reactive protein are synergistic risk factors for metabolic syndrome in a large-scale middle-aged to elderly population: the Shimanami Health Promoting Program Study. J Clin Endocrinol Metab. 2008;93:715–722. doi: 10.1210/jc.2007-0397. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima R, Kamei N, Yamane K, Nakanishi S, Nakashima A, et al. Decreased total and high molecular weight adiponectin are independent risk factors for the development of type 2 diabetes in Japanese-Americans. J Clin Endocrinol Metab. 2006;91:3873–3877. doi: 10.1210/jc.2006-1158. [DOI] [PubMed] [Google Scholar]
- 11.Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 13.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franks PW, Brage S, Luan J, Ekelund U, Rahman M, et al. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes Res. 2005;13:1476–1484. doi: 10.1038/oby.2005.178. [DOI] [PubMed] [Google Scholar]
- 15.Sun Q, van Dam RM, Meigs JB, Franco OH, Mantzoros CS, et al. Leptin and Soluble Leptin Receptor Levels in Plasma and Risk of Type 2 Diabetes in US Women: A Prospective Study Diabetes. 2009;59:611–618. doi: 10.2337/db09-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingelsson E, Larson MG, Yin X, Wang TJ, Meigs JB, et al. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. J Clin Endocrinol Metab. 2008;93:3149–3157. doi: 10.1210/jc.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sepilian VP, Crochet JR, Nagamani M. Serum soluble leptin receptor levels and free leptin index in women with polycystic ovary syndrome: relationship to insulin resistance and androgens. Fertil Steril. 2006;85:1441–1447. doi: 10.1016/j.fertnstert.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Ukkola O, Kesaniemi YA. Leptin and high-sensitivity C-reactive protein and their interaction in the metabolic syndrome in middle-aged subjects. Metabolism-Clinical and Experimental. 2007;56:1221–1227. doi: 10.1016/j.metabol.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Soderberg S, Zimmet P, Tuomilehto J, Chitson P, Gareeboo H, et al. Leptin predicts the development of diabetes in Mauritian men, but not women: a population-based study. International Journal of Obesity. 2007;31:1126–1133. doi: 10.1038/sj.ijo.0803561. [DOI] [PubMed] [Google Scholar]
- 20.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, et al. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr. 2007;86:353–359. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 21.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31:177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 23.Yang W, Lu J, Weng J, Jia W, Ji L, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Yu Z, Ye X, Zou S, Li H, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care. 2010;33:1925–1932. doi: 10.2337/dc10-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhai F, Yang X, editors. Beijing: People's Medical Publishing House; 2006. The nutrition and health status of the Chinese people 2002: diet and nutrients intake. [Google Scholar]
- 26.Committee IR. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) wwwipaqkise. 2009 [Google Scholar]
- 27.Ye X, Yu Z, Li H, Franco OH, Liu Y, et al. Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Am Coll Cardiol. 2007;49:1798–1805. doi: 10.1016/j.jacc.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Rennie KL, Gu W, Li H, Yu Z, et al. Independent associations of body-size adjusted fat mass and fat-free mass with the metabolic syndrome in Chinese. Ann Hum Biol. 2009;36:110–121. doi: 10.1080/03014460802585079. [DOI] [PubMed] [Google Scholar]
- 29.Yu Z, Ye X, Wang J, Qi Q, Franco OH, et al. Associations of physical activity with inflammatory factors, adipocytokines, and metabolic syndrome in middle-aged and older chinese people. Circulation. 2009;119:2969–2977. doi: 10.1161/CIRCULATIONAHA.108.833574. [DOI] [PubMed] [Google Scholar]
- 30.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 31.Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2007;9:282–289. doi: 10.1111/j.1463-1326.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 32.Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, et al. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585–590. doi: 10.2337/diabetes.53.3.585. [DOI] [PubMed] [Google Scholar]
- 33.Furler SM, Gan SK, Poynten AM, Chisholm DJ, Campbell LV, et al. Relationship of adiponectin with insulin sensitivity in humans, independent of lipid availability. Obesity (Silver Spring) 2006;14:228–234. doi: 10.1038/oby.2006.29. [DOI] [PubMed] [Google Scholar]
- 34.Koska J, Stefan N, Permana PA, Weyer C, Sonoda M, et al. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr. 2008;87:295–302. doi: 10.1093/ajcn/87.2.295. [DOI] [PubMed] [Google Scholar]
- 35.Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond) 2010;34:S4–S17. doi: 10.1038/ijo.2010.234. [DOI] [PubMed] [Google Scholar]
- 36.Zhuo Q, Wang ZQ, Fu P, Piao JH, Tian Y, et al. Comparison of adiponectin, leptin and, leptin to adiponectin ratio as, diagnostic marker for metabolic syndrome in older adults of Chinese major cities. Diabetes Research and Clinical Practice. 2009;84:27–33. doi: 10.1016/j.diabres.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–988. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Wang Z, Li C. Modulation of circulating leptin levels by its soluble receptor. Journal of Biological Chemistry. 2001;276:6343–6349. doi: 10.1074/jbc.M009795200. [DOI] [PubMed] [Google Scholar]
- 39.Shimamura M, Matsuda M, Ando Y, Koishi R, Yasumo H, et al. Leptin and insulin down-regulate angiopoietin-like protein 3, a plasma triglyceride-increasing factor. Biochem Biophys Res Commun. 2004;322:1080–1085. doi: 10.1016/j.bbrc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 40.Huang W, Dedousis N, Bandi A, Lopaschuk GD, O'Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147:1480–1487. doi: 10.1210/en.2005-0731. [DOI] [PubMed] [Google Scholar]
- 41.Kim JY, De Wall EV, Laplante M, Azzara A, Trujillo ME, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. Journal of Clinical Investigation. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of participants 1. Abbreviations: MetS = metabolic syndrome, hsCRP = high sensitive C-reactive protein, HMW-adiponectin = high-molecular-weight adiponectin, sOB-R = soluble leptin receptor. 1 Data were Mean ± SD or Median (IQR) or Number (percentage). 2 Adjusted for age and sex. 3 Data were available for 956 participants.
(DOC)
Odds ratio (95% CI) of modified metabolic syndrome according to sex-specific tertile of HMW-adiponectin, leptin and sOB-R in BMI-stratified analyses 1. 1 Definition of metabolic syndrome was modified: having 2 or more components of metabolic syndrome without central obesity. Tertiles were based on sex-specific levels in BMI-stratified subgroup. NO. of cases/control were 160/338 in normal weight group and 414/143 in overweight group. 2 Adjusted for the same variables in Table 2. Model 1: including BMI; Model 2: including FMI; Model 3: adjustment for log-transformed trunk fat percentage. 3 Data were available for 956 participants. NO. of cases/control were 147/307 in normal weight group and 369/133 in overweight group.
(DOC)
Odds ratio (95% CI) of metabolic syndrome according to sex-specific tertile of HMW-adiponectin, leptin and sOB-R in subgroup analyses 1. 1 Tertiles were based on sex-specific levels in each subgroup. Adjusted for the same variables as Model 4 in Table 2, including FMI. Data were available for 956 participants. 2 Median concentration of FMI was 5.87 for men and 7.79 for women. Definition of metabolic syndrome was modified: having 2 or more components of metabolic syndrome without central obesity. No adjustment for FMI. 3 Median concentration of hsCRP was 0.87 mg/L. 4 Median level of HOMA-IR was 1.08.
(DOC)