Abstract
Milk fat globules (MFG) are accepted primarily as triacylglycerol delivery systems. The identification of nanometer-sized lipid-protein particles termed ‘lactosomes’ that do not contain triacylglycerol raises the question of their possible functions. MFGs were isolated by slow centrifugation and lactosomes were isolated by ultracentrifugation at a density equivalent to plasma HDL (d > 1.063 g/ml) from human milk obtained from six volunteers at different lactation stages. Isolated lactosomes were analyzed and compared with MFGs for their size distribution, lipidome, proteome, and functional activity. Lactosomes from early milk—day 8, were found to be similar in size as those from mature milk >28 days averaging ~25 nm in diameter. In total, 97 non-redundant proteins were identified in the MFG and lactosome fractions, 46 of which were unique to the MFG fraction and 29 of which were unique to the lactosome fraction. The proteins identified in the lactosome and MFG fractions were enriched with proteins identified with immunomodulatory pathways. Unlike MFGs and GM1 laden reconstituted high- density lipoprotein (rHDL) that served as a positive control, lactosomal binding capacity to cholera toxin was weak. Lipidomic analyses found that lactosomes were devoid of triacylglycerol and gangliosides, unlike MFGs, but rich in a variety of phospholipid species. The data found differences in structure, composition and function between lactosomes and MFG suggesting these two particles are derived from different biosynthetic and/or secretory pathways. The results reveal a bioactive lipid-protein, nanometer-length scale particle that is secreted into milk not to supply energy to the infant, but to play unique, protective and regulatory roles.
Keywords: Milk, lipidome, proteome, fat globule, lactosome
INTRODUCTION
Throughout evolution, mammalian milk has been subjected to constant Darwinian selective pressure, driving its nutritional values, bioactivities and complex structures toward an optimization of maternal cost and infant survival (1). Synthesis and secretion of complex supramolecular structures into milk are only of value to the extent that they protect the mother and/or encourage the success of the infant. Of all complex structures in milk, our understanding of milk lipid macrostructures is oversimplified, relating primarily to the content of pure triacylglycerols (Tg) and their fatty acid composition. The nutritional properties of milk lipids are treated simply as bulk oils, and typically compared to plant-derived oils. However, milk lipids are secreted as ensembles of complex lipid-protein ensembles, termed milk fat globules (MFGs) (2).
Unlike other tissues that secrete lipid- protein ensembles in the nanometer length sclae (i.e. lipoproteins), the mammary gland exploits a distinctive synthetic and secretion pathway to deliver lipids throughout an aqueous environment. MFGs are synthesized by the rough endoplasmic reticulum (ER) and exocytosed from the apical side of mammary epithelia enrobed by a tri-layer of polar lipids forming the MFG membrane (MFGM) (3). The MFGM consists mainly of phospholipids, glycolipids and sphingomyelin, derived from the ER and the apical membrane of the secreting mammary epithelial cells (2, 4, 5). These MFGM constituents, both lipids and proteins, have been assigned roles beyond simple nutrition, such as protection against enteropathogens (6). The diameters of secreted MFGs range from < 0.2 μm to > 15 μm (7, 8) and particle size distribution is altered under different physiological and metabolic conditions (9-11), implying that these disparate particles exert different functions than to simply deliver bulk fat to the infant.
The complexity of the MFG-secretion pathway coupled with structural analytical methods ill-equipped to measure structure-function relationships on a nanometer scale, have led to the common perception that MFGs of all sizes share the same synthetic and secretory pathways. In addition, MFGs of varying sizes are accepted as comparable in composition and function. However, in a recent study we found that MFGs derived from human milk varied in their lipid composition according to their size (12). These results challenged the perception that all MFGs are compositionally identical. Moreover, a class of lipid particles that was not described before was identified on the nanometer scale. These lipid-protein assemblies were termed ‘lactosomes’ to distinguish them from Tg-rich MFGs, a major source of energy for the infant. According to their alleged composition (i.e. lipid-protein, Tg-poor) which is similar to plasma HDL, lactosomes were isolated in human milk by sequential flotation from the density range corresponding to that of plasma HDL.
In plasma, lipid protein assemblies within the density of HDL have known functions as transporters of hydrophobic materials form peripheral tissue to the liver (i.e. HDL and albumin). In contrast, the lipid protein assemblies that play a role in secretion pathway and transport of Tg have lower densities such as low density lipoprotein and MFG in plasma and milk respectively. Therefore, the presence of lipid-protein assemblies secreted into milk that are found in milk’s high-density range is puzzling and raises questions about their biological roles.
The discovery of a this class of lipid particles—lactosomes--in milk that are free of Tg and therefore do not offer a nutritional role to the neonate led us to elucidate their structure and composition to generate new hypotheses with respect to their function.
MATERIALS AND METHODS
Sample Collection and Lipid Particle Separation
Milk samples were collected from 6 mothers at approximately 10 d, 28 d and approximately 6 mos. postpartum. Donors were instructed to fully pump one breast and aliquot 10 ml of milk after gently inverting. Milk samples were stored at −80°C until further analysis. Samples are denoted by the subject ID--1000, 1004, 1005, 1006, 1007, 1008 and postpartum day beginning 24 hours after labor--08, 09, 28, 169, and 198.
Milk lipid particles were separated according to their density. Whole milk was first separated by slow centrifugation at 1000 rpm for 10 min separating skim milk and MFGs as cream. The MFG fraction was gently removed using a wide-opening pipette, to prevent fractionation of the large, Tg-rich milk lipid globules. The skim fraction was respun at 1000 rpm for 10 min to enrich the skim fraction with small particles. This second skim fraction was subsequently adjusted to a density corresponding to that of HDL, 1.063 g/ml (13) using KBr solution, and centrifuged for 2 h 5 min at 120,000g. The opaque layer formed at the top 300 μL of the vial underneath the fat pad was identified as the lactosome (12).
Determining Particle Structure and Sizes
Scanning Electron Microscopy (SEM) images were obtained of the MFG fraction by washing in 0.1M phosphate buffer pH 7.35 and applying to a poly-L-lysine treated coverslips (14).
Transmission Electron Microscopy (TEM) was achieved by negative staining the lactosome sample (15). A 10 μL drop was added to a 400mesh form var/carbon grid and incubated for 10 min. 10 μL of 1% phosphotungstic acid in DDH2O was added and rapidly blotted from the grid. The particle size for MFG samples was determined by dynamic light scattering using Microtrac S3500 (Microtrac, Montgomeryville, PA) and cited elsewhere (16). Particle size data of lactosomes was obtained as described in the SI text and based on previous lipid vesicles size analysis (17).
Identifying MFG and Lactosome Proteome
Lactosomes from samples 1000d08, 1005d08, 1008d08 were delipidated according to the method of Wessel and Flugge (18) and the precipitated protein was solubilized in a solution of 8 M urea, 1% (w/v) SDS, and 100 mM Tris-HCl. Resolubilized protein was then reduced with 200 mM dithiothreitol at 55°C for 45 min before cooling and alkylation at room temperature with 40 mM iodoacetamide for 20 min in the dark. Reduced, alkylated proteins were then diluted fivefold with 18 MΩ water, and digested overnight with sequencing-grade modified trypsin (Promega, Madison, WI) at 37°C. The sample was then adjusted to pH 3 with formic acid and desalted on a C18 ZipTip.
Analysis was performed using an Agilent 6200 Series HPLC-Chip/TOF MS system equipped with the Agilent 1200 series microwell-plate autosampler, capillary pump, nanopump and HPLC-Chip interface as described in the SI text. The applied protein identification was achieved by using x! Hunter (version 2009.08.01.3, http://www.thegpm.org/hunter/index.html) to query the swiss-prot database (release 15.11). Carbamidomethylation of cysteine residues was selected as a constant modification. Variable modifications included deamidation of asparagine and glutamine residues, oxidation of methionine residues, and acetylation of the N-terminus. Semi-style cleavage was not allowed. A single missed tryptic cleavage was allowed for peptide identifications. The species was restricted to Homo sapiens.
Analysis of the Lactosome and MFG Proteomes
For each protein in each sample, a percent relative abundance was calculated as [(number of total peptides in the sample representing the sequence) / (number of total peptides identified in the sample)] * 100. Using ‘R’ statistical software (http://www.r-project.org/), paired two-sample t-tests were applied to determine whether each protein’s percent relative abundance differed significantly between the lactosome and MFG fractions. The resulting P-values are referred to as the unadjusted P-values, for which P ≤ 0.05 denotes marginal significance. To improve the stringency of the tests, a Benjamini-Hochberg multiple testing correction was applied to the unadjusted P- values with functions from the multi-test library (19). A Benjamini-Hochberg adjusted P-value ≤ 0.05 was used to determine significance.
Ingenuity Pathways Analysis software (http://www.ingenuity.com/) was used to identify metabolic and signaling pathways that are over-represented by the MFG or lactosome proteomes relative to all annotated genes. A Fischer’s exact test followed by a Benjamini-Hochberg multiple testing correction were used to calculate the P-value which represents the probability that the enrichment of the function or pathway with the gene set of interest is explained by chance alone. Functions or pathways reported to be statistically significant were those with a Benjamini-Hochberg adjusted P ≤ 0.05.
Identification of Particle Function in Binding Cholera Toxin
Spectroscopic analysis of fluorescent probes was performed with an LS 55 fluorescence spectrometer (Perkin-Elmer, Waltham, MA). The primary probe used was 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate (TMA-DPH) from Invitrogen (Carlsbad, CA) which only fluoresces from within a hydrophobic environment. After a 30-min delay to allow DPH partitioning into the lipid portion of the sample, cholera toxin subunit B (CTB, Sigma-Aldrich, St Louis, MO) modified with a fluorescent tag (fluorescein isothiocyanate or FITC) was added. As FITC-CTB associates with GM1 at the particle surface, the close proximity of the FITC dye molecule to the DPH contained in the lipid bilayer of the particle induces non-radiative energy transfer from donor (DPH) to acceptor (FITC); also known as FRET or Forester Resonance Energy Transfer. The association of CTB with milk particles can thus be identified by measuring the reduction in DPH emission and a qualitative comparison generated between samples and controls. Fluorescence emission was monitored using a fluorescence spectrometer at a wavelength of 490 nm which corresponds to peak FITC absorption (20). Donor- acceptor (i.e. TMA-DPH and FITC-CTB) concentration ratios were kept constant across all samples. In addition, a similar lipid- to – probe ratio was maintained in each sample in order to minimize systematic variation in donor- acceptor spacing and to ensure a qualitative comparison remains valid. All scans were performed at a temperature of 37°C and pH 7.4. Reconstituted HDL (rHDL) were produced as previously described (21) using purified wild-type apolipoprotein AI (apoAI) that was a gift from Dr. John C. Voss’s lab.
Identifying the lipidome of MFG and lactosome fractions
MFG fraction lipids (n=3) were extracted by the Folch method with the final lipid extract dissolved in 1mL of CHCl3 and stored at −80 °C until analyzed (22). A modified Folch extraction was used to extract lipids from lactosome samples that matched the MFG fractions analyzed (n=3) as described in the SI text.
Mass spectrometric comparison of lipid extracts
The MFG fraction lipid extracts were prepared for analysis by mass spectrometry by diluting the sample 500-fold using a solution of CH3Cl:MeOH (1:2 v/v) spiked with 10 mM NH4Ac. Lactosome lipid extracts were prepared for mass spectrometric analysis by performing a 5-fold dilution using the same solution as that used for the MFG fraction extracts. MFG fraction samples were analyzed in the positive mode whereas the lactosome samples were analyzed in both the positive and negative mode using electrospray ionization on a Varian 920 TQ-FTMS Fourier transform ion cyclotron resonance mass spectrometer (ESI FT-ICR MS) (Varian Inc., Walnut Creek, CA) equipped with a 9.4T superconducting magnet (Cryomagnetics, Oak Ridge, TN) and as described in the SI text.
Determining the presence of gangliosides in MFG and lactosome fractions
Gangliosides were extracted from desalted MFG and lactosome fractions as previously described with several modifications (23, 24). Briefly, samples were mixed with 22.5 volumes of water/chloroform/methanol 1.2:1:2 (by volume), centrifuged at 2000 rpm, and the aqueous upper layer was collected. The pellet was re-extracted with water/chloroform/methanol 1.2:1:2 (by vol.), centrifuged, and the aqueous layer was combined. The gangliosides were then enriched and purified by DEAE-Sephadex anion exchange column (GE Health Care Bio-Sciences, Little Chalfont, UK) and C8 solid phase extraction (Supelco, Bellefonte, PA, USA). The dried samples were solubilized in a 50:50 (v/v) methanol/water solution prior to mass spectrometry analysis.
Mass spectra were acquired on an IonSpec Pro MALDI FT-ICR mass spectrometer with a 7.0 Tesla superconducting magnet (IonSpec, Irvine, CA, USA) in positive and negative ion mode. Microliter quantities of an analyte solution and a matrix solution were mixed on a sample plate. 50 mg/ml 2,5-dihydroxybenzoic acid in a 50:50 acetonitrile/water was used as the matrix. A standard ganglioside mixture was used for external calibration.
RESULTS
Characterization of Human MFG and Lactosome Fractions
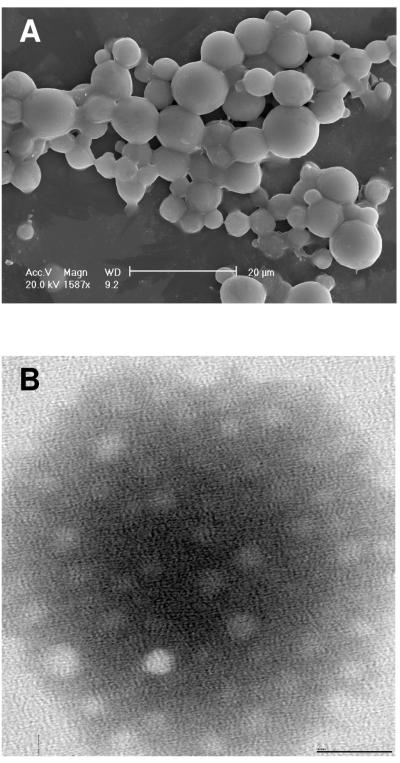
The morphology of MFGs in cream isolated from mature milk was analyzed by SEM and lactosome particles isolated from the same milk samples were analyzed by TEM. The size distribution of MFGs was within the size range of previously reported data (Figure 1A) (16). Unlike MFGs, the size distribution of lactosomes was strikingly homogenous and an order of magnitude smaller (~25nm) (Figure 1B).
Figure 1.
Images of milk fat lgobules (MFG) and lactosome fractions.
(A) Scanning electron microscopy of the MFG fraction at 169 days postpartum.
(B) Transmission electron microscopy of the lactosome fraction at 169 days postpartum.
Dynamic light scattering was used to confirm the size ranges for both MFGs and lactosome particles from the same milk samples. Mean diameters of MFGs in the cream fractions were 2.7 ± 0.9 μm, 1.5 ± 0.7 μm and 1.3 ± 0.6 μm, from samples 1000d9, 1004d8 and 1005d8 respectively. Dynamic light scattering confirmed the size and homogeneity of lactosomes visualized by TEM (Table 1) using a 90Plus Particle Size Analyzer (Brookhaven, Holtsville, NY). Inter-quartile ranges for lactosome size distributions were generated from repeated particle dynamic size measurements for each sample (Figure 2) and indicated a narrow size distribution. Due to unequal numbers of size values generated among early and mature lactosomes, an Independent Samples t-test that assumed heteroscedasticity found significant differences between early and mature lactosomes for sample 1004 (P ≤ 0.05), however there were no significant differences between lactation stages for samples 1000 and 1005.
Table 1.
Size characteristics of lactosomes1
| (nm) | |||
|---|---|---|---|
|
| |||
| Sample | Mean | Median | Range |
| 1000d09 | 30.5 ± 14.7 | 28.5 | (8.6-81.1) |
| 1000d28 | 26.1 ± 13.1 | 23.8 | (5.2-54.8) |
| 1004d08 | 34.2 ± 16.4 | 33.1 | (10.0-74.5) |
| 1004d28 | 24.2 ± 12.0* | 22.2 | (8.8-69.0) |
| 1005d08 | 24.5 ± 13.6 | 19.9 | (9.4-61.3) |
| 1005d28 | 28.3 ± 16.4 | 22.8 | (8.5-67.0) |
Data displayed as means ± standard deviation; different from day 08,
P < 0.001.
Figure 2.
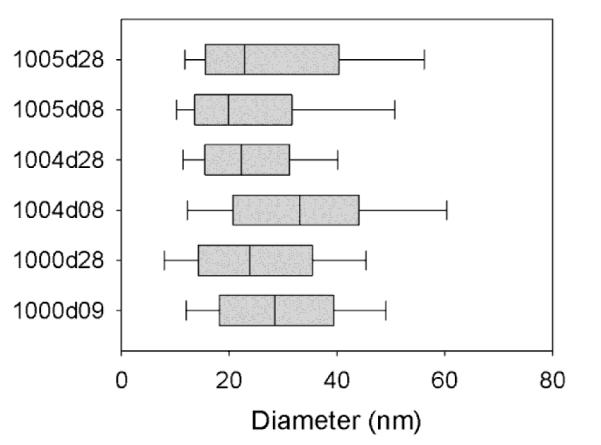
The size distribution of lactosomes derived from early and mature milks from various donors. Each box plot represents the distribution of repeated particle diameter measurement using a commercial dynamic light scattering device. The first four digits denote the sample donor (1000, 1004, 1005) while the final two indicate the postpartum day after labor (08, 09 or 28).
The Proteome of MFG and Lactosome Fractions Differ in Composition and Share Functions in Immuno-modulation
In addition to characterizing the morphologic structure of lactosomes, proteomic analyses of three lactosome samples and paired MFG fractions were determined by LC-MS-MS. The proteins identified in the lactosome and MFG fractions included caseins, abundant whey proteins (including α-lactalbumin, lactoferrin, osteopontin, and bile-salt-stimulated lipase), and several known MFG proteins (butyrophilin and various apolipoproteins). In total, 97 non-redundant proteins were identified in the MFG and lactosome fractions, 46 of which were unique to the MFG fraction and 29 of which were unique to the lactosome fraction (Online Supplemental Tables 1-6).
To elucidate functions of MFGs and lactosomes, their proteomes were analyzed for the enrichment of functions and signaling pathways. Significant molecular and cellular functions associated with the lactosome fraction are listed in Table 2 in order of decreasing significance. With the exception of “Amino Acid Metabolism”, MFGs shared these functions. In addition the MFG proteome was associated with “Cell Signaling” and “Free Radical Scavenging”. Two signaling pathways were enriched with proteins identified in the lactosome: “Acute Phase Response Signaling” and “Caveolar-mediated Endocytosis Signaling”. Both of these pathways, as well as a number of other immunomodulatory pathways were enriched with proteins identified in MFGs. Both MFG and lactosome proteins clearly have many possible immunomodulatory functions, suggesting that lactosomes, like MFG, are important to the protection of the neonate.
Table 2.
Significant molecular and cellular functions associated with the lactosome fraction
| Significant Function | Associated Proteins |
|---|---|
| Lipid Metabolism | APOE, APOB, APOA1, CLU, BTN1A1, LALBA, CEL, ALB, LTF, PLIN2, PSAP, SERPINA1, FABP3 |
| Molecular Transport | B2M, APOE, TTR, APOB, APOA1, CLU, BTN1A1, LALBA, CEL, ALB, LTF, PLIN2, CSN2, PSAP, FABP3 |
| Small Molecule Biochemistry | APOE, GNPDA1, TTR, APOB, APOA1, CLU, LALBA, BTN1A1, LYZ, CEL, ALB, LTF, PLIN2, PSAP, SERPINA1, FABP3, KRT1 |
| Protein Synthesis | APOE, APOB, APOA1, CSN2, LALBA |
| Cellular Movement | APOE, CD59, SPP1, APOB, CFL1, APOA1, CLU, LIMS1, KRT10, PIGR, ACTG1, ALB, SERPINA1, C4B |
| Cellular Assembly and Organization |
B2M, APOE, CD59, TTR, SPP1, CFL1, APOA1, SEPT7, CLU, MFGE8, KRT9, CST3, PLIN2 |
| Cell Death | PKM2, B2M, APOE, TTR, CD59, APOB, SPP1, CFL1, APOA1, CLU, YWHAZ, LIMS1, LALBA, MFGE8, LYZ, KRT10, PIGR, ALB, CST3, PSAP |
| Carbohydrate Metabolism | PKM2, CEL, APOE, ALB, GNPDA1, SPP1, APOA1, PSAP, LALBA, LYZ, FABP3 |
| Amino Acid Metabolism | APOE, TTR, ALB |
| Cell-To-Cell Signaling and Interaction |
B2M, APOE, TTR, CD59, SPP1, APOB, CFL1, APOA1, CLU, MFGE8, LIMS1, LYZ, PIGR, ALB, LTF, KRT2, KRT1, C4B |
| Antigen Presentation | APOE, APOB, SPP1, APOA1, KRT1, PIGR, C4B |
| Cellular Growth and Proliferation |
B2M, APOE, CD59, SPP1, CFL1, TFG, APOA1, CLU, MFGE8, KRT10, MYOF, PIGR, CST3, KRT2, SERPINA1, FABP3, KRT4 |
| Protein Trafficking | APOE, APOA1, CSN2, LALBA |
| Gene Expression | PIP, SPP1, CLU, YWHAZ, LALBA |
| Cellular Compromise | APOE, SPP1, CFL1, SEPT7, CLU |
| Cell Morphology | B2M, APOE, SPP1, LTF, CFL1 |
| Cellular Development | B2M, APOE, CD59, SPP1, CST3, CLU |
| Cellular Function and Maintenance |
KRT9, APOE, CD59, SPP1, CFL1, CST3, CLU |
| Post-Translational Modification | CEL, APOE, ALB, C4B |
| Vitamin and Mineral Metabolism |
APOE, CEL, APOA1, PLIN2, KRT1 |
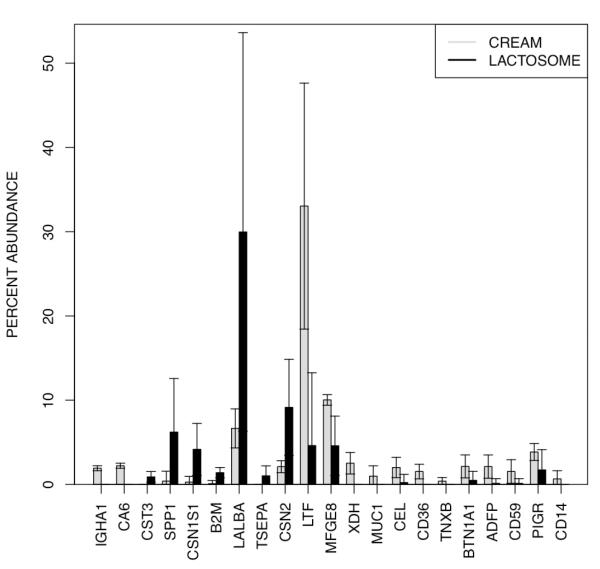
To determine whether relative abundances of proteins differ between the MFG and lactosome fractions, paired two-sample t-tests were applied to abundances of the 97 non-redundant proteins in the MFG and lactosome samples. Of the 97 proteins, 21 were marginally significant (unadjusted P ≤ 0.05), two of which, immunoglobulin heavy constant alpha 1 (IGHA1) and carbonic anhydrase 6 (CA6), were significant after a multiple testing correction (B-H adjusted P ≤ 0.05). The percent abundances of these 21 proteins in the two fractions are depicted in Figure 3.
Figure 3.
Differences in protein abundance between milk fat globules (MFG) and lactosome fractions. The relative abundances of proteins differbetween the MFG and lactosome fractions (marginal significance, unadjusted P ≤ 0.05). Two of these proteins, immunoglobulin heavy constant alpha 1 (IGHA1) and carbonic anhydrase 6 (CA6), were significant after a multiple testing correction (B-H adjusted P ≤ 0.05).
The MFG and Lactosome Fractions Differ in Lipid Composition
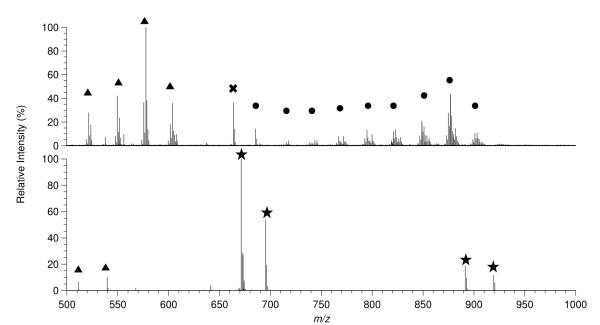
To further our understanding about the functional roles of human milk particles, preliminary lipidomic analyses were generated and compared between MFGs in cream and lactosomes from the same three subjects during the same lactation stage. Based on the reported literature concerning the lipid composition of human milk fat, the decision was made to collect positive mode spectra of the MFG fraction as this fraction was expected to be composed largely of Tg (25, 26), which are best detected in the positive mode. The positive mode mass spectra of the lipids in the MFG fraction showed several clusters of protonated masses separated by 28 mass units with each cluster being composed of several distinct masses in the m/z 700-900 range. These clusters can be seen in Figure 4a, an ESI FT-ICR spectrum of the MFG fraction from sample 1008 day 8. It was determined that each of these clusters was a family of lipids differing only in the degree of saturation. The high resolving power and mass accuracy afforded by FT-ICR MS allowed these lipids to be identified as glycerides, specifically Tg. While the class of the lipids in the spectra could be assigned on the basis of accurate mass measurements the constituent fatty acids, and their position within the lipid, could not be conclusively identified. Further analysis of the lipids by tandem mass spectrometry will be required to obtain these results. Dimers of fatty acids were also seen in the positive mode spectra of the MFG fractions in the range of m/z 500-600 (Figure 4a, marked with triangles). The formation of lipid dimers, trimers and higher aggregates during the electrospray process is well known and not unexpected when the total lipid content of the electrospray solution is greater than 0.1 ng/mL (27). Tandem mass spectrometry experiments confirmed these ions as being dimers of long chain fatty acids (Supplemental Figure 1). It should be noted that although monoacylglycerol, diacylglycerol, phospholipids, and glycolipids were not identified in these spectra they are most likely in the MFG fraction but cannot be detected due to limited dynamic range of FT-ICR MS. The negative mode spectra of the MFG fraction showed few detectable lipid masses, with the few detected lipids being the deprotonated analogs of the fatty acid clusters in the positive mode spectra. Since the lipidome of human milk is known (28), it was decided to proceed with MFG analysis in positive mode only since MFG are predominately Tg.
Figure 4.
ESI FT-ICR MS spectra of milk fat globule (MFG) and lactosome fractions from sample 1008 day 8 showing marked distinctions. (A) Positive mode spectrum of the MFG fraction. The MFG spectra are dominated by triglyceride (Tg, marked with a circle) and dimers of long chain fatty acids (marked with a triangle). Lipid heterogeneity can clearly be seen in the Tg species. (B) Negative mode spectrum of the lactosome fraction showing phospholipid (Pl, marked with a star) and fatty acid dimers (marked with a triangle). The phospholipids identified in this spectrum were phosphatidylinositol (m/z 890-920), bisphosphotidylinositol (m/z 670-700), and phosphatidic acid (m/z 640-650).
The positive mode spectra of the lactosomes showed possible ammoniated phosphatidylcholine, phosphatidic acid, and cholesterol ester species in very low abundance (< 1% of the spectral intensity) in the range of m/z 700-900 while the dominant species in the spectra were non-lipid (Supplemental Figure 2). Comparison of the positive mode spectra for the MFG and lactosome fractions clearly shows that the two have different lipid compositions. Due to the extremely low abundance of the potential lipid ions further study is needed to determine an enrichment procedure in order to elucidate their structure and determine if they are truly cholesterol esters.
The predominant lipid species in the negative mode spectra of the lactosome fractions were identified as inositol species and phosphatidic acids (Figure 4b, marked with a star). Fatty acid dimers were also seen in the negative mode spectra but at much less abundance than in the MFG fractions. Based on these preliminary results it can be seen that the MFG and lactosome fractions are clearly different with regards to lipid composition. A more in depth lipidomic analysis as well as the development of methods to enrich the lactosome lipid extracts will be the topic of future work.
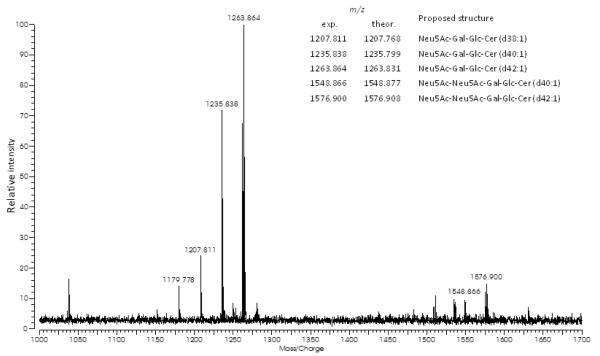
Mass spectrum of the purified MFG gangliosides shows individual ganglioside species and high heterogeneities in ceramide structures (Figure 5). Each peak was tentatively assigned based on accurate mass measurements with the postulation of core structures. For example, the [M-H]− ion at m/z 1235.799 and the [M+Na−2H]− ion at m/z 1548.877 corresponded to Neu5Ac-Gal-Glc-Cer (d40:1) and Neu5Ac-Neu5Ac-Gal-Glc-Cer (d40:1), respectively. Sequences of the gangliosides reported herein are consistent with those of the previously reported human milk ganglioside GM3 and GD3 (29). However, following the same procedure, only noise peaks were observed and gangliosides were not detectable from lactosomes (Supplemental Figure 3).
Figure 5.
Negative mode spectrum of human milk fat globule (MFG) gangliosides for 1005d28. Legend in the figure lists the assignment of the major detected structures shown.
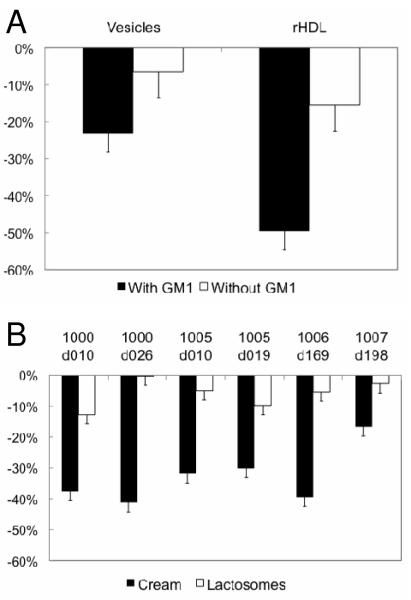
Milk Fat Globules in Cream Offer Protection against Cholera Toxin Binding
To directly probe whether human MFGs or lactosomes impart protective biological functions, a FRET-based assay was performed to assess cholera toxin-receptor binding. Positive controls of vesicles and rHDL (Figure 6a) synthesized with DMPC and the cholera toxin receptor, GM1 showed binding while negative controls of vesicles and rHDL without GM1 displayed little or no measurable binding. The addition of CTB to the MFG fraction derived from human milk led to a significant decrease in fluorescence indicating binding. However, after addition of CTB to paired lactosomes, the magnitude in fluorescence was comparable to that of the negative controls (Figure 6b). These data indicate that MFGs, known to contain gangliosides (25) bind pathogenic toxins (30, 31) and hence are capable of intercepting pathogenic toxins specific to cholera. Lactosomes, although rich in phospholipids, do not contain appreciable concentrations of gangliosides and may provide biological protection through immuno-modulation rather than as a vector to thwart pathogen or toxin binding.
Figure 6.
Cholera Toxin Binding Assay. (A) Positive and negative controls for the cholera toxin binding assay. Dimyristoylphosphatidylcholine (DMPC) vesicles and rHDL (containing DMPC and apolipoprotein A-I) were prepared for comparative analyses with the milk fat globule (MFG) and lactosome fractions. Positive controls were laden with 1mol% GM1 (toxin receptor) while negative controls did not contain any GM1. The graph shows the percent drop in DPH fluorescence at 490 nm for each control sample. (B) Cholera toxin binding in response to MFG and lactosomes. The percent drop in donor emission (DPH) after introduction of FITC-labeled cholera toxin is shown here. The lactosome fractions do not show a significant reduction relative to the negative controls while the MFG fractions are comparable to the positive controls.
DISCUSSION
In this study, an interdisciplinary approach was applied to compare the structure, composition and putative functions of a unique lipidated nanometer-length particles, in human milk, recently termed lactosomes (12). Proteomic shotgun analysis combined with lipidomics and fluorescence based techniques yielded the initial functional characterization of human lactosomes.
Using transmission electron microscopy, isolated lactosomes from milk collected at 10 days postpartum were visualized as spherical particles of approximately 25 nm in diameter. To acquire a more accurate size distribution of the lactosomes from varying lactation stages (days 8, 9 and 28) from three subjects, dynamic light scattering was used. Lactosomes isolated from early and later lactation stages were about 30 nm in diameter which is similar to that of stable vesicles, yet dissimilar in size to plasma HDL (32). Similarly, lactosomes diameter and size distribution were much smaller than other milk-derived nano-vesicles of unknown origin—exosomes (33).
Lactosomes are spherical lipid particles in milk, and it was initially suspected that they were compositionally and functionally similar to MFGs. Because the MFG size distribution is sufficiently wide such that the smallest MFG can overlap with the largest lactosome particles, size alone cannot determine if lactosomes are small MFGs. Therefore, a shotgun proteomic analysis was conducted to compare functional differences between lactosome and MFGs from paired samples. In a first pass annotation approach, Ingenuity Pathways Analysis was used to connect the complex array of proteins that were identified in the lipid particles to biological processes. Admittedly, this approach is limited since most genes and the proteins they encode have not been annotated for cellular functions and not milk and lactation-related functions. Nevertheless, the plethora of molecular functions represented by proteins in the lactosome fraction coupled with the absence of triglyceride content suggests that lactosomes may provide a unique delivery mechanism for transfer of signaling proteins between mother and offspring.
To interrogate the possibility that lactosomes do not share an identical secretion pathway with Tg-rich MFGs, a manual screening of proteins with differing abundance between MFGs and lactosomes (Figure 3) was conducted using the amiGO website (http://amigo.geneontology.org/cgi-bin/amigo/search.cgi). The proteomic results confirm that several membrane bound proteins that are well described as part of the apical membrane of the mammary gland epithelial cell were only found in the MFGs and not in the lactosomes. MUC1 (mucin 1), which was significantly associated with the MFGs and not the lactosomes, is found on the apical membrane of mammary epithelial cells. Its presence in the MFGs is in agreement with the known and unique secretion pathway in which globules bud from mammary epithelium enveloped by the apical membrane of the cells, including its cargo of proteins. The absence of MUC1 from the lactosomes proteome further distinguishes it from milk exosomes, which were reported to carry a substantial amount of this glycoprotein on their surface (33). Similarly, IGHA1 is also associated with the MFG and not the lactosomes. IGHA1 is associated with the endoplasmic reticulum—the site of Tg synthesis-- of the mammary epithelial cell. On the other hand, MFGE8 (milk fat globule-EGF factor 8 protein), a protein that interacts specifically and non-covalently with phosphatidylserine and phosphatidylethanolamine was found in both MFGs and lactosomes. The presence of this protein in both MFGs and lactosomes confirms that phospholipids surround both particles. Finally, two well-defined proteins, XDH (xanthine dehydrogenase) and BTN1A1 (butyrophilin), known for their major role in the secretion pathway of Tg-rich MFGs (34), were unequally associated with each of the studied particles. XDH was found only in the MFG whereas BTN1A1 was found in both MFGs and lactosomes, however in much lower abundance in the latter. These two proteins and the interaction between them are required for the secretion of Tg-rich MFGs and their absence or low abundance in lactosomes suggest that lactosomes are secreted by a different pathway.
The annotated functions of those proteins with greater abundance (unadjusted P ≤ 0.05) in the lactosomes relative to the MFGs suggest that lactosomes have putative protective roles in immunity and cell regulation. SPP1 (osteopontin), an extracellular glycoprotein associated with the aqueous phase of colostrum (35) involved in the early innate (36) and adaptive immune responses (37-39). Another lactosome-associated protein found in the aqueous phase of human colostrum (35) with immune-modulatory function is B2M (Beta-2-microglobulin). B2M is a component of the major histocompatibility complex (MHC) class 1, associated with the acute phase response (40) and shown to be critical for protection against bacterial infection. In addition to carrying a protein cargo associated with immune protection, lactosomes offer a putative role in protection against dysregulated cellular proliferation. Lactosomes contained a relatively high abundance (unadjusted P ≤ 0.05) of LALBA (α-Lactalbumin), a major secretory protein of human whey (35) and a component to lactose synthase. In the presence of oleic acid, partial unfolding of α-Lactalbumin is engineered into HAMLET (human alpha-lactalbumin made lethal to tumor cells) which acts as an apoptotic factor that has the ability to kill abnormal cells while sparing healthy ones (41, 42). Thus, it is possible that lactosomes have a distinct function in the protection of the neonate gut.
To confirm that the lipid components of MFGs and lactosomes are compositionally different, mass spectrometry analysis was performed. The analysis of the mass spectra obtained from MFGs and lactosome samples found fundamental differences between the two fractions. The MFG predominantly contained triacyl- and diacylglycerol which were absent in lactosomes. The predominant lipid classes found in lactosomes were phospholipids. Based on the absence of a Tg core, an energy delivery role cannot be assigned to lactosomes unlike that for MFGs.
To elucidate the functional role associated with both MFGs and lactosome particles, both were tested in a positive and negative controlled cholera binding functional assay. Lipid protein assemblies from plasma (i.e. lipoproteins) (43, 44) and from milk (45) exert protection against endotoxemia by binding and neutralizing bacterial-secreting toxins. In spite of their low abundance in milk as part of the MFG membrane, gangliosides play an important role in the prevention of infection and immunity (31, 46). Therefore, we tested if milk-derived lipid-protein assemblies could bind cholera toxin compared with rHDL and MFGs. Since GM1 binds endotoxins with a high affinity (47), the positive and negative controls for this assay included rHDL and phospholipid vesicles laden with and without ganglioside mono-sialyic (GM1). Interestingly, while Tg-rich MFGs showed significant binding capacity compared with the positive controls, the lactosomes showed little interaction with cholera toxin. Not knowing if this was a result of the compositional or structural configuration of lactosomes, gangliosides were measured and compared between lactosomes and MFGs.
Gangliosides were not detected in the lactosomes; however GM3 and GD3 were identified in MFGs. These data together with the proteomic analyses do not support the current accepted theory in which all milk-derived lipid particles originate from the same synthetic and secretory pathways in the mammary gland (2). Furthermore, the difference in ganglioside composition-- a signature of the apical mammary epithelial cell membrane--suggests that lactosomes do not share the secretory pathway with MFGs.
Our data indicate that milk contains at least two different species of lipid-protein assemblies; MFGs and lactosomes. Based on their different lipid and protein compositions, MFGs and lactosomes appear to be secreted from different synthetic and secretory pathways. Using shotgun proteomic analysis, both particles were found to carry a cargo of proteins involved in inflammatory modulation, yet possibly through different pathways. In addition, lactosomes could exert protection by regulating cellular function at the level of the infant gut. Furthermore, these two particles differ in their nutritive roles such that MFGs are a main source of energy for the infant (i.e. Tg- rich cream particles) while lactosomes potentially offer immunomodulatory functionality independent of macronutrient supply. Future mechanistic studies are required to determine what physiological and pathological conditions dictate the function of lactosomes throughout lactation.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institute of Environmental Health Sciences (NIEHS) grant R37 ES02710, the NIEHS Superfund Basic Research Program P42 ES04699, the University of California Davis Center for Children’s Environmental Health, NIEHS grant P01 ES11269, the University of California Discovery Program, California Dairy research Foundation 09 GEB-01-NH and U.S. Department of Energy, Office of Basic Energy Sciences through a grant ( DE-FG02-04ER46173) from the Biomolecular Materials Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.German JB, Dillard CJ. Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Crit Rev Food Sci Nutr. 2006;46:57–92. doi: 10.1080/10408690590957098. [DOI] [PubMed] [Google Scholar]
- 2.Mather IH, Keenan TW. Origin and secretion of milk lipids. J Mammary Gland Biol Neoplasia. 1998;3:259–73. doi: 10.1023/a:1018711410270. [DOI] [PubMed] [Google Scholar]
- 3.Wooding FB. The mechanism of secretion of the milk fat globule. J Cell Sci. 1971;9:805–21. doi: 10.1242/jcs.9.3.805. [DOI] [PubMed] [Google Scholar]
- 4.Mather IH, Weber K, Keenan TW. Membranes of mammary gland. XII. Loosely associated proteins and compositional heterogeneity of bovine milk fat globule membrane. J Dairy Sci. 1977;60:394–402. doi: 10.3168/jds.S0022-0302(77)83878-2. [DOI] [PubMed] [Google Scholar]
- 5.Cavaletto M, Giuffrida MG, Conti A. Milk fat globule membrane components--a proteomic approach. Adv Exp Med Biol. 2008;606:129–41. doi: 10.1007/978-0-387-74087-4_4. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Juanes F, Alonso JM, Zancada L, Hueso P. Glycosphingolipids from bovine milk and milk fat globule membranes: a comparative study. Adhesion to enterotoxigenic Escherichia coli strains. Biol Chem. 2009;390:31–40. doi: 10.1515/BC.2009.003. [DOI] [PubMed] [Google Scholar]
- 7.Michalski MC, Cariou R, Michel F, Garnier C. Native vs. damaged milk fat globules: membrane properties affect the viscoelasticity of milk gels. J Dairy Sci. 2002;85:2451–61. doi: 10.3168/jds.S0022-0302(02)74327-0. [DOI] [PubMed] [Google Scholar]
- 8.Scow RO, Blanchette-Mackie EJ, Smith LC. Transport of lipid across capillary endothelium. Fed Proc. 1980;39:2610–7. [PubMed] [Google Scholar]
- 9.Michalski MC, Briard V, Michel F, Tasson F, Poulain P. Size distribution of fat globules in human colostrum, breast milk, and infant formula. J Dairy Sci. 2005;88:1927–40. doi: 10.3168/jds.S0022-0302(05)72868-X. [DOI] [PubMed] [Google Scholar]
- 10.Couvreur S, Hurtaud C, Lopez C, Delaby L, Peyraud JL. The linear relationship between the proportion of fresh grass in the cow diet, milk fatty acid composition, and butter properties. J Dairy Sci. 2006;89:1956–69. doi: 10.3168/jds.S0022-0302(06)72263-9. [DOI] [PubMed] [Google Scholar]
- 11.Lopez C, Briard-Bion V, Menard O, Rousseau F, Pradel P, Besle JM. Phospholipid, sphingolipid, and fatty acid compositions of the milk fat globule membrane are modified by diet. J Agric Food Chem. 2008;56:5226–36. doi: 10.1021/jf7036104. [DOI] [PubMed] [Google Scholar]
- 12.Argov N, Wachsmann-Hogiu S, Freeman SL, Huser T, Lebrilla CB, German JB. Size-dependent lipid content in human milk fat globules. J Agric Food Chem. 2008;56:7446–50. doi: 10.1021/jf801026a. [DOI] [PubMed] [Google Scholar]
- 13.Wyne KL, Woollett LA. Transport of maternal LDL and HDL to the fetal membranes and placenta of the Golden Syrian hamster is mediated by receptor-dependent and receptor-independent processes. J Lipid Res. 1998;39:518–30. [PubMed] [Google Scholar]
- 14.Hayat M. Introduction to biological scanning electron microscopy. University Park Press; Baltimore: 1978. [Google Scholar]
- 15.Hayat M. Principles and techniques of electron microscopy: biological applications. Cambridge University Press; 2000. [Google Scholar]
- 16.Argov N, Wachsmann-Hogiu S, Freeman SL, Huser T, Lebrilla CB, German JB. Size-Dependent Lipid Content in Human Milk Fat Globules. J Agric Food Chem. 2008 doi: 10.1021/jf801026a. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki K, Murase O, Sugishita K, Yoneyama S, Akada K, Ueha M, Nakamura A, Kobayashi S. Optical characterization of liposomes by right angle light scattering and turbidity measurement. Biochim Biophys Acta. 2000;1467:219–26. doi: 10.1016/s0005-2736(00)00223-6. [DOI] [PubMed] [Google Scholar]
- 18.Wessel D, Flugge UI. A Method for the Quantitative Recovery of Protein in Dilute-Solution in the Presence of Detergents and Lipids. Analytical Biochemistry. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 19.Pollard KS, Dudoit S, van der Laan MJ. Multiple Testing Procedures: R multtest Package and Applications to Genomics Using R and Bioconductor. Bioinformatical and Computational Biology. 2005:251–272. [Google Scholar]
- 20.Davenport L, Dale R, Bisby R, Cundall R. Transverse location of the fluorescent probe 1, 6-diphenyl-1, 3, 5-hexatriene in model lipid bilayer membrane systems by resonance excitation energy transfer. Biochemistry. 1985;24:4097–4108. doi: 10.1021/bi00336a044. [DOI] [PubMed] [Google Scholar]
- 21.Vinchurkar MS, Bricarello DA, Lagerstedt JO, Buban JP, Stahlberg H, Oda MN, Voss JC, Parikh AN. Bridging across length scales: multi-scale ordering of supported lipid bilayers via lipoprotein self-assembly and surface patterning. J Am Chem Soc. 2008;130:11164–9. doi: 10.1021/ja803110v. [DOI] [PubMed] [Google Scholar]
- 22.Folch J, Lees M, Stanley G. H. Sloane. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 23.Smith D, Prieto P. Special considerations for glycolipids and their purification. In: Ausubel Frederick M., et al., editors. Current protocols in molecular biology. 2001. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen L. A liquid chromatography/tandem mass spectrometric approach for the determination of gangliosides GD3 and GM3 in bovine milk and infant formulae. Rapid Communications in Mass Spectrometry. 2006;20:3625–3633. doi: 10.1002/rcm.2775. [DOI] [PubMed] [Google Scholar]
- 25.Jensen R. The lipids in human milk. Progress in lipid research. 1996;35:53–92. doi: 10.1016/0163-7827(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 26.Jensen RG, Thompson MP. Handbook of Milk Composition. Academic Press; San Diego: 1995. [Google Scholar]
- 27.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 28.Jensen RG. Lipids in human milk. Lipids. 1999;34:1243–71. doi: 10.1007/s11745-999-0477-2. [DOI] [PubMed] [Google Scholar]
- 29.Iwamori M, Takamizawa K, Momoeda M, Iwamori Y, Taketani Y. Gangliosides in human, cow and goat milk, and their abilities as to neutralization of cholera toxin and botulinum type A neurotoxin. Glycoconjugate Journal. 2008;25:675–683. doi: 10.1007/s10719-008-9128-6. [DOI] [PubMed] [Google Scholar]
- 30.Desai P, Walsh M, Weimer B. Solid-Phase Capture of Pathogenic Bacteria by Using Gangliosides and Detection with Real-Time PCR?†. Applied and Environmental Microbiology. 2008;74:2254–2258. doi: 10.1128/AEM.02601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newburg D, Ruiz-Palacios G, Morrow A. Human milk glycans protect infants against enteric pathogens. 2005. [DOI] [PubMed]
- 32.Kaess B, Fischer M, Baessler A, Stark K, Huber F, Kremer W, Kalbitzer H, Schunkert H, Riegger G, Hengstenberg C. The lipoprotein subfraction profile: heritability and identification of quantitative trait loci. The Journal of Lipid Research. 2008;49:715. doi: 10.1194/jlr.M700338-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Admyre C, Johansson S, Qazi K, Filen J, Lahesmaa R, Norman M, Neve E, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. The Journal of Immunology. 2007;179:1969. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 34.Mather IH, Jack LJ. A review of the molecular and cellular biology of butyrophilin, the major protein of bovine milk fat globule membrane. J Dairy Sci. 1993;76:3832–50. doi: 10.3168/jds.s0022-0302(93)77726-7. [DOI] [PubMed] [Google Scholar]
- 35.Palmer DJ, Kelly VC, Smit AM, Kuy S, Knight CG, Cooper GJ. Human colostrum: identification of minor proteins in the aqueous phase by proteomics. Proteomics. 2006;6:2208–16. doi: 10.1002/pmic.200500558. [DOI] [PubMed] [Google Scholar]
- 36.Alain K, Karrow N, Thibault C, St-Pierre J, Lessard M, Bissonnette N. Osteopontin: an early innate immune makers ofEscherichia coli mastitis harbors genetic polymorphisms with possible links with resistance to mastitis. BMC genomics. 2009;10:444. doi: 10.1186/1471-2164-10-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashkar S, Weber G, Panoutsakopoulou V, Sanchirico M, Jansson M, Zawaideh S, Rittling S, Denhardt D, Glimcher M, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 38.Chabas D, Baranzini S, Mitchell D, Bernard C, Rittling S, Denhardt D, Sobel R, Lock C, Karpuj M, Pedotti R. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 39.Weber G, Ashkar S, Glimcher M, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi S, Oki J, Miyamoto A, Moriyama T, Asano A, Inyaku F, Okuno A. Beta-2-microglobulin and ferritin in cerebrospinal fluid for evaluation of patients with meningitis of different etiologies. Brain and Development. 1999;21:192–199. doi: 10.1016/s0387-7604(99)00017-0. [DOI] [PubMed] [Google Scholar]
- 41.Pettersson-Kastberg J, Mossberg A, Trulsson M, Joong Y, Min S, Lim Y, O’Brien J, Svanborg C, Mok K. alpha-Lactalbumin, Engineered to be Non-native and Inactive, Kills Tumor Cells when in Complex with Oleic Acid: A new biological function resulting from partial unfolding. Journal of molecular biology. 2009 doi: 10.1016/j.jmb.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 42.Svensson M, HÃ¥kansson A, Mossberg A-K, Linse S, Svanborg C. Conversion of α-lactalbumin to a protein inducing apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4221–4226. doi: 10.1073/pnas.97.8.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaby R. Lipopolysaccharide-binding molecules: transporters, blockers and sensors. Cellular and Molecular Life Sciences (CMLS) 2004;61:1697–1713. doi: 10.1007/s00018-004-4020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris H, Grunfeld C, Feingold K, Rapp J. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. Journal of Clinical Investigation. 1990;86:696. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desai P, Walsh M, Weimer B. Solid phase capture of pathogenic bacteria using gangliosides and detection with real time PCR. Applied and Environmental Microbiology. 2008 doi: 10.1128/AEM.02601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rueda R. The role of dietary gangliosides on immunity and the prevention of infection. British Journal of Nutrition. 2007;98:68–73. doi: 10.1017/S0007114507832946. [DOI] [PubMed] [Google Scholar]
- 47.Shi J, Yang T, Kataoka S, Zhang Y, Diaz A, Cremer P. GM Clustering Inhibits Cholera Toxin Binding in Supported Phospholipid Membranes. Biol. Chem. 1997;272:5533–5538. doi: 10.1021/ja069375w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.