Abstract
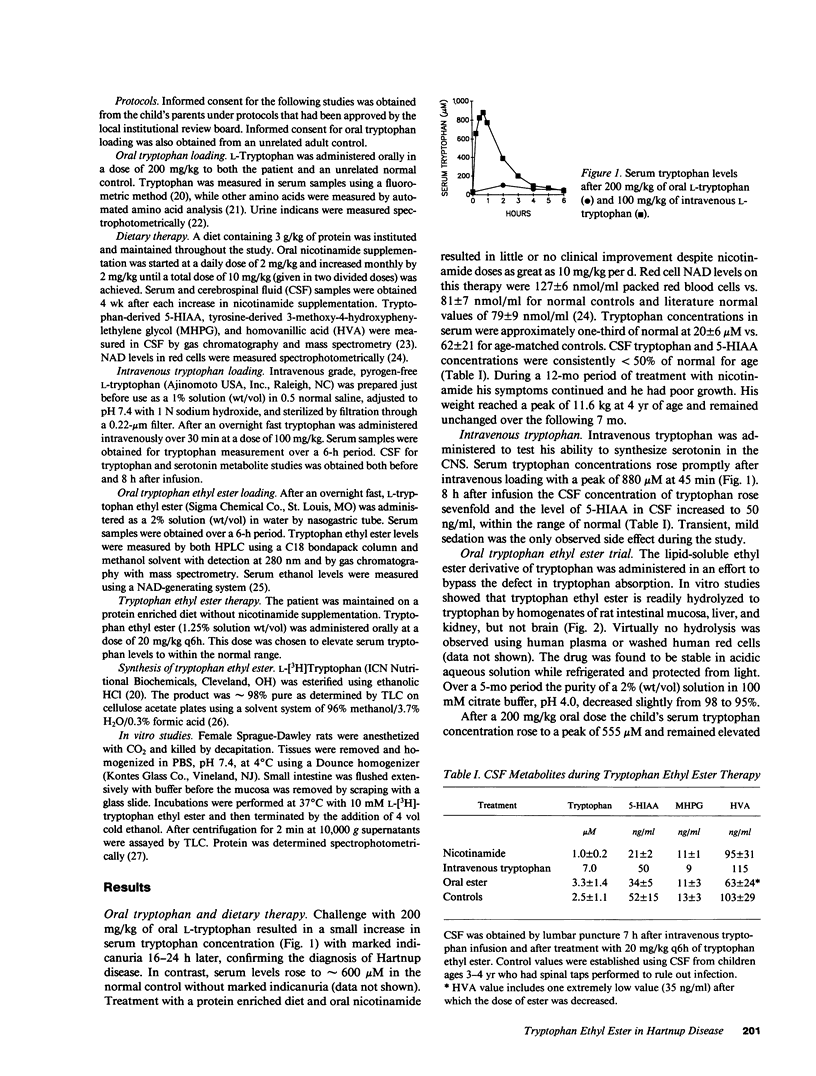
Tryptophan ethyl ester, a lipid-soluble tryptophan derivative, was used to bypass defective gastrointestinal neutral amino acid transport in a child with Hartnup disease. The child's baseline tryptophan concentrations in serum (20 +/- 6 microM) and cerebrospinal fluid (1.0 +/- 0.2 microM) were persistently less than 50% of normal values. Cerebrospinal fluid 5-hydroxyindoleacetic acid (5-HIAA), a serotonin metabolite, was also less than 50% of normal (21 +/- 2 ng/ml). Serum tryptophan concentrations increased only modestly and briefly after an oral challenge with 200 mg/kg of oral L-tryptophan, reflecting the absorptive defect. An oral challenge with 200 mg/kg of tryptophan ethyl ester resulted in a prompt increase in serum tryptophan to a peak of 555 microM. Sustained treatment with 20 mg/kg q6h resulted in normalization of serum (66 +/- 15 microM) and cerebrospinal fluid tryptophan concentrations (mean = 2.3 microM). Cerebrospinal fluid 5-HIAA increased to more normal concentrations (mean = 33 ng/ml). No toxicity was observed over an 8-mo period of treatment, chronic diarrhea resolved, and body weight, which had remained unchanged for 7 mo before ester therapy, increased by approximately 26%. We concluded that tryptophan ethyl ester is effective at circumventing defective gastrointestinal neutral amino acid transport and may be useful in the treatment of Hartnup disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asatoor A. M., Cheng B., Edwards K. D., Lant A. F., Matthews D. M., Milne M. D., Navab F., Richards A. J. Intestinal absorption of two dipeptides in Hartnup disease. Gut. 1970 May;11(5):380–387. doi: 10.1136/gut.11.5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Moorhouse S. R., Pratt O. E. Amino acid precursors of monoamine neurotransmitters and some factors influencing their supply to the brain. Psychol Med. 1976 May;6(2):277–286. doi: 10.1017/s0033291700013830. [DOI] [PubMed] [Google Scholar]
- Denckla W. D., Dewey H. K. The determination of tryptophan in plasma, liver, and urine. J Lab Clin Med. 1967 Jan;69(1):160–169. [PubMed] [Google Scholar]
- Eccleston D., Ashcroft G. W., Crawford T. B. Effect of tryptophan administration on 5HIAA in cerebrospinal fluid in man. J Neurol Neurosurg Psychiatry. 1970 Apr;33(2):269–272. doi: 10.1136/jnnp.33.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom J. D., Wurtman R. J. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971 Jul 9;173(3992):149–152. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- Fernstrom J. D., Wurtman R. J. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. 1972 Oct 27;178(4059):414–416. doi: 10.1126/science.178.4059.414. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Bashan N., Tietze F., Bernardini I., Schulman J. D. Cystine transport is defective in isolated leukocyte lysosomes from patients with cystinosis. Science. 1982 Sep 24;217(4566):1263–1265. doi: 10.1126/science.7112129. [DOI] [PubMed] [Google Scholar]
- Gillman P. K., Bartlett J. R., Bridges P. K., Kantamaneni B. D., Curzon G. Relationships between tryptophan concentrations in human plasma, cerebrospinal fluid and cerebral cortex following tryptophan infusion. Neuropharmacology. 1980 Dec;19(12):1241–1242. doi: 10.1016/0028-3908(80)90217-8. [DOI] [PubMed] [Google Scholar]
- Greenwood M. H., Friedel J., Bond A. J., Curzon G., Lader M. H. The acute effects of intravenous infusion of L-tryptophan in normal subjects. Clin Pharmacol Ther. 1974 Sep;16(3):455–464. doi: 10.1002/cpt1974163part1455. [DOI] [PubMed] [Google Scholar]
- Jonas A. J. Cystine transport in purified rat liver lysosomes. Biochem J. 1986 Jun 15;236(3):671–677. doi: 10.1042/bj2360671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A. J., Smith M. L., Schneider J. A. ATP-dependent lysosomal cystine efflux is defective in cystinosis. J Biol Chem. 1982 Nov 25;257(22):13185–13188. [PubMed] [Google Scholar]
- MILNE M. D., CRAWFORD M. A., GIRAO C. B., LOUGHRIDGE L. W. The metabolic disorder in Hartnup disease. Q J Med. 1960 Jul;29:407–421. [PubMed] [Google Scholar]
- Oldendorf W. H. Saturation of blood brain barrier transport of amino acids in phenylketonuria. Arch Neurol. 1973 Jan;28(1):45–48. doi: 10.1001/archneur.1973.00490190063008. [DOI] [PubMed] [Google Scholar]
- Poklis A., Mackell M. A. Evaluation of a modified alcohol dehydrogenase assay for the determination of ethanol in blood. Clin Chem. 1982 Oct;28(10):2125–2127. [PubMed] [Google Scholar]
- Pomeroy J., Efron M. L., Dayman J., Hoefnagel D. Hartnup disorder in a New England family. N Engl J Med. 1968 May 30;278(22):1214–1216. doi: 10.1056/NEJM196805302782207. [DOI] [PubMed] [Google Scholar]
- Reeves J. P. Accumulation of amino acids by lysosomes incubated with amino acid methyl esters. J Biol Chem. 1979 Sep 25;254(18):8914–8921. [PubMed] [Google Scholar]
- SCRIVER C. R. HARTNUP DISEASE: A GENETIC MODIFICATION OF INTESTINAL AND RENAL TRANSPORT OF CERTAIN NEUTRAL ALPHA-AMINO ACIDS. N Engl J Med. 1965 Sep 2;273:530–532. doi: 10.1056/NEJM196509022731005. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Mahon B., Levy H. L., Clow C. L., Reade T. M., Kronick J., Lemieux B., Laberge C. The Hartnup phenotype: Mendelian transport disorder, multifactorial disease. Am J Hum Genet. 1987 May;40(5):401–412. [PMC free article] [PubMed] [Google Scholar]
- Seifert W. E., Jr, Foxx J. L., Butler I. J. Age effect on dopamine and serotonin metabolite levels in cerebrospinal fluid. Ann Neurol. 1980 Jul;8(1):38–42. doi: 10.1002/ana.410080106. [DOI] [PubMed] [Google Scholar]
- Tahmoush A. J., Alpers D. H., Feigin R. D., Armbrustmacher V., Prensky A. L. Hartnup disease. Clinical, pathological, and biochemical observations. Arch Neurol. 1976 Dec;33(12):797–807. doi: 10.1001/archneur.1976.00500120001001. [DOI] [PubMed] [Google Scholar]
- Wilcken B., Yu J. S., Brown D. A. Natural history of Hartnup disease. Arch Dis Child. 1977 Jan;52(1):38–40. doi: 10.1136/adc.52.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. W., Lambert A. M., Pillai P. M., Jones P. M. Observations on nicotinic acid therapy in Hartnup disease. Arch Dis Child. 1967 Dec;42(226):642–646. doi: 10.1136/adc.42.226.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerez C. R., Lee S. J., Tanaka K. R. Spectrophotometric determination of oxidized and reduced pyridine nucleotides in erythrocytes using a single extraction procedure. Anal Biochem. 1987 Aug 1;164(2):367–373. doi: 10.1016/0003-2697(87)90506-9. [DOI] [PubMed] [Google Scholar]


