Abstract
The T-cell response to antigen depends upon coordinate signaling between costimulatory and inhibitory receptors. Altered function of either may underlie the pathophysiology of autoimmune and/or chronic inflammatory diseases and manipulation of these pathways is an important emerging area of therapeutics. We report here that the immunosuppressant drug CTLA4-Ig inhibits the effector phase of allergic airway inflammation through a CD28-independent, nitric oxide synthase dependent mechanism. Using mice deficient in both B and T lymphocyte attenuator (BTLA) and CD28, we demonstrate that simultaneous deficiency of an inhibitory receptor can rescue the in vivo but not the in vitro CD28-deficient phenotype. Furthermore, we demonstrate that inflammation in the CD28/BTLA-double-deficient mice is suppressed by CTLA4-Ig. This suppression is reversed by treatment with the Nitric Oxide Synthase (NOS) inhibitor, N6-methyl-L-arginine acetate (L-NMMA). In addition CTLA4-Ig was ineffective at inhibiting inflammation in NOS2-deficient mice when given at the effector phase. Thus, CD28 and BTLA coordinately regulate the in vivo response to inhaled allergen, and CTLA4-Ig binding to B7-proteins inhibits the effector phase of inflammation by a CD28-independent, NOS-dependent mechanism.
Keywords: Costimulation, CTLA4-Ig, T cell activation, allergic lung inflammation
Introduction
The outcome of a T cell's encounter with antigen is determined by the integration of multiple signals delivered through the T cell antigen receptor as well as via costimulatory and inhibitory pathways (for review, see [1, 2]). CD28 is one of the best characterized costimulatory receptors and its engagement potently augments the T cell response to sub-mitogenic levels of antigen [3–5]. Following initial activation, additional costimulatory receptors such as Inducible Costimulator (ICOS) and TNF receptor family members, as well as inhibitory receptors including CTLA-4 (CD152) and B and T lymphocyte attenuator (BTLA), are expressed. [6–10]. The coordinate regulation of the expression of these opposing receptors is important in modulating the intensity and duration of the T cells response.
Inflammation in vivo can be manipulated by altering the expression of and/or signaling through costimulatory and inhibitory receptors. For example, we had previously shown that mice deficient in BTLA develop prolonged airway inflammation following a single allergen challenge [11]. In contrast, mice that lack CD28 or in which CD28:ligand interactions are pharmacologically blocked do not develop lung inflammation [12–15]. However, less is known about the effects of a combinatorial blockade of both inhibitory and activating receptors on immune responses. To explore this, we generated mice deficient in both CD28 and BTLA. While T cells from the CD28/BTLA-double-deficient (CD28−/−BTLA−/−) mice did not respond to in vitro stimulation with anti-CD3, the double knockout mice developed significant lung inflammation following in vivo allergen challenge.
CTLA4-Ig is a recombinant fusion protein that binds CD80 and CD86 preventing ligation of the counter-receptors CD28 and CTLA4 [16] and is effective in inhibiting inflammation in both animals and humans. Abatacept, a humanized version of CTLA4-Ig, is now an approved treatment for rheumatoid arthritis [17]. The prevention of CD28-mediated costimulation has been presumed to be the primary mechanism by which CTLA4-Ig functions in vivo. However, an alternative explanation has been put forth which depends upon B7-dependent expression of indoleamine 2,3 dioxygenase (IDO) [18, 19]. The CD28−/−BTLA−/− mice presented a novel opportunity to directly test if CTLA4-Ig could inhibit inflammation in the absence of CD28. In fact, airway inflammation in the double knockout mice was suppressed by CTLA4-Ig, but this was dependent on nitric oxide synthase 2 (NOS2) activity, not IDO. Thus, these data demonstrate 1) that unopposed inhibitory signaling by BTLA in the CD28−/− mice suppresses inflammation in vivo and 2) that CTLA4-Ig engagement of B7-proteins inhibits inflammation through a nitric oxide dependent, CD28 independent mechanism. These findings have important implications for both understanding the pathophysiology of inflammatory diseases and understanding the mechanism of current therapeutic agents.
Results
CTLA4-Ig terminates established airway inflammation
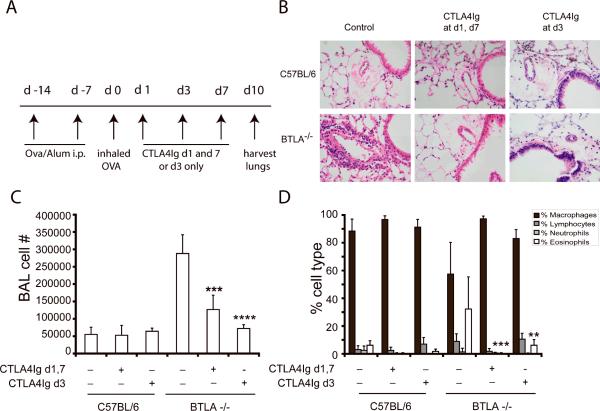
Blocking of B7:CD28 interactions by CTLA4-Ig prevents productive T lymphocyte activation and therefore the initiation of a T cell directed immune response. However, whether this intervention can alter the course of already established inflammation is not known. We used a well characterized T cell and CD28 dependent model of allergic lung inflammation to examine this question[12, 20]. We had previously shown that mice deficient for the inhibitory receptor, BTLA, had airway inflammation for up to 21 days following a single allergen challenge, whereas WT mice resolved the infiltrate by day 7 [11]. We took advantage of this prolonged inflammatory response to test whether CTLA4-Ig could accelerate the resolution of allergic airway inflammation. We tested 2 different dosing schemes, one in which CTLA4-Ig was administered at the time of challenge and then redosed 7 days later to account for the half life of the drug, and an alternate regimen in which a single dose of CTLA4-Ig was administered 3 days following challenge, a time point at which we typically observe abundant inflammation. In these experiments, tissue was collected for analysis 10 days following challenge as opposed to 3 days in the remaining experiments. As shown in figure 1, administration of a single dose of CTLA4-Ig at the time of peak inflammation (day 3) led to a clearing of tissue inflammation 7 days later, whereas untreated BTLA−/− mice had persistent inflammation. In fact, a single dose at day 3 was as effective as administration throughout the entire challenge phase. These data demonstrate that CTLA4-Ig can promote the termination and resolution of the inflammatory process.
Figure 1. CTLA4-Ig promotes the resolution of airway inflammation in BTLA−/− mice.
C57BL/6 or BTLA−/− deficient mice were sensitized and challenged with OVA. Groups of mice (n=5/group) received either control or CTLA4-Ig (100 μg, ip) on either day 1 and day 7 or on day 3 only following challenge. Samples were collected for analysis 10 days following the inhaled challenge. A) Schematic representation of the experimental protocol. B) Representative lung sections stained with H&E. C) Total BAL cell numbers and D) differential analysis of cells recovered from the BAL fluid. Data show mean + SD (n=5) and are representative of more than 5 independent experiments, **p<0.01, ***p<0.0005, ****p<0.0001 as compared to BTLA−/− mice that had not received CTLA4-Ig.
BTLA-deficiency rescues the phenotype of CD28-deficient T cells in vivo but not in vitro
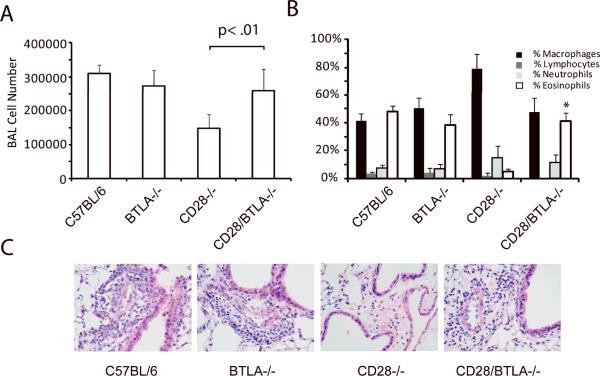
The ability of CTLA4-Ig to terminate inflammation in the BTLA−/− mice is consistent with a model in which the inflammation is driven by persistent, unopposed B7:CD28 mediated T cell activation. From this it follows that the converse situation, in which there is a loss of costimulation through CD28, might result in dominance of inhibitory signals through BTLA, thereby suppressing T cell function. To test this, we bred mice that were deficient in both CD28 and BTLA. The response of these mice to an in vivo antigen challenge was tested. Mice of each genotype were sensitized and challenged with OVA and samples collected 72 hours following the inhaled challenge (Figure 2). Consistent with our previous work, acute inflammation in the BTLA−/− mice was similar to that seen in the WT (Figure 2A) [11, 21]. As expected, the CD28−/− mice did not show any evidence of inflammation in either the BAL or in histological examination of lung sections. In contrast, challenge of the CD28−/−BTLA−/− mice resulted in significant airway inflammation, detected histologically as well as by an increase in both the total number of cells and an increased percentage of eosinophils recovered in the BAL (Figure 2). Flow cytometric analysis of cells recovered in the BAL showed similar percentages of total CD4+ T cells (4.3% ± 0.6% in WT vs. 5.3% ± 1.5% in CD28−/−BTLA−/−) as well as CD25hi CD4+ T cells (17% ± 2% in WT vs. 13% ± 4% in CD28−/−BTLA−/−) suggesting that T cells were recruited and activated. In contrast, no CD25hi cells were detected in the BAL from CD28−/− mice. Thus, despite the lack of CD28-expression, the mice responded to an in vivo challenge to a similar extent as WT mice. However, not all aspects of the in vivo response were similarly restored, as no OVA-specific IgG1 was detected in the serum of sensitized CD28−/−BTLA−/− mice (data not shown). These data suggest that unopposed inhibitory signaling through BTLA suppresses inflammation in the CD28−/− mice, and that elimination of this BTLA-mediated suppression enabled restoration of some aspects of the inflammatory response despite an absence of CD28-mediated costimulation.
Figure 2. CD28−/−BTLA−/− mice respond to allergen challenge in vivo.
Mice of each genotype were systemically sensitized with OVA/Alum then given an inhaled challenge of OVA as described in the Materials and methods. Samples were collected for analysis 3 days following the inhaled challenge. A) Total BAL cell number and B) differential analysis of cells recovered from the BAL fluid. C) Histologic examination of H&E-stained sections confirms peribronchial and perivacular inflammation in the lungs of the C57BL/6, BTLA−/− and CD28−/−BTLA−/− mice, but not the CD28−/− mice. Data show mean + SD (n=5 mice per group) and are representative of more than 5 independent experiments, *p<0.01 compared to CD28−/− mice.
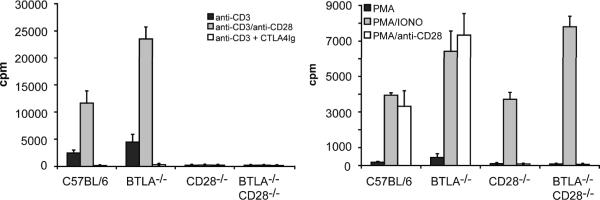
We next assayed the proliferative response of splenocytes from the CD28−/−BTLA−/− mice to in vitro stimulation (Figure 3). Consistent with previous reports, cells isolated from BTLA−/− mice proliferated to a greater extent than WT cells. However, both CD28−/− and CD28−/−BTLA−/− splenocytes failed to proliferate when stimulated with anti-CD3 or anti-CD3/CD28 (Figure 3A), while cells from all genotypes did proliferate in response to PMA and ionomycin (Figure 3B). Thus, despite an ability to respond to antigen in vivo, the lack of CD28 dominates the cellular response to in vitro stimulation. These data also suggest that factors present in vivo that allow for T cell function in the double knockout are not fully recapitulated in vitro.
Figure 3. CD28−/−BTLA−/− T cells do not proliferate to anti-CD3 in vitro.
Splenocytes were isolated from C57BL/6, BTLA−/−, CD28−/−, or CD28−/−BTLA−/− mice and stimulated with anti-CD3 alone, anti-CD3 + anti-CD28 or anti-CD3 + CTLA4-Ig. Alternatively splenocytes were stimulated with PMA, PMA + ionomycin, or PMA + anti-CD28. Proliferation was determined by tritiated thymidine incorporation. Data show mean ± SD (n=4) and are representative of 3 independent experiments.
As both CD28 and BTLA are expressed on cells other than T lymphocytes, it is possible that the inflammation observed in the double knockout mice is not a T cell dependent or antigen specific response. This might also account for the discrepancy between the in vivo and in vitro observations. We reasoned that if this were the case, the double knockout mice should respond in an antigen non-specific manner to inhaled challenge. To test this, we systemically sensitized mice to OVA and then administered an inhaled challenge with either OVA or hen egg lysozyme (HEL). Both WT and CD28−/−BTLA−/− mice developed inflammation when challenged with OVA, but neither did when challenged with HEL (Supporting Information Figure 1). These data support that the inflammation is in fact antigen specific and therefore likely dependent on T cell function.
CTLA4-Ig inhibits allergic airway inflammation independent of CD28
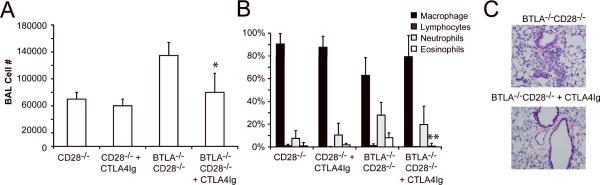
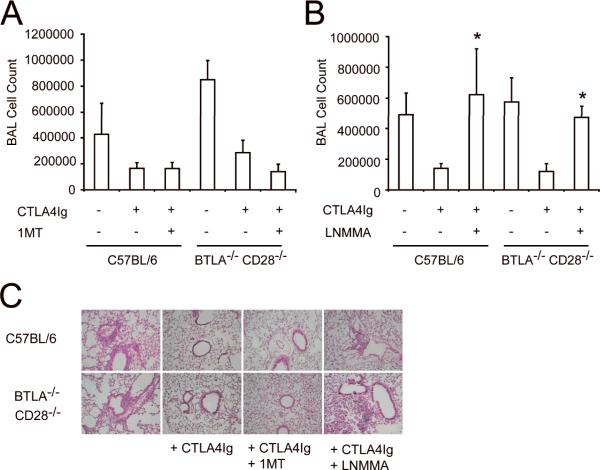
The predominant mechanism by which CTLA4-Ig is thought to modulate inflammation is by binding CD80 and CD86 and preventing engagement of CD28 [16, 22, 23]. However, alternative mechanisms have been proposed[18]. The CD28−/−BTLA−/− mice provided a unique opportunity to test whether CTLA4-Ig could inhibit inflammation independent of CD28. Allergen challenged CD28−/−BTLA−/− mice were treated with CTLA4-Ig and inflammation determined by BAL and histology. Surprisingly, eosinophilic inflammation in the CD28−/−BTLA−/− mice was efficiently inhibited by CTLA4-Ig, as determined by a decrease in the number of eosinophils recovered in the BAL as well as a resolution of the cellular infiltrate seen by histological examination (Figure 4). Inflammation was also effectively inhibited in allergen challenged WT and BTLA−/− mice by CTLA4-Ig, although for the sake of clarity, these data are not presented in the figure. Consistent with our previous work, no inflammation was induced in the CD28−/− mice and CTLA4-Ig had no effect in these animals [13]. As CD28−/−BTLA−/− mice genetically lack CD28, this established that a second, novel mechanism of CTLA4-Ig immune suppression must be present that is independent of the prevention of B7:CD28-mediated costimulation.
Figure 4. CTLA4-Ig inhibits airway inflammation in CD28−/−BTLA−/− mice.
Mice of each genotype were systemically sensitized with OVA/Alum then given an inhaled challenge of OVA as described in the Materials and methods. For clarity, only data from the CD28−/− and CD28−/−BTLA−/− groups is presented, although C56BL/6 and BTLA−/− mice were also tested, and the data were similar to that presented in Fig.1. CTLA4-Ig was administered (100 μg, i.p.) on the day of inhaled challenge. Samples were collected for analysis 3 days later. A) Total cell number and B) differential cell analysis. C) Histology of H&E-stained lung sections. Data show mean + SD (n=5) and are representative of 5 experiments, *p<0.05, **p<0.005 compared to CD28−/−BTLA−/− mice that had not received CTLA4-Ig.
Engagement of B7 by CTLA4-Ig has been shown to induce indoleamine 2,3 dioxygenase (IDO), which catalyzes the rate limiting step in tryptophan metabolism [18, 19]. IDO activity can suppress inflammation through direct inhibition of effector T cells and by inducing the generation of regulatory T cells [19, 24–27]. The effect of IDO can be reversed in vivo by treatment with a competitive inhibitor, 1-methyl tryptophan (1-MT) [28]. To test if IDO mediated the CD28-independent effect of CTLA4-Ig, allergen challenged mice were treated with CTLA4-Ig alone or in combination with 1-MT. However, as shown in figure 5, 1-MT had no effect on the ability of CTLA4-Ig to inhibit lung inflammation as determined by BAL cell counts and histology. No effect of 1-MT was seen whether it was administered by i.p. injection or in the drinking water (data not shown).
Figure 5. Inhibition of airway inflammation by CTLA4-Ig is dependent on NOS.
Mice of each genotype were systemically sensitized with OVA/Alum then given an inhaled challenge of OVA as described in the Materials and methods. CTLA4-Ig was administered (100 μg, i.p.) on the day of inhaled challenge. Samples were collected for analysis 3 days later. Some mice also received daily injections of (A) 1-MT (10 mg, i.p.) or (B) the NOS inhibitor L-NMMA (2 mg, i.p.) from the time of challenge until harvest. No effect of L-NMMA was seen in mice that did not receive CTLA4-Ig (not shown). Data show mean + SD (n=5), *p<0.05 as compared to mice of the same genotype that received CTLA4-Ig but not L-NMMA. C) H&E-stained lung sections from mice of each group. All data are representative of 3 independent experiments.
The lack of effectiveness of IDO inhibition suggested that an alternative mechanism must be responsible for CD28-independent immunosuppression mediated by CTLA4-Ig. NO has been demonstrated to have broad effects in many different cell types both pro- and anti-inflammatory (for review see [29]). To test the role of NO in this model, mice were systemically administered L-NMMA, which broadly inhibits NOS function, during the challenge phase. In both WT and CD28−/−BTLA−/− mice, treatment with L-NMMA reversed the immunosuppressive effect of CTLA4-Ig (Figure 5B and C). This was evident in both the cellularity of the BAL as well as in the histology of lung sections. Inflammation was not inhibited in WT or CD28−/−BTLA−/− mice given L-NMMA in the absence of CTLA4-Ig (data not shown).
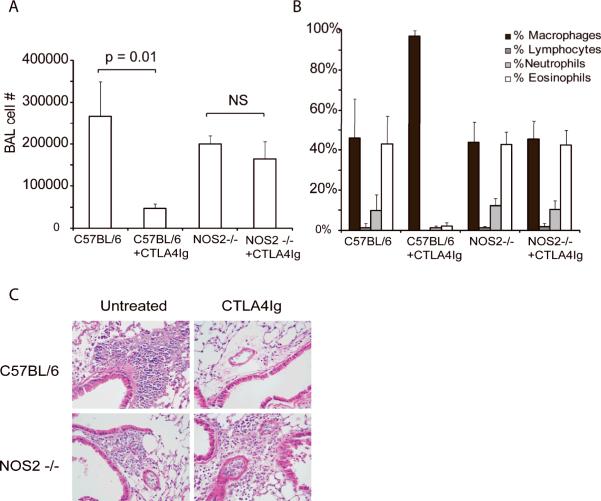
Using mice genetically deficient in NOS2, we further examined the requirement for the NO pathway in the ability of CTLA4-Ig to modulate airway inflammation (Figure 6). Following OVA challenge, the NOS2−/− mice had slightly lower cell recoveries in the BAL although this was not statistically significant. Importantly, challenge of the NOS2−/− mice still resulted in a marked increase in overall cell and eosinophil recruitment to the lung as determined by both BAL cell counts and histology. However, CTLA4-Ig was not effective in suppressing inflammation in the NOS2−/− mice, whereas it abrogated inflammation in WT mice. Thus, these data clearly implicate the NOS/NO system as an important component of the immunosuppressive mechanism by which CTLA4-Ig functions.
Figure 6. CTLA4-Ig does not inhibit inflammation in NOS2−/− mice.
C57BL/6 or NOS2−/− mice were sensitized and challenged with OVA. CTLA4-Ig was administered at the time of challenge and specimens collected 3 days later. CTLA4-Ig effectively suppressed allergic airway inflammation in the C57BL/6 mice but not the NOS2−/− mice as shown by A) BAL cell number, B) differential analysis and C) histology of H & E stained lung sections. Data are representative of 2 independent experiments. N= 5 mice per group in each experiment.
We next tested whether NOS2 was required for CTLA4-Ig to prevent inflammation when administered only at priming. WT, NOS2-deficient and CD28−/−BTLA−/− mice were sensitized and challenged with OVA and groups of mice received either no CTLA4-Ig, CTLA4-Ig at the time of i.p. sensitization only, or at the time of inhaled challenge only. Specimens were collected 3 days following challenge (Supporting Information Figure 2). These data demonstrate CTLA4-Ig inhibits priming by blocking CD28-dependent costimulation, but when administered only during the effector phase, inflammation is suppressed by a CD28-independent, NOS2-dependent mechanism.
Discussion
CTLA4-Ig is a fusion protein that can inhibit T cell responses in vitro and inflammation in vivo [16, 22, 23, 30–34]. The presumed mechanism of action for CTLA4-Ig has been that it prevents binding of CD80 or CD86 to CD28 and therefore the delivery of a costimulatory signal to the T cell, effectively blocking the priming stage of an immune response. However, in most human diseases, priming has occurred prior to any opportunity for therapeutic intervention. The effectiveness of CTLA4-Ig to modulate inflammation in these circumstances suggests that a different mechanism may be operative. Whether CTLA4-Ig can regulate T cell function independent of CD28 has been difficult to experimentally address. The ability of the CD28−/−BTLA−/− mice to mount an in vivo inflammatory response provided us an opportunity to do so. We found that CTLA4-Ig was as effective in suppressing inflammation in the CD28−/−BTLA−/− mice as it was in the WT mice. In some systems, CTLA4-Ig has been suggested to work through induction of indoleamine 2,3 dioxygenase (IDO), which catalyzes the rate limiting step in tryptophan metabolism [18, 19]. Induction of IDO results in the local depletion of tryptophan, which is then sensed by neighboring T cells. This induces expression of GCN2 kinase in the effector T cell, leading to cell cycle arrest [27]. In addition to this mechanism, IDO expressing dendritic cells promote the differentiation of naive T cells to Treg cells [35, 36]. However, IDO does not appear to play a role in our system, as inhibition of IDO activity with 1-methyl tryptophan had no effect.
In contrast to the lack of effectiveness of IDO inhibition, when a competitive inhibitor of NOS activity was given along with CTLA4-Ig, the immunosuppressive effects of CTLA4-Ig were completely reversed both in WT and CD28−/−BTLA−/− mice. Strikingly, CTLA4-Ig was completely ineffective in inhibiting inflammation induced in NOS2−/− mice, demonstrating a strict requirement for this enzyme in the suppressive function of CTLA4-Ig in this system. Importantly there are significant differences in our experimental system from some others in which CTLA4-Ig has been studied. In these experiments, we administered CTLA4-Ig only at the time of inhaled challenge, and not during the sensitization step when T cell priming occurs. Thus, it may be that the mechanism by which CTLA4-Ig works differs depending on whether it is administered during priming (CD28-dependent) or effector (CD28-independent, NOS-dependent) phases. This is in fact supported by our finding that CTLA4-Ig administered at priming was effective at preventing inflammation in the NOS2−/− mice but not the CD28−/−BTLA−/− mice (Supporting Information Figure 2). Thus, these data clearly implicate the NOS/NO system as an important component of the immunosuppressive mechanism by which CTLA4-Ig functions during the effector phase of inflammation.
There are several possibilities as to how CTLA4-Ig may induce NOS2. It is feasible that direct signaling through CD80 or CD86 may result in upregulation of NOS in the same cell, or alternatively the effect may be indirect, mediated by induction of a cytokine such as IFN-γ which might induce NOS2 in a different cell. It is conceivable that there may be several steps and cell types in between binding of CTLA4-Ig to CD80 and CD86 and induction of NOS2. While this will be important to elucidate in future studies, the data presented does establish a novel CD28-independent, NOS2-dependent mechanism for the drug. Interestingly, a related compound, belatacept, has been shown to induce the secretion of HLA-G by dendritic cells and by this mechanism contribute to the suppression of immune responses in humans [37, 38].
NO has been demonstrated to have broad effects in many different cell types both pro-and anti-inflammatory (for review see [29]). NO promotes the development of a population of myeloid suppressor cells which dampen inflammation [39]. In addition, NO mediated nitrosylation of signaling proteins, including JAK3 and STAT5 downstream of the IL-2 receptor pathway, directly inhibits T cell function [40, 41]. Lastly, in a manner similar to IDO, generation of NOS activity can deplete the local milieu of arginine, inducing GCN2 kinase in neighboring effector T cells leading to cell cycle arrest [42, 43]. In fact, synergy between NO and IDO has been suggested as an important component of transplant immunosuppression by CTLA4-Ig [44].
Using a murine model, several groups have examined the requirement for NOS in allergic airway inflammation with conflicting results, demonstrating either no effect from NOS-deficiency or inhibition or a reduction in airway inflammation[45–47]. We found a subtle but reproducible decrease in overall lung inflammation in the NOS2−/− mice, however the changes were not statistically significant.
A large body of data supports the importance of CD28 signaling in the initiation of an inflammatory response. Blockade of B7:CD28 engagement inhibits T cell priming in vivo and CD28−/− mice fail to mount antibody responses to T-dependent antigens [16, 22, 23, 48]. Additional data has suggested that CD28 signaling may be important in maintaining inflammation once established [13]. As CD28 is continuously expressed on the T cell and B7-proteins can be induced on both immune and non-immune cells at the site of inflammation, the potential for ongoing interactions exist.
During the course of an immune response a number of receptors are expressed by the T cell which can either activate or inhibit function. Receptors of multiple classes may be expressed simultaneously, requiring the T cell to integrate potentially conflicting signals. BTLA is expressed following activation and is required for the normal termination of a model immune response to inhaled allergen, an inflammatory response that is also dependent on CD28 [11, 49]. Using this system, we explored the interrelationship of CD28 and BTLA signaling. We generated mice deficient in both CD28 and BTLA and tested their response to in vivo allergen challenge. While CD28−/− mice fail to respond at all, somewhat surprisingly, the CD28−/−BTLA−/− mice mounted significant lung inflammation that was equivalent to that seen in WT mice. Thus, a simultaneous lack of the inhibitory receptor, BTLA, effectively rescued the in vivo phenotype of the CD28−/− mice. In contrast, however, the T cells from the double knockout fail to respond to in vitro activation with anti-CD3. While the reason for this discrepancy has not been identified, it is likely that additional signals present in the in vivo milieu but not recapitulated in vitro are important. Nonetheless, this finding clearly demonstrates the importance and interdependence of activating and inhibitory signaling in determining the outcome of an encounter with antigen.
Both CD28 and BTLA as well as their ligands are expressed on several cell types, including hematopoietic and non-hematopoietic cells. CD28 has been detected not only on T cells, but on plasma cells as well as human but not murine neutrophils and eosinophils [13, 50–54]. However, the function in these cell types is not well described. BTLA has multiple polymorphisms and can be expressed by both T and B cells, as well as by dendritic cells, macrophages and NK cells in some mouse strains [49]. The fact that the counter-receptors for CD28 and BTLA, B7-proteins and HVEM respectively, can also be expressed by a variety of cell types adds additional layers of complexity to the system. Thus, while the model we have chosen to examine is predominantly T cell dependent, it is possible that the genetic and pharmacologic manipulations have altered the function of a non-T cell population and in that manner changed the in vivo immune response to inhaled allergen challenge. However, the observation that the inflammation in the CD28/BTLA double knockout is antigen specific demonstrates that T cell recognition of antigen is required, and that this is not a non-specific inflammatory response.
This study highlights the dynamic interplay of activating and inhibitory signaling through CD28 and BTLA. While mice deficient in either CD28 or BTLA have the expected phenotype for the loss of a costimulatory or inhibitory receptor, we found that the simultaneous lack of BTLA and CD28 reversed the phenotype of CD28−/− mice, restoring responsiveness to an in vivo antigen challenge. We further demonstrated a novel, CD28-independent, NO-dependent, mechanism of action for the immunosuppressive drug CTLA4-Ig. This is of particular importance as this drug is now in therapeutic use for the treatment of refractory rheumatoid arthritis, marketed as abatacept (Orencia™, Bristol Myers Squibb). Further studies as to how CTLA4-Ig regulates NOS activity may provide greater insight as to the potential for manipulation of these pathways as treatment for immune mediated disease.
Materials and Methods
Mice
BTLA−/− mice on the C57BL/6 background were obtained from K. Murphy (Washington University, St. Louis, MO). CD28−/− mice on the C57BL/6 background were originally obtained from C. Thompson (University of Pennsylvania, Philadelphia, PA). Mice deficient in BTLA and CD28 on the C57BL/6 background were generated by a simple cross of the two strains and bred in our animal facility. C57BL/6 and NOS2−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were bred and housed in specific pathogen-free facilities at Washington University School of Medicine. All animal studies have been approved by the Washington University Animal Studies Committee.
Antibodies
All antibodies were purchased from eBioscience (San Diego, CA), unless otherwise specified.
Allergic airway inflammation
Mice were primed and challenged with OVA, as previously described [13, 20]. Briefly, mice received i.p. injections of OVA adsorbed to alum on days 0 and 7. On day 14, the mice were intranasally challenged with 50 μl of 2% OVA in PBS in the morning and afternoon. Lung and bronchoalveolar lavage (BAL) specimens were harvested and analyzed as previously described [13]. Murine CTLA4-Ig (100 μg/injection, either purchased from Sigma Chemical Corporation, St Louis, MO or provided by Bristol Myers Squibb, Princeton, NJ) was given by i.p. injection at the time of challenge or the indicated time points. In some experiments, 1-methyl tryptophan (1-MT Sigma Chemical Corp, St Louis, MO), 10 mg/day, or L-NMMA (2 mg/day in PBS by i.p. injection, Sigma Chemical, St Louis, MO) were given at the time of challenge and re-administered daily until harvest. The 1-MT was dissolved first in 1N NaOH then diluted to a final concentration with PBS and pH adjusted to 7.0 prior to i.p. injection. Each experiment consisted of at least 5 mice per group and has been repeated a minimum of 3 times. Representative data from one experiment is shown.
Proliferation Assays
Splenocytes were isolated by density gradient centrifugation over Lympholyte M (Accurate Chemical, Westbury, NY) and then plated at 1×105 cells per well and stimulated with anti-CD3 alone or in combination with anti-CD28 (1.0 μg/ml) or CTLA4-Ig (10 μg/ml). Replicate wells were stimulated with PMA (5 ng/ml) alone, PMA plus ionomycin (0.1 μg/ml), or PMA plus anti-CD28. Quadruplicate wells were plated for all conditions. After 48 hours, the cultures were pulsed with tritiated thymidine (3H-TdR, 1 μCi/well) overnight then harvested onto glass microfiber filters and counts determined by liquid scintillation counting. Data presented is the mean of the quadruplicate wells +/− the standard deviation. All experiments have been repeated a minimum of three times and representative data presented.
Statistical analysis
All data presented is the mean +/− std dev of independent replicates. All statistics were analyzed using a 2-tailed students T test with Microsoft Excel software (Microsoft Corporation, Seattle, WA)
Supplementary Material
Acknowledgments
We thank Dr Ken Murphy and Dr Theresa Murphy for providing us with the BTLA−/− mice and for helpful discussion and Steven Nadler MD, Bristol Myers Squibb Corporation (Princeton, NJ) for providing murine CTLA4-Ig. CMD and AP are supported by NIH grant T32 HL07317. This work was supported by NIH grants U19AI070489 and HL062683 (JMG).
Footnotes
Conflict of Interest: There authors declare no financial or commercial conflict of interest.
References
- 1.Carreno BM, Collins M. The B7 family of ligands and its receptors: New Pathways for Costimulation and Inhibition of Immune Responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 2.Keir ME, Sharpe AH. The B7/CD28 costimulatory family in autoimmunity. Immunol Rev. 2005;204:128–143. doi: 10.1111/j.0105-2896.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 3.Lesslauer W, Koning F, Ottenhoff T, Giphart M, Goulmy E, van Rood JJ. T90/44 (9.3 antigen). A cell surface molecule with a function in human T cell activation. Eur J Immunol. 1986;16:1289–1296. doi: 10.1002/eji.1830161017. [DOI] [PubMed] [Google Scholar]
- 4.Pierres A, Lopez M, Cerdan C, Nunes J, Olive D, Mawas C. Triggering CD 28 molecules synergize with CD 2 (T 11.1 and T 11.2)- mediated T cell activation. Eur J Immunol. 1988;18:685–690. doi: 10.1002/eji.1830180505. [DOI] [PubMed] [Google Scholar]
- 5.Damle NK, Doyle LV, Grosmaire LS, Ledbetter JA. Differential regulatory signals delivered by antibody binding to the CD28 (Tp44) molecule during the activation of human T lymphocytes. J. Immunol. 1988;140:1753–1761. [PubMed] [Google Scholar]
- 6.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 7.Toennies HM, Green JM, Arch RH. Expression of CD30 and Ox40 on T lymphocyte subsets is controlled by distinct regulatory mechanisms. J Leukoc Biol. 2004;75:350–357. doi: 10.1189/jlb.0803401. [DOI] [PubMed] [Google Scholar]
- 8.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 9.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 11.Deppong C, Juehne TI, Hurchla M, Friend LD, Shah DD, Rose CM, Bricker TL, Shornick LP, Crouch EC, Murphy TL, Holtzman MJ, Murphy KM, Green JM. Cutting edge: B and T lymphocyte attenuator and programmed death receptor-1 inhibitory receptors are required for termination of acute allergic airway inflammation. J Immunol. 2006;176:3909–3913. doi: 10.4049/jimmunol.176.7.3909. [DOI] [PubMed] [Google Scholar]
- 12.Burr JS, Kimzey SL, Randolph DR, Green JM. CD28 and CTLA4 coordinately regulate airway inflammatory cell recruitment and T-helper cell differentiation after inhaled allergen. Am J Respir Cell Mol Biol. 2001;24:563–568. doi: 10.1165/ajrcmb.24.5.4375. [DOI] [PubMed] [Google Scholar]
- 13.Kimzey SL, Liu P, Green JM. Requirement for CD28 in the effector phase of allergic airway inflammation. J Immunol. 2004;173:632–640. doi: 10.4049/jimmunol.173.1.632. [DOI] [PubMed] [Google Scholar]
- 14.Harris N, Campbell C, Le Gros G, Ronchese F. Blockade of CD28/B7 costimulation by mCTLA4-Hγ1 inhibits antigen- induced lung eosinophilia but not Th2 cell development or recruitment in the lung. Eur. J. Immunol. 1997;27:155–161. doi: 10.1002/eji.1830270123. [DOI] [PubMed] [Google Scholar]
- 15.Keane-Myers A, Gause WC, Linsley PS, Chen SJ, Wills-Karp M. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J. Immunol. 1997;158:2042–2049. [PubMed] [Google Scholar]
- 16.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by the soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 17.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229:307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 18.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 19.Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM. Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood. 2005;105:1574–1581. doi: 10.1182/blood-2004-06-2089. [DOI] [PubMed] [Google Scholar]
- 20.Kung TT, Jones H, Adams GK, 3rd, Umland SP, Kreutner W, Egan RW, Chapman RW, Watnick AS. Characterization of a murine model of allergic pulmonary inflammation. Int. Arch. Allergy. Immunol. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 21.Deppong C, Degnan JM, Murphy TL, Murphy KM, Green JM. B and T lymphocyte attenuator regulates T cell survival in the lung. J Immunol. 2008;181:2973–2979. doi: 10.4049/jimmunol.181.5.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 23.Turka LA, Linsley PS, Lin H, Brady W, Leiden JM, Wei R-Q, Gibson ML, Zheng X-G, Myrdal S, Gordon D, Bailey T, Bolling SF, Thompson CB. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc. Natl. Acad. Sci. USA. 1992;89:11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr., Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 25.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting Edge: Induced Indoleamine 2,3 Dioxygenase Expression in Dendritic Cell Subsets Suppresses T Cell Clonal Expansion. J Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 26.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 27.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 30.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 31.Khoury SJ, Akalin E, Chandraker A, Turka LA, Linsley PS, Sayegh MH, Hancock WW. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155:4521–4524. [PubMed] [Google Scholar]
- 32.Harris N, Peach R, Naemura J, Linsley PS, Le Gros G, Ronchese F. CD80 costimulation is essential for the induction of airway eosinophilia. J. Exp. Med. 1997;185:177–182. doi: 10.1084/jem.185.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Oosterhout AJ, Hofstra CL, Shields R, Chan B, Van Ark I, Jardieu PM, Nijkamp FP. Murine CTLA4-IgG treatment inhibits airway eosinophilia and hyperresponsiveness and attenuates IgE upregulation in a murine model of allergic asthma. Am. J. Respir. Cell. Mol. Biol. 1997;17:386–392. doi: 10.1165/ajrcmb.17.3.2679. [DOI] [PubMed] [Google Scholar]
- 34.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 35.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahri R, Naji A, Menier C, Charpentier B, Carosella ED, Rouas-Freiss N, Durrbach A. Dendritic Cells Secrete the Immunosuppressive HLA-G Molecule upon CTLA4-Ig Treatment: Implication in Human Renal Transplant Acceptance. J Immunol. 2009;183:7054–7062. doi: 10.4049/jimmunol.0803054. [DOI] [PubMed] [Google Scholar]
- 38.Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6—STAT3 signaling pathway. Proceedings of the National Academy of Sciences. 2008;105:8357–8362. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 40.Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- 41.Duhe RJ, Evans GA, Erwin RA, Kirken RA, Cox GW, Farrar WL. Nitric oxide and thiol redox regulation of Janus kinase activity. Proc Natl Acad Sci U S A. 1998;95:126–131. doi: 10.1073/pnas.95.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Currie GA, Gyure L, Cifuentes L. Microenvironmental arginine depletion by macrophages in vivo. Br J Cancer. 1979;39:613–620. doi: 10.1038/bjc.1979.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill M, Zagani R, Voisine C, Usal C, Anegon I. Nitric oxide and indoleamine 2,3-dioxygenase mediate CTLA4Ig-induced survival in heart allografts in rats. Transplantation. 2007;84:1060–1063. doi: 10.1097/01.tp.0000285293.75911.56. [DOI] [PubMed] [Google Scholar]
- 45.Xiong Y, Karupiah G, Hogan SP, Foster PS, Ramsay AJ. Inhibition of Allergic Airway Inflammation in Mice Lacking Nitric Oxide Synthase 2. J Immunol. 1999;162:445–452. [PubMed] [Google Scholar]
- 46.Trifilieff A, Fujitani Y, Mentz F, Dugas B, Fuentes M, Bertrand C. Inducible Nitric Oxide Synthase Inhibitors Suppress Airway Inflammation in Mice Through Down-Regulation of Chemokine Expression. J Immunol. 2000;165:1526–1533. doi: 10.4049/jimmunol.165.3.1526. [DOI] [PubMed] [Google Scholar]
- 47.De Sanctis GT, MacLean JA, Hamada K, Mehta S, Scott JA, Jiao A, Yandava CN, Kobzik L, Wolyniec WW, Fabian AJ, Venugopal CS, Grasemann H, Huang PL, Drazen JM. Contribution of Nitric Oxide Synthases 1, 2, and 3 to Airway Hyperresponsiveness and Inflammation in a Murine Model of Asthma. J. Exp. Med. 1999;189:1621–1630. doi: 10.1084/jem.189.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahinian A, Pfeffer K, Lee KP, Kündig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 49.Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 50.Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, Capron M. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med. 1999;190:487–495. doi: 10.1084/jem.190.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woerly G, Lacy P, Younes AB, Roger N, Loiseau S, Moqbel R, Capron M. Human eosinophils express and release IL-13 following CD28-dependent activation. J Leukoc Biol. 2002;72:769–779. [PubMed] [Google Scholar]
- 52.Venuprasad K, Chattopadhyay S, Saha B. CD28 signaling in neutrophil induces T-cell chemotactic factor(s) modulating T-cell response. Hum Immunol. 2003;64:38–43. doi: 10.1016/s0198-8859(02)00689-4. [DOI] [PubMed] [Google Scholar]
- 53.Venuprasad K, Parab P, Prasad DV, Sharma S, Banerjee PR, Deshpande M, Mitra DK, Pal S, Bhadra R, Mitra D, Saha B. Immunobiology of CD28 expression on human neutrophils. I. CD28 regulates neutrophil migration by modulating CXCR-1 expression. Eur J Immunol. 2001;31:1536–1543. doi: 10.1002/1521-4141(200105)31:5<1536::AID-IMMU1536>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 54.Lee KP, Taylor C, Petryniak B, Turka LA, June CH, Thompson CB. The genomic organization of the CD28 gene. Implications for the regulation of CD28 mRNA expression and heterogeneity. J Immunol. 1990;145:344–352. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.