Abstract
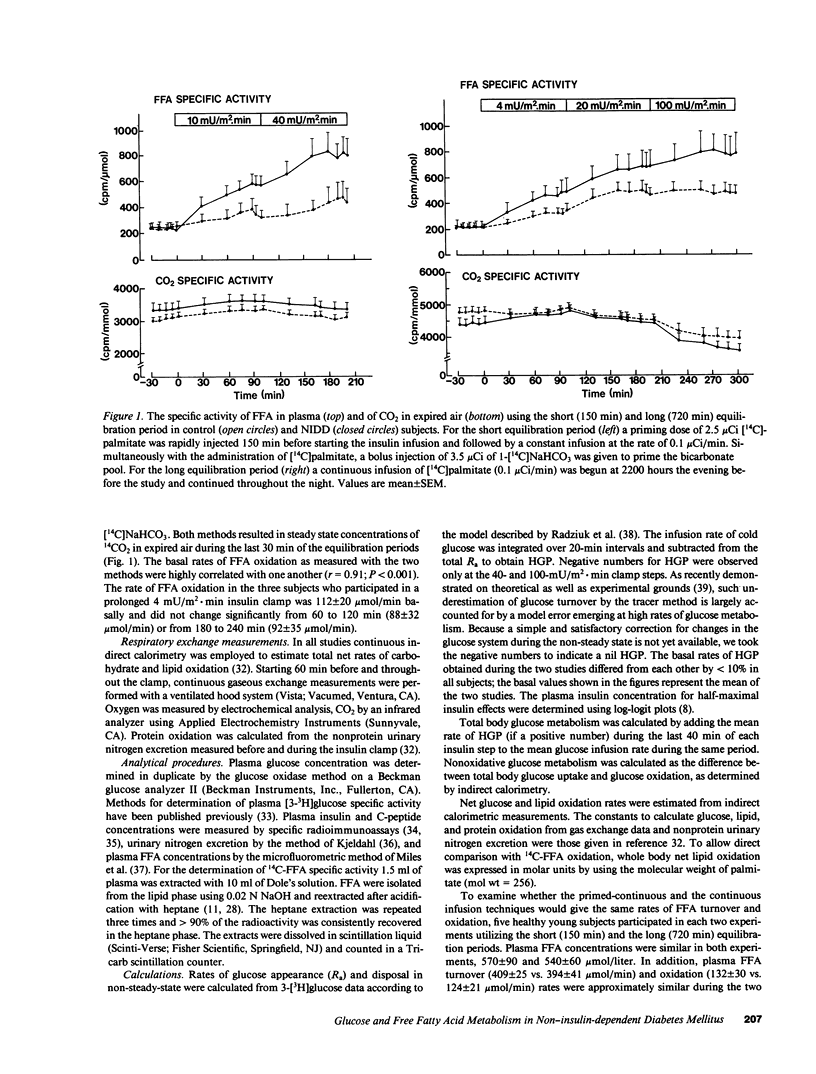
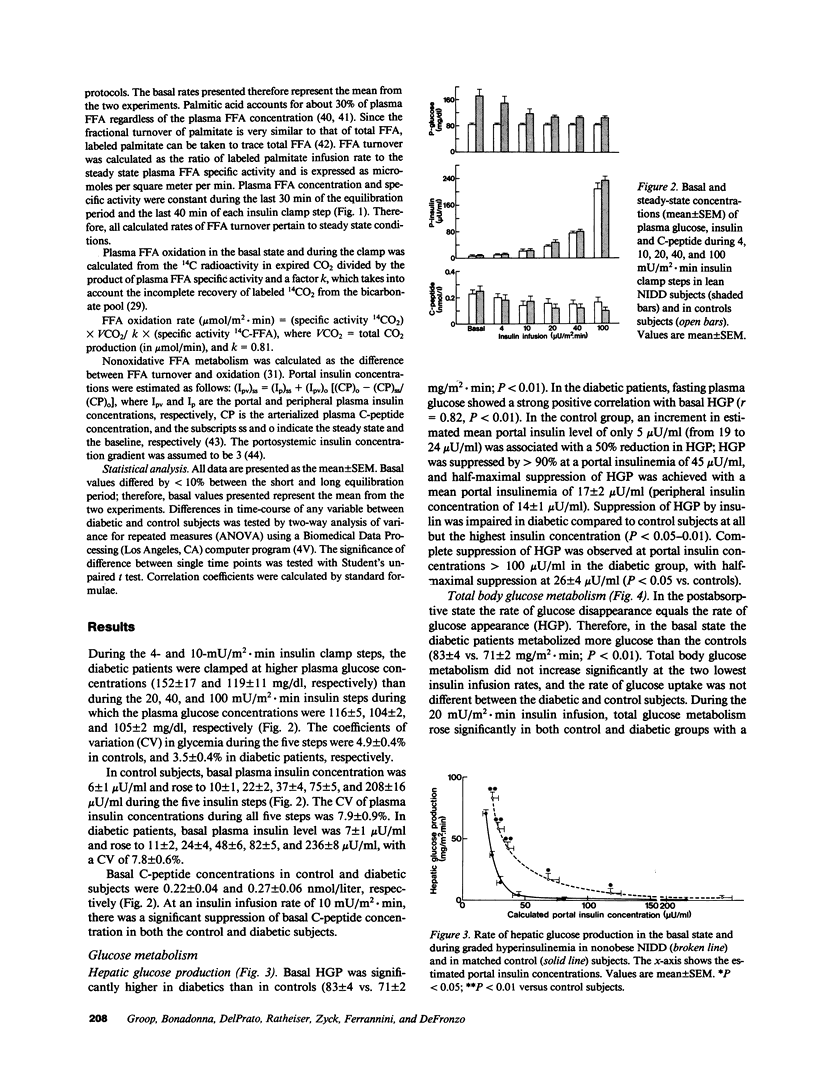
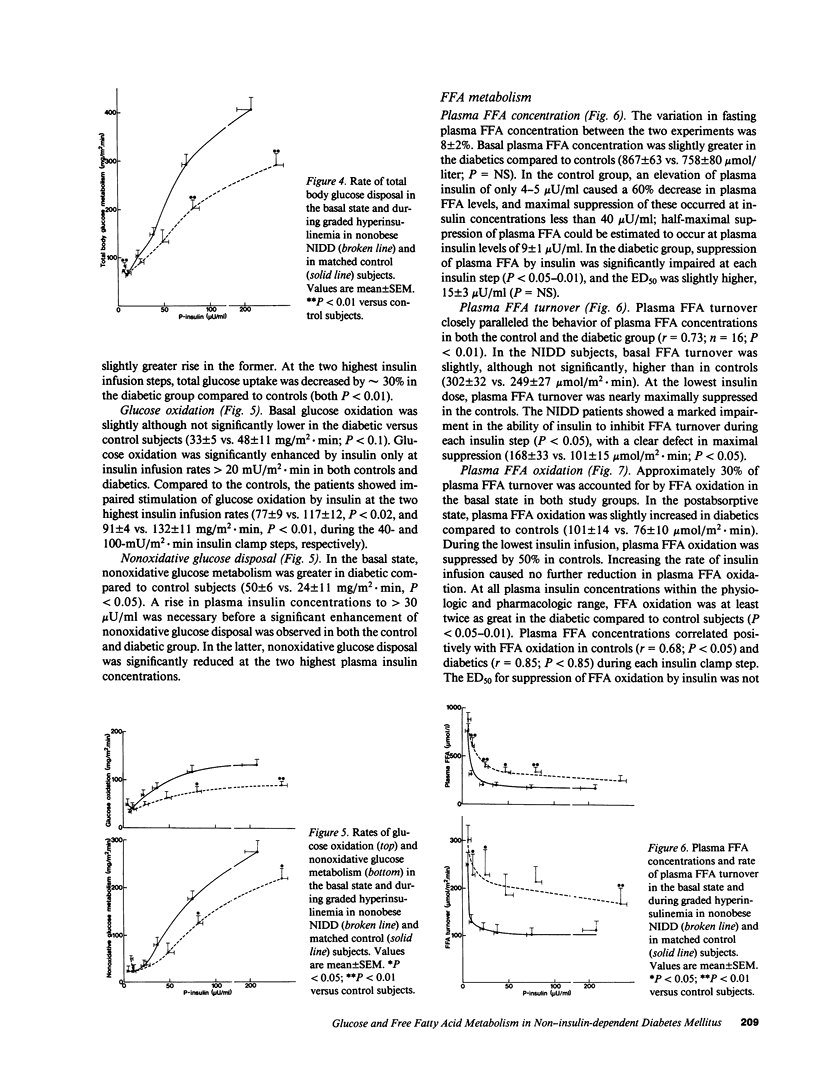
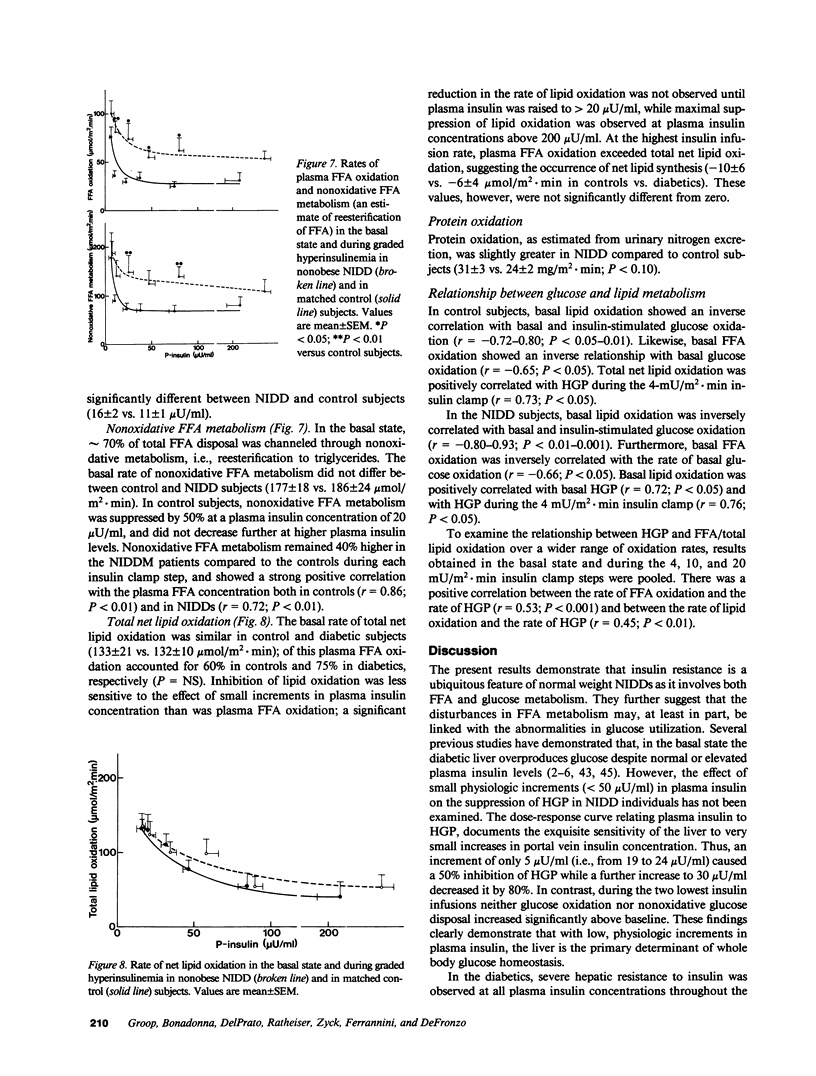
The effect of graded, physiologic hyperinsulinemia (+5, +15, +30, +70, +200 microU/ml) on oxidative and nonoxidative pathways of glucose and FFA metabolism was examined in nine lean non-insulin dependent diabetic patients (NIDDM) and in eight age- and weight-matched control subjects. Glucose and FFA metabolism were assessed using stepwise insulin clamp in combination with indirect calorimetry and infusion of [3H]3-glucose/[14C]palmitate. The basal rate of hepatic glucose production (HGP) was higher in NIDDM than in control subjects, and suppression of HGP by insulin was impaired at all but the highest insulin concentration. Glucose disposal was reduced in the NIDD patients at the three highest plasma insulin concentrations, and this was accounted for by defects in both glucose oxidation and nonoxidative glucose metabolism. In NIDDs, suppression of plasma FFA by insulin was impaired at all five insulin steps. This was associated with impaired suppression by insulin of plasma FFA turnover, FFA oxidation (measured by [14C]palmitate) and nonoxidative FFA disposal (an estimate of reesterification of FFA). FFA oxidation and net lipid oxidation (measured by indirect calorimetry) correlated positively with the rate of HGP in the basal state and during the insulin clamp. In conclusion, our findings demonstrate that insulin resistance is a general characteristic of glucose and FFA metabolism in NIDDM, and involves both oxidative and nonoxidative pathways. The data also demonstrate that FFA/lipid and glucose metabolism are interrelated in NIDDM, and suggest that an increased rate of FFA/lipid oxidation may contribute to the impaired suppression of HGP and diminished stimulation of glucose oxidation by insulin in these patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Arner P., Bolinder J., Engfeldt P., Ostman J. The antilipolytic effect of insulin in human adipose tissue in obesity, diabetes mellitus, hyperinsulinemia, and starvation. Metabolism. 1981 Aug;30(8):753–760. doi: 10.1016/0026-0495(81)90020-2. [DOI] [PubMed] [Google Scholar]
- BIERMAN E. L., DOLE V. P., ROBERTS T. N. An abnormality of nonesterified fatty acid metabolism in diabetes mellitus. Diabetes. 1957 Nov-Dec;6(6):475–479. doi: 10.2337/diab.6.6.475. [DOI] [PubMed] [Google Scholar]
- Balasse E. O., Neef M. A. Operation of the "glucose-fatty acid cycle" during experimental elevations of plasma free fatty acid levels in man. Eur J Clin Invest. 1974 Aug;4(4):247–252. doi: 10.1111/j.1365-2362.1974.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal S. A. Stimulation of gluconeogenesis by palmitic acid in rat hepatocytes: evidence that this effect can be dissociated from the provision of reducing equivalents. Metabolism. 1983 Oct;32(10):971–976. doi: 10.1016/0026-0495(83)90137-3. [DOI] [PubMed] [Google Scholar]
- Boden G., Ray T. K., Smith R. H., Owen O. E. Carbohydrate oxidation and storage in obese non-insulin-dependent diabetic patients. Effects of improving glycemic control. Diabetes. 1983 Nov;32(11):982–987. doi: 10.2337/diab.32.11.982. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolinder J., Ostman J., Arner P. Postreceptor defects causing insulin resistance in normoinsulinemic non-insulin-dependent diabetes mellitus. Diabetes. 1982 Oct;31(10):911–916. doi: 10.2337/diab.31.10.911. [DOI] [PubMed] [Google Scholar]
- Chen Y. D., Golay A., Swislocki A. L., Reaven G. M. Resistance to insulin suppression of plasma free fatty acid concentrations and insulin stimulation of glucose uptake in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987 Jan;64(1):17–21. doi: 10.1210/jcem-64-1-17. [DOI] [PubMed] [Google Scholar]
- Cobelli C., Mari A., Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol. 1987 May;252(5 Pt 1):E679–E689. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- Dagenais G. R., Tancredi R. G., Zierler K. L. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest. 1976 Aug;58(2):421–431. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Koivisto V. New concepts in the pathogenesis and treatment of noninsulin-dependent diabetes mellitus. Am J Med. 1983 Jan 17;74(1A):52–81. doi: 10.1016/0002-9343(83)90654-x. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E. Regulation of hepatic glucose metabolism in humans. Diabetes Metab Rev. 1987 Apr;3(2):415–459. doi: 10.1002/dmr.5610030204. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- Felber J. P., Ferrannini E., Golay A., Meyer H. U., Theibaud D., Curchod B., Maeder E., Jequier E., DeFronzo R. A. Role of lipid oxidation in pathogenesis of insulin resistance of obesity and type II diabetes. Diabetes. 1987 Nov;36(11):1341–1350. doi: 10.2337/diab.36.11.1341. [DOI] [PubMed] [Google Scholar]
- Felber J. P., Meyer H. U., Curchod B., Iselin H. U., Rousselle J., Maeder E., Pahud P., Jéquier E. Glucose storage and oxidation in different degrees of human obesity measured by continuous indirect calorimetry. Diabetologia. 1981;20(1):39–44. doi: 10.1007/BF00253814. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Fraze E., Donner C. C., Swislocki A. L., Chiou Y. A., Chen Y. D., Reaven G. M. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab. 1985 Nov;61(5):807–811. doi: 10.1210/jcem-61-5-807. [DOI] [PubMed] [Google Scholar]
- Golay A., Swislocki A. L., Chen Y. D., Reaven G. M. Relationships between plasma-free fatty acid concentration, endogenous glucose production, and fasting hyperglycemia in normal and non-insulin-dependent diabetic individuals. Metabolism. 1987 Jul;36(7):692–696. doi: 10.1016/0026-0495(87)90156-9. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenfeldt L. Turnover of individual free fatty acids in man. Fed Proc. 1975 Dec;34(13):2246–2249. [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Human forearm muscle metabolism during exercise. VII. FFA uptake and oxidation at different work intensities. Scand J Clin Lab Invest. 1972 Dec;30(4):429–436. doi: 10.3109/00365517209080281. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J., Pernow B., Räf L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest. 1972 Sep;51(9):2324–2330. doi: 10.1172/JCI107043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- Horwitz D. L., Starr J. I., Mako M. E., Blackard W. G., Rubenstein A. H. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest. 1975 Jun;55(6):1278–1283. doi: 10.1172/JCI108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. V., Savage P. J., Nagulesparan M., Bennion L. J., Unger R. H., Bennett P. H. Evidence for marked sensitivity to the antilipolytic action of insulin in obese maturity-onset diabetics. Metabolism. 1979 Jul;28(7):744–750. doi: 10.1016/0026-0495(79)90180-x. [DOI] [PubMed] [Google Scholar]
- ISSEKUTZ B., Jr, MILLER H. I., PAUL P., RODAHL K. SOURCE OF FAT OXIDATION IN EXERCISING DOGS. Am J Physiol. 1964 Sep;207:583–589. doi: 10.1152/ajplegacy.1964.207.3.583. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Bortz W. M., Miller H. I., Paul P. Turnover rate of plasma FFA in humans and in dogs. Metabolism. 1967 Nov;16(11):1001–1009. doi: 10.1016/0026-0495(67)90093-5. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Paul P., Miller H. I., Bortz W. M. Oxidation of plasma FFA in lean and obese humans. Metabolism. 1968 Jan;17(1):62–73. doi: 10.1016/s0026-0495(68)80008-3. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Bogardus C., Mott D. M., Kennedy A. L., Knowler W. C., Howard B. V. Relationship between insulin-mediated glucose disposal and lipid metabolism in man. J Clin Invest. 1985 Apr;75(4):1106–1115. doi: 10.1172/JCI111804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Zawadzki J. K., Young A. A., Abbott W. G., Bogardus C. Glucose storage is a major determinant of in vivo "insulin resistance" in subjects with normal glucose tolerance. J Clin Endocrinol Metab. 1986 May;62(5):922–927. doi: 10.1210/jcem-62-5-922. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Digirolamo M., Krotkiewski M., Smith U. Insulin binding and responsiveness in fat cells from patients with reduced glucose tolerance and type II diabetes. Diabetes. 1983 Aug;32(8):748–754. doi: 10.2337/diab.32.8.748. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Meylan M., Henny C., Temler E., Jéquier E., Felber J. P. Metabolic factors in the insulin resistance in human obesity. Metabolism. 1987 Mar;36(3):256–261. doi: 10.1016/0026-0495(87)90185-5. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Ellman M. G., McClean K. L., Jensen M. D. Validation of a new method for determination of free fatty acid turnover. Am J Physiol. 1987 Mar;252(3 Pt 1):E431–E438. doi: 10.1152/ajpendo.1987.252.3.E431. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Nankervis A., Proietto J., Aitken P., Harewood M., Alford F. Differential effects of insulin therapy on hepatic and peripheral insulin sensitivity in Type 2 (non-insulin-dependent) diabetes. Diabetologia. 1982 Oct;23(4):320–325. doi: 10.1007/BF00253737. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Measurement and validation of nonsteady turnover rates with applications to the inulin and glucose systems. Fed Proc. 1974 Jul;33(7):1855–1864. [PubMed] [Google Scholar]
- Reitsma W. D. The relationship between serum free fatty acids and blood sugar in non-obese and obese diabetics. Acta Med Scand. 1967 Sep;182(3):353–361. doi: 10.1111/j.0954-6820.1967.tb11535.x. [DOI] [PubMed] [Google Scholar]
- Robin A. P., Nordenström J., Askanazi J., Carpentier Y. A., Elwyn D. H., Kinney J. M. Influence of parenteral carbohydrate on fat oxidation in surgical patients. Surgery. 1984 May;95(5):608–618. [PubMed] [Google Scholar]
- Ruderman N. B., Toews C. J., Shafrir E. Role of free fatty acids in glucose homeostasis. Arch Intern Med. 1969 Mar;123(3):299–313. [PubMed] [Google Scholar]
- Ruderman N., Shafrir E., Bressler R. Relation of fatty acid oxidation tgluconeogenesis: effect of pentenoic acid. Life Sci. 1968 Oct 15;7(20):1083–1089. doi: 10.1016/0024-3205(68)90145-8. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Williams P. E., Liljenquist J. E., Lacy W. W., Keller U., Cherrington A. D. Effect of hyperglycemia independent of changes in insulin or glucagon on lipolysis in the conscious dog. Metabolism. 1980 Apr;29(4):317–320. doi: 10.1016/0026-0495(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Taskinen M. R., Bogardus C., Kennedy A., Howard B. V. Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest. 1985 Aug;76(2):637–644. doi: 10.1172/JCI112016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud D., Jacot E., DeFronzo R. A., Maeder E., Jequier E., Felber J. P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982 Nov;31(11):957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- Waterhouse C., Baker N., Rostami H. Effect of glucose ingestion on the metabolism of free fatty acids in human subjects. J Lipid Res. 1969 Sep;10(5):487–494. [PubMed] [Google Scholar]
- Williamson J. R., Kreisberg R. A., Felts P. W. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966 Jul;56(1):247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]



