Abstract
Phonological density refers to the number of words that can be generated by replacing a phoneme in a target word with another phoneme in the same position. Although the precise nature of the phonological neighborhood density effect is not firmly established, many behavioral psycholinguistic studies have shown that visual recognition of individual words is influenced by the number and type of neighbors the words have. This study explored neurobehavioral correlates of phonological neighborhood density in skilled readers of English using near infrared spectroscopy. On the basis of a lexical decision task, our findings showed that words with many phonological neighbors (e.g., FRUIT) were recognized more slowly than words with few phonological neighbors (e.g., PROOF), and that words with many neighbors elicited significantly greater changes in blood oxygenation in the left than in the right hemisphere of the brain, specifically in the areas BA 22/39/40. In previous studies these brain areas have been implicated in fine‐grained phonological processing in readers of English. The present findings provide the first demonstration that areas BA 22/39/40 are also sensitive to phonological density effects. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: optical imaging, phonological processing, phonological neighbors, lexical decision, hemodynamic measure, blood oxygenation, NIRS, visual word recognition
INTRODUCTION
Behavioral and neurocognitive studies of reading are increasingly being enlisted for their potential contribution to efforts to develop evidence‐based interventions for dyslexic readers [Gabrieli, 2009]. On a theoretical level, reading researchers have long debated the extent and nature of the role of phonological processing in visual word recognition. The present study offers an initial neurobehavioral investigation of this issue with particular reference to the variable of phonological neighborhood density. Phonological neighborhood density (PND) is a measure of neighborhood size that is based on the number of words that can be generated by replacing a phoneme in a target word with another phoneme in the same position [Yates, 2005]. For example, phonological neighbors of the word urge are edge, age, earl, earn, and earth. Phonological neighborhood density effects provide potentially important insights into the organization and operation of the mental lexicon [Grainger et al., 2005; Yates, 2005].
The nature of the phonological neighborhood effect is currently not well understood. In one of the first studies of this variable, Yates et al. [2004] examined phonological neighborhood density (PND) while controlling for orthographic neighborhood characteristics. They found a facilitative effect on a lexical decision task; that is, participants were faster at making lexical decisions to words with many vs. few phonological neighbors [Yates et al., 2004]. Using a different set of stimuli, Yates [2005] again obtained a facilitative phonological neighborhood density effect in lexical decision, as well as in a naming task and a semantic categorization task.
Nevertheless, the presence of an apparent facilitative effect of phonological neighborhood density runs contrary to predictions of interactive activation models of visual word recognition. These models propose that inhibitory connections exist between lexical representations within a given layer of the mental lexicon [Andrews, 1997; Coltheart et al., 2001; Grainger and Jacobs, 1996]. Similarly, the cross‐code consistency view of lexical representation proposed by Grainger et al. [2005] argues against a facilitative effect of phonological neighborhood density. The notion of cross‐code consistency was invoked by Grainger et al. to account for a pattern of findings they observed when examining the relationship between orthographic neighborhood density and phonological neighborhood density in visual word recognition. Orthographic neighborhood density refers to the number of words that can be generated by replacing one letter from a target word in the same letter position [Coltheart et al., 1977]; for example, gap, cup, and cat are all orthographic neighbors of the target word, cap. In a lexical decision task with readers of French, Grainger et al. [2005] found that the effect of phonological neighborhood density was facilitative for words with high orthographic neighborhood density but was inhibitory for words with low orthographic neighborhood density.
According to the cross‐code consistency account proposed by Grainger et al. [2005], words with high orthographic and high phonological neighborhood density and words with low orthographic and low phonological neighborhood density tend to have more consistent orthographic to phonological representations compared with words that are high in one type of neighborhood density but low in the other. Higher cross‐code consistency is in turn associated with faster response times. In this view, words that have a high orthographic neighborhood density will show more consistent cross‐code mapping if they also have high phonological neighborhood density, which should, in turn, lead to faster response latencies and a facilitative phonological neighborhood density effect, as has been reported by Yates et al. [2004] and Yates [2005]. Conversely, when words have a low orthographic neighborhood density, the higher the phonological neighborhood density of a word, the less consistent the cross‐code mapping and thus the slower the response should be. This should result in an inhibitory phonological neighborhood density effect. This latter prediction has not been tested to date. The present study, therefore, tested the prediction of an inhibitory phonological neighborhood density effect when orthographic neighborhood density is kept very low.
In addition to testing cross‐code consistency‐based predictions, this study sought to explore whether there is a neural correlate of phonological density effects detectable using neuroimaging techniques. Recent developments in brain activity recording methods have made it possible for researchers to examine the neural correlates of a number of psycholinguistically‐relevant variables influencing word recognition. Two neuroimaging studies using magnetoencephalography (MEG) have studied phonological density effects. Pylkkänen et al. [2002] found that, of the different components studied, the M350 response component was particularly sensitive to phonological neighborhood density [see also Pylkkänen and Marantz, 2003]. Pylkkänen et al. [2002] also found that people responded to words with high phonological neighborhood density more slowly than they did to words with low phonological neighborhood density. However, this finding was not replicated in a subsequent study by Stockall et al. [2004], in which a null effect of phonological neighborhood density was obtained. It is important to note that these MEG studies of phonological neighborhood density did not control for orthographic neighborhood size, making it difficult to know whether the effects were solely due to phonological neighborhood density.
This study explored neural correlates of phonological neighborhood density effects using the hemodynamic based measure of near infrared spectroscopy (NIRS). To our knowledge, no previous hemodynamic study in visual word recognition has specifically examined this variable. Hemodynamic changes in the brain can potentially provide corroborating evidence for the existence of a distinct phonological neighborhood density effect. NIRS measures changes in the concentration of oxy‐hemoglobin and deoxy‐hemoglobin in the brain regions of interest by shining near‐infrared light (650–950 nm) through the scalp and analyzing the characteristics of its subsequent absorption and scattering. When a brain area engages in a mental operation, an increase in the concentration of the oxy‐hemoglobin should be observed [see Strangman et al., 2002a, for a review]. Previous studies suggest that NIRS data are highly consistent with fMRI data [Strangman et al., 2002b]. NIRS is a portable and affordable alternative to fMRI, and provides reasonable temporal and spatial resolution [Hochman, 2000; Strangman et al., 2002a]. The temporal resolution of the NIRS system is a 200 Hz sampling rate, which is better than fMRI but is worse than that of EEG. The spatial resolution of NIRS can be as precise as 5 mm, which is better than EEG but is worse than fMRI.
Because it is not possible to monitor blood oxygenation changes in the whole brain using NIRS, one needs to identify a brain region of interest (ROI). Two recent meta‐analyses of functional neuroimaging studies using fMRI have identified several brain areas related to phonological processing [Bolger et al., 2005; Tan et al., 2005]. With English words, brain activation was found to be more pronounced in the left dorsal temporoparietal system, including posterior regions of the left superior temporal gyrus (BA22), the angular gyrus (BA39), and the supramarginal gyrus (BA40). These areas are thought to mediate grapheme‐to‐phoneme conversion and fine‐grained phonemic processing in alphabetic writing systems [Bolger et al., 2005; Tan et al., 2005]. Because phonological neighborhood effects, as conceptualized by the cross‐code consistency account, relate to fine‐grained phonemic processing and to grapheme‐to‐phoneme conversion, the brain areas of BA22/39/40 were selected as the ROI in this study which examined whether hemodynamic measures are sensitive to phonological neighborhood density effects in English readers.
To examine the effect of phonological neighborhood density effect as cleanly as possible, we controlled for orthographic neighborhood density by keeping it very low. This allowed us to test the assumption of interactive activation models of inhibitory connections between similar representations and the prediction based on cross‐code consistency of an inhibitory effect of phonological neighborhood density. Moreover, whereas previous NIRS studies of language processing have used a block design approach, we used an event‐related design. Although a block design provides superior statistical power to detect subtle differences [Friston et al., 1999], an event‐related design has the advantage of allowing randomized presentation of stimuli. This is desirable in investigations where the carryover effects typical of block designs may introduce response artifacts [Chee et al., 2003]. Using an event‐related design in the present study also allowed us to determine whether event‐related designs using NIRS are sensitive to the subtle changes typically observed in word recognition experiments.
METHOD
Participants
Fifteen native English readers from a large southwestern U.S. university participated in the experiment to receive course credit. All were right‐handed with normal or corrected‐to‐normal vision. The study was approved by the university institutional review board and participants gave their written consent.
MATERIALS
Thirty‐two monosyllabic, single‐morpheme English words ranging from four to seven letters in length were selected as the stimuli. They were subdivided into two lists consisting of 16 high‐ and 16 low‐phonological neighborhood density categories. Words in the high phonological density list contained six or more phonological neighbors; those in the low phonological density list had fewer than six neighbors. Stimuli in the two lists were matched in number of letters, number of phonemes, word frequency, orthographic neighborhood density, bigram frequency, mean frequency of orthographic neighbors, and mean frequency of phonological neighbors. Since previous research with the orthographic neighborhood density effect has found that it is generally restricted to low frequency words [Andrews, 1989, 1992], in this study, only low frequency words (those with a frequency count of under 35) were selected. In addition, 32 pronounceable nonwords were selected and intermixed with the experimental trials. Each participant received a different randomized sequence from a list consisting of 64 trials that included 16 words with higher phonological neighborhood density, 16 words with lower phonological neighborhood density, and 32 nonwords. All values of linguistic characteristics were determined by consulting the English lexicon project [Balota et al., 2007] and the Irvine Phonotactic Online Dictionary (IPhOD) [Vaden et al., 2005]. See Table I for a summary of stimulus characteristics.
Table I.
Characteristics of the stimuli used in the lexical decision task (mean values)
| Characteristic | High OND | Low OND |
|---|---|---|
| Number of letters | 5.63 | 5.88 |
| Number of phonemes | 4.00 | 4.38 |
| Word frequency | 10.06 | 10.75 |
| OND | 0.00 | 0.00 |
| PND | 13.44 | 3.13 |
| BF | 2498.25 | 2409.44 |
| Mean frequency of ON | 0.00 | 0.00 |
| Mean frequency of PN | 17.63 | 18.06 |
OND, orthographic neighborhood density; BF, bigram frequency; ON, orthographic neighbors; PN, phonological neighbors; PND, phonological neighborhood density.
Apparatus
The experiment was administered on a personal computer using the E‐Prime software package [Schneider et al., 2002]. The optical signals were collected by an electronic control box serving both as the source and the receiver of the near‐infrared light (NIRS 4 × 4 CW4 System, TechEn Inc.). Studies of Homan et al. [1987], Okamota et al. [2004], and Koessler et al. [2009] have suggested that the international 10–20 system has an appropriate relationship with the cortical anatomy. On the basis of a recent estimation by Koessler et al. [2009], the grand standard deviation for using the international 10–10 system, which is an extension of the international 10–20 system, is 4.6 mm in x, 7.1 mm in y, and 7.8 mm in z. In relation to the spatial resolution of NIRS system used here, the variability of neuroanatomical localization is acceptable for using the international 10–20 system as a means of NIRS probe placement. Strips placed on the regions corresponding to the presumed locations of BA 22/39/40 for each hemisphere were designed to hold two laser emitters, which directed the two wavelengths of near‐infrared light through the scalp, and four laser detectors, which received the returning near‐infrared light (see Fig. 1). These were placed on participants' heads to record changes in blood oxygenation during the lexical decision task. Specifically, two emitters were located at TP3 and TP4 following the international 10/20 system. TP3 and TP4 are located midway between regions P3 and T3 and between P4 and T4 which have been hypothesized to be relevant to language function [Homan et al., 1987]. The locations the emitters were placed, the TP3 and TP4, have been suggested by Homan et al. [1987], Okamota et al. [2004], and Koessler et al. [2009] to cover BA 22/39/40.
Figure 1.
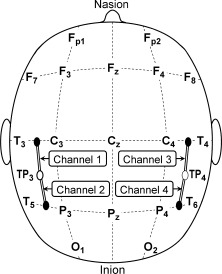
Positions of the probes used. Open circles denote laser emitters and filled circles denote laser detectors. Two laser emitters were placed at TP3 and TP4 following International 10/20 system. The distances between laser emitters and detectors were set at 3 cm.
Four detectors were located 3 cm away from the centrally located emitters. This is the distance suggested to ensure detection of near‐infrared light following penetration of the adult neocortex [Hedden and Delpy, 1997]. Four channels, formed by different pairs of emitters and detectors, covered the brain areas thought to be related to phonological processing. Specifically, Channels 1 and 2 covered the left superior temporal gyrus (BA22), the supramarginal gyrus (BA40), and the angular gyrus (BA39), whereas Channels 3 and 4 covered the homologous regions of the right hemisphere [Homan et al., 1987; Okamoto et al., 2003]. These areas have been identified as playing an important role in grapheme‐to‐phoneme processing in English [Bolger et al., 2005; Tan et al., 2005]. A chin rest was used to reduce motion artifacts.
Each emitter contained two light sources with a wavelength of 690 nm and 830 nm, respectively. The former is more sensitive to deoxy‐hemoglobin and the latter is more sensitive to oxy‐hemoglobin. Another laptop computer was programmed to control and record the signals received by the electronic control box. The data recorded by the control box were then converted to relative concentrations of oxy‐hemoglobin and deoxy‐hemoglobin using the modified Beer‐Lambert law [Strangman et al., 2002a].
Procedure
The task was a go/no go version of the lexical decision task. For each trial, participants, tested individually, first saw a fixation signal presented at the center of the screen. They were to press a button, using their dominant hand, upon seeing the fixation signal. This was followed by the stimulus which was presented at the center of the screen. Participants were instructed to make a speeded lexical decision response and press the response button only if they thought the stimulus formed an actual English word. Each stimulus appeared on the screen for 2 s and was followed by a blank screen. Reaction time (RT) was recorded from the onset of stimulus presentation until the participant pressed a button. In between trials a blank screen was presented for either 12, 14, 16, or 18 s. The variation in exposure time of the fixation stimulus was introduced to hold participants' attention and prevent them from guessing. Participants received at least 10 practice trials until they got used to the procedure before the experiment was initiated.
RESULTS
Behavioral Data
In calculating the mean RTs of correct responses for each condition for each participant, those trials with RTs less than 200 ms or higher than 1800 ms were discarded. These cutoffs led to the rejection of less than 1% of the observations. Table II shows the accuracy and recomputed means for correct RTs for each experimental condition. The results of a t‐test indicated a significant effect of phonological neighborhood density in RT, t(14) = 1.88, P < 0.05, with slower RTs for words with more phonological neighbors compared with those with fewer phonological neighbors. In other words, phonological neighborhood density showed an inhibitory effect. No significant effect was found in accuracy, t(14) < 1.
Table II.
Mean reaction time (ms) and accuracy (%) in lexical decision task
| High PND | Low PND | PND effect | |
|---|---|---|---|
| RT | 798.66 (31.65) | 762.83 (33.62) | 35.83 (19.02) |
| Accuracy | 91.67 (1.80) | 90.48 (2.17) | 1.19 (1.76) |
RT, reaction time; PND, phonological neighborhood density. Standard errors are reported in parentheses.
Optical Imaging Data
The NIRS data from 4 channels were digitally recorded at a 200 Hz sampling rate. The data were then converted into optical density units that were digitized and low‐pass‐filtered at 1 Hz and high‐pass‐filtered at 0.02 Hz to reduce noise for systemic physiology (e.g., pulse). The filtered data were then converted to reflect the concentration of both the oxy‐hemoglobin and deoxy‐hemoglobin; these served as the data used for advanced analysis. The converted data were analyzed in 17‐s epochs including 2 s before and 15 s after the onset of the stimuli. Data conversion was conducted using HomER software [Huppert and Boas, 2005].
NIRS data were down‐sampled to 2 Hz and then averaged in further analyses. Analyses were first conducted by averaging data from Channels 1 and 2 to estimate the blood oxygenation change in the left hemisphere and by averaging data from Channels 3 and 4 to estimate the blood oxygenation change in the right hemisphere. The estimation of changes in cerebral flow of oxy‐hemoglobin, with family‐wise error rate controlled by the Bonferroni test, is depicted in Figure 2. Black lines in the figure represent data for words with high phonological neighborhood density and gray lines represent data for words with low phonological neighborhood density. Circles on the black lines (high phonological neighborhood density) indicate time points at which the changes in blood oxygenation were significantly different from zero. Circles on the gray lines (low phonological neighborhood density) indicate time points at which the changes in blood oxygenation were significantly different from zero. The asterisks above the standard error bars indicate time points at which the changes in blood oxygenation were significantly different between words with high vs. low phonological neighborhood density. Figure 3 shows the estimation of changes in cerebral flow of oxy‐hemoglobin for nonwords for comparison with that for words. For evaluating the overall quality of the raw data, the estimation of changes in the concentration in oxy‐hemoglobin and deoxy‐hemoglobin in both left and right hemispheres for two representative participants is presented in Figure 4.
Figure 2.
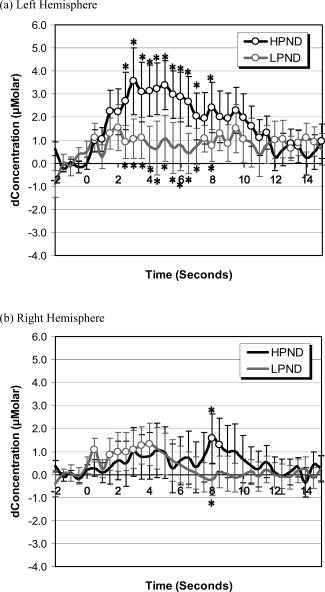
Grand average changes of the concentration in oxy‐hemoglobin in (a) left hemisphere and in (b) right hemisphere. The data were analyzed in 17‐second epochs with 2 seconds before and 15 seconds after the onset of the stimuli. Black lines represent data for words with high phonological neighborhood density and gray lines represent data for words with low phonological neighborhood density. Circles on the black lines indicate time points at which the changes in blood oxygenation were significantly different from zero for words with high phonological neighborhood density. Circles on the gray lines indicate time points at which the changes in blood oxygenation were significantly different from zero for words with low phonological neighborhood density. The bars on each time point show the standard errors. The asterisks above and below the standard error bars indicate time points at which the changes in blood oxygenation were significantly different between words with high vs. low phonological neighborhood density. These analyses were controlled by the Bonferroni test for family‐wise error rate.
Figure 3.
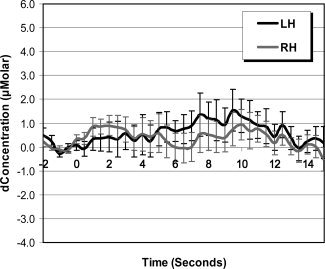
Grand average changes of the concentration in oxy‐hemoglobin in both left (LH) and right (RH) hemispheres for nonwords.
Figure 4.
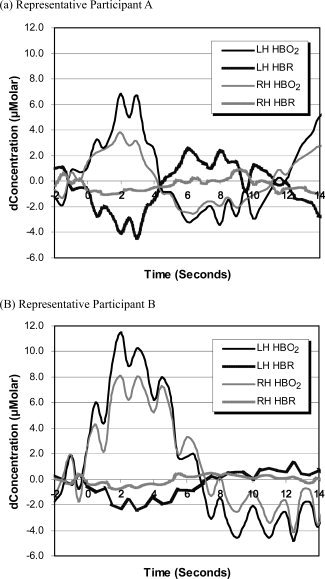
Grand average changes of the concentration in oxy‐hemoglobin (HBO2) and deoxy‐hemoglobin (HBR) in both left (LH) and right (RH) hemispheres for two representative participants.
Hemodynamic Response to Words With High Phonological Neighborhood Density Per Hemisphere
An analysis of blood oxygenation changes per hemisphere for words with high phonological neighborhood density showed a significant increase in oxy‐hemoglobin concentration in the left hemisphere, t(14) = 3.69, P < 0.001, but no increase in the right hemisphere, t(14) < 1. When analyzing at each sampled time point, with family wise error rate controlled by the Bonferroni test, the increases in oxy‐hemoglobin concentration were found to be significantly different from zero in the left hemisphere for the time periods 0.5 to 11.5 s and 13 to 13.5 s after the onset of the stimuli. Oxy‐hemoglobin concentration significantly increased from zero in the right hemisphere between 8 and 8.5 s after the onset of the stimuli.
Hemodynamic Response to Words With Low Phonological Neighborhood Density Per Hemisphere
For words with low phonological neighborhood density, a significant increase in oxy‐hemoglobin concentration was found in the left hemisphere, t(14) = 2.56, P < 0.05, with no corresponding increase in the right hemisphere, t(14) < 1. When analyzing data for each sampled time point, with family‐wise error rate controlled by the Bonferroni test, the increases in oxy‐hemoglobin concentration were significantly different from zero in the left hemisphere at 0.5 s, 1.5 to 3.5 s, 7.5 to 10 s, and 12 to 14.5 s after the onset of the stimuli. Oxy‐hemoglobin concentration significantly increased from zero in the right hemisphere at 0.5 and at 1.5 to 4 s after the onset of the stimuli.
Hemodynamic Response to Words With High vs. Low Phonological Neighborhood Density Per Hemisphere
When directly comparing activation data for words with high vs. low phonological neighborhood density, stronger activation was found for high than low phonological neighborhood density words in the left hemisphere, t(14) = 1.86, P < 0.05; no difference in activation was found between the two word types in the right hemisphere, t(14) < 1. With family‐wise error rate controlled by the Bonferroni test, words with high phonological neighborhood density showed stronger activation than those with low phonological neighborhood density at 2.5 to 7 s and at 8 s in the left hemisphere, whereas in the right hemisphere words with high phonological neighborhood density only showed stronger activation than those with low phonological neighborhood density at 8 s after stimulus onset.
Although interpretation of direct comparison of hemodynamic responses across brain areas must be done with caution [e.g., Logothetis and Wandell, 2004], we present an analysis of NIRS data that directly compared activation patterns between the two hemispheres. Specifically, we conducted a three‐way ANOVA with Experiment Period (stimuli present vs. absent‐baseline), Phonological Neighborhood Density (high vs. low), and Hemisphere (left vs. right) as within‐subjects factors. Baseline was set from 2 s before the onset of the stimuli to the onset of the stimuli. This analysis was conducted following the suggestion of Lloyd‐Fox et al. [2009] to compare the shape of the response to different stimuli (i.e., words with high vs. low phonological neighborhood density). A main effect of Experiment Period was found, F(1,14) = 7.36, P < 0.05, suggesting that blood oxygenation changes increased significantly after the presence of the stimuli relative to baseline. An interaction of Experiment Period and Hemisphere was also found, F(1,14) = 6.58, P < 0.05. Further simple effect analyses suggested that the blood oxygenation changes significantly increased relative to baseline after the presence of the stimuli only in the left hemisphere for words with high phonological neighborhood density, F(1,56) = 13.36, P < 0.001; there was no increase in the left hemisphere for words with low phonological neighborhood density, F(1,56) = 2.98, P = 0.09, in the right hemisphere for words with high phonological neighborhood density, F(1,56) = 1.12, P = 0.3, or in the right hemisphere for words with low phonological neighborhood density, F(1,56) < 1. Simple effect analyses also suggested that a phonological neighborhood density effect was only obtained in the left hemisphere, F(1,56) = 3.95, P < 0.05, during the experimental period following stimulus presentation. In other words, the phonological neighborhood density effect was more evident in the left hemisphere in the brain regions of BA 22/39/40 monitored in this study.
DISCUSSION
Phonological neighborhood density was examined in isolation in the present study by keeping orthographic neighborhood density as low as possible. The behavioral results showed an inhibitory effect of phonological neighborhood density. This effect is consistent with predictions based on interactive activation models of word recognition [e.g., Coltheart et al., 2001; Grainger and Jacobs, 1996] and based on a cross‐code consistency account [Grainger et al., 2005]. The NIRS data also provided evidence of changes in blood oxygenation volume in relation to changes in phonological neighborhood density, both within and across hemispheres. First, there was significant left hemisphere activation in the brain regions of BA 22/39/40 but no significant right hemisphere activation in these regions. Furthermore, in the left hemisphere, words with high phonological neighborhood density generated stronger blood oxygenation changes in BA 22/39/40 than those with low phonological neighborhood density.
It is possible that some of the left hemisphere activation observed may reflect use of the right hand for response, but it is unlikely that the asymmetry is entirely due to hand used for responding. What is not possible to establish at present is whether the strong blood oxygenation changes observed for high PND words should be interpreted as reflecting an effect of inhibition or facilitation. Given that the behavioral data showed an inhibitory effect of neighborhood density, we tentatively conclude that stronger blood oxygenation changes for words with high phonological neighborhood density likely reflect inhibition from intralevel lateral connections among words. Stated differently, our results indicate that higher phonological neighborhood density was associated with longer response times, and that the associated longer duration of neural activity caused a larger hemodynamic response. Whereas earlier neuroimaging studies of phonological neighborhood density using MEG yielded inconclusive results, largely because they did not control for potential influences of orthographic neighborhood density, this study obtained a clear effect of phonological neighborhood density using a hemodynamic measure, after carefully controlling for characteristics of orthographic neighborhood. Indeed, the present study is the first hemodynamic study demonstrating a neural correlate of the phonological neighborhood density effect in visual word recognition.
Research has suggested that the brain areas of BA 22/39/40 in the left hemisphere are important for mediating grapheme‐to‐phoneme conversion and fine‐grained phonemic processing in alphabetic writing systems like English [Bolger et al., 2005; Tan et al., 2005]. The finding of a phonological neighborhood density effect in this area suggests that this effect is closely related to grapheme‐to‐phoneme conversion. This is in line with what was proposed by the cross‐code consistency account [Grainger et al., 2005]. In the latter account, consistency is calculated in terms of lexical and sublexical representations and in terms of orthographic and phonological representations. All coactivated representations can affect lexical decision. The higher the level of compatibility across coactivated representations, the better the resonance that can be established across these representations. In this study, words with low orthographic and low phonological neighborhood density would tend to have more consistent orthographic to phonological representations (thereby producing faster responses) whereas words with low orthographic but high phonological neighborhood density would tend to have less consistent orthographic to phonological representations (producing slower responses), resulting in an inhibitory phonological neighborhood density effect. However, since the stimuli used in the present study were mainly low frequency words, future studies are needed to determine if the present findings will generalize to words in other frequency ranges.
Even with the less powerful event‐related design used here (but one that guards against carry‐over effects), our results suggest that the NIRS method is capable of detecting subtle hemodynamic changes in complex language experiments, as suggested also by Chen et al. [2008]. Because other studies have suggested that NIRS data are highly consistent with fMRI data, we anticipate seeing more research in the future using NIRS, as it is a more portable and affordable alternative to fMRI, as long as the ROI has been identified and is within the range of spatial resolution provided by NIRS [Strangman et al., 2002a].
CONCLUSION
This study examined the phonological neighborhood density effect in English visual word recognition using a NIRS‐based event‐related design. With an English stimulus set in which orthographic neighborhood density was carefully controlled, our NIRS data provide evidence for phonological neighborhood density effects in BA 22/39/40, which is a region previously identified as important in mediating grapheme‐to‐phoneme conversion in English. This finding shows that it is possible to identify distinct neural correlates of neighborhood density modulating visual word recognition.
Stimuli used in this study:
Words with high phonological neighborhood density: braille, bronze, chaise, chrome, froze, fruit, lapse, proud, quake, shield, sleeve, sphere, waltz, whoop, whoosh, writhe.
Words with low phonological neighborhood density: blithe, blouse, glance, glib, hertz, lounge, prompt, proof, scourge, scratch, sketch, sluice, sparse, spouse, swerve, warmth.
REFERENCES
- Andrews S ( 1989): Frequency and neighborhood effects on lexical lexical access: Activation or search? J Exp Psychol Learn Mem Cogn 15: 802–814. [Google Scholar]
- Andrews S ( 1992): Frequency and neighborhood effects in lexical access: Lexical similarity or orthographic redundancy? J Exp Psychol Learn Mem Cogn 18: 234–254. [Google Scholar]
- Andrews S ( 1997): The effect of orthographic similarity on lexical retrieval: Resolving neighborhood conflicts. Psychon Bull Rev 4: 439–461. [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KI, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Treiman R ( 2007): The English lexicon project. Behav Res Methods 39: 445–459. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W ( 2005): Cross‐cultural effect on the brain revisited: Universal structures plus writing system variation. Hum Brain Mapp 25: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Venkatraman V, Westphal C, Siong SC ( 2003): Comparison of block and event‐related fMRI designs in evaluating the word‐frequency effect. Hum Brain Mapp 18: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H‐C, Vaid J, Bortfeld H, Boas DA ( 2008): Optical imaging of phonological processing in two distinct orthographies. Exp Brain Res 184: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M, Davelaar E, Jonasson JT, Besner D ( 1977): Access to the internal lexicon In: Dornic S, editor. Attention and Performance VI. New York: Academic Press; pp 535–555. [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J ( 2001): DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol Rev 108: 204–256. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM ( 1999): Stochastic designs in event‐related fMRI. Hum Brain Mapp 10: 607–619. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE ( 2009): Dyslexia: A new synergy between education and cognitive neuroscience. Science 325: 280–283. [DOI] [PubMed] [Google Scholar]
- Grainger J, Jacobs AM ( 1996): Orthographic processing in visual word recognition: A multiple read‐out model. Psychol Rev 103: 518–565. [DOI] [PubMed] [Google Scholar]
- Grainger J, Muneaux M, Farioli F, Ziegler JC ( 2005): Effects of phonological and orthographic neighbourhood density interact in visual word recognition. Q J Exp Psychol A 58: 981–998. [DOI] [PubMed] [Google Scholar]
- Hedden JC, Delpy DT ( 1997): Diagnostic imaging with light. Br J Radiol 70: S206–S214. [DOI] [PubMed] [Google Scholar]
- Hochman DW ( 2000): Optical monitoring of neuronal activity: Brain‐mapping on a shoestring. Brain Cog 42: 56–59. [DOI] [PubMed] [Google Scholar]
- Homan RW, Herman J, Purdy P ( 1987): Cerebral location of international 10‐20 system electrode placement. Electroenceph Clin Neurophysiol 66: 376–382. [DOI] [PubMed] [Google Scholar]
- Huppert T, Boas DA ( 2005): Hom ER: Hemodynamic Evoked Response NIRS data analysis GUI. Available from the Photon Migration Imaging Lab, Martinos Center for Biomedical Imaging, http://www.nmr.mgh.harvard.edu/PMI/.
- Koessler L, Maillard L, Benhadid A, Vignal JP, Felblinger J, Vespignani H, Braun M ( 2009): Automated cortical projection of EEG sensors: Anatomical correlation via the international 10‐10 system. Neuroimage 46: 64–72. [DOI] [PubMed] [Google Scholar]
- Lloyd‐Fox S, Blasi A, Elwell CE ( 2010): Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev 34: 269–284. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA ( 2004): Interpreting the BOLD signal. Annu Rev Physiol 66: 735–769. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Oda I, Isobe S, Suzuki T, Kohyama K, Dan I ( 2003): Three‐dimensional probabilistic anatomical cranio‐cerebral correlation via the international 10‐20 system oriented for transcranial functional brain mapping. Neuroimage 21: 99–111. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L, Marantz A ( 2003): Tracking the time course of word recognition with MEG. Trends Cogn Sci 7: 187–189. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L, Stringfellow A, Marantz A ( 2002): Neuromagnetic evidence for the timing of lexical activation: An MEG component sensitive to phonotactic probability but not to neighborhood density. Brain Lang 81: 666–678. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A ( 2002): E‐Prime User's Guide. Pittsburgh: Psychology Software Tools. [Google Scholar]
- Stockall L, Stringfellow A, Marantz A ( 2004): The precise time course of lexical activation: MEG measurements of the effects of frequency, probability, and density in lexical decision. Brain Lang 90: 88–94. [DOI] [PubMed] [Google Scholar]
- Strangman G, Boas DA, Sutton JP ( 2002a): Non‐invasive neuroimaging using near‐infrared light. Biol Psychiatry 52: 679–693. [DOI] [PubMed] [Google Scholar]
- Strangman G, Culver JP, Thompson JH, Boas DA ( 2002b): A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. NeuroImage 17: 719–731. [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT ( 2005): Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta‐analysis. Hum Brain Mapp 25: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaden KI, Hickok GS, Halpin HR ( 2005): Irvine Phonotactic Online Dictionary. [Data file]. Available at: http://www.iphod.com.
- Yates M ( 2005): Phonological neighbors speed visual word recognition: Evidence from multiple tasks. J Exp Psychol Learn Mem Cogn 31: 1385–1397. [DOI] [PubMed] [Google Scholar]
- Yates M, Locker L, Simpson GB ( 2004): The influence of phonological neighborhood on visual word recognition. Psychon Bull Rev 11: 452–457. [DOI] [PubMed] [Google Scholar]


