Abstract
A better understanding of the neural systems underlying impulse control is important for psychiatry. While most impulses are motivational or emotional rather than motoric per se, it is research into the neural architecture of motor response control that has made the greatest strides. This article reviews recent developments in the cognitive neuroscience of stopping responses. Most research of this kind has focused on reactive control – i.e. how subjects stop a response outright when instructed by a signal. It is argued that reactive paradigms are limited as models of control relevant to psychiatry. Instead, a set of paradigms is advocated that begins to model proactive inhibitory control – i.e. how a subject prepares to stop an upcoming response tendency. Proactive inhibitory control is generated according to the goals of the subject, rather than by an external signal, and it can be selectively targeted at a particular response tendency. This may have wider validity than reactive control as an experimental model for stopping inappropriate responses.
Keywords: executive function, cognitive control, prefrontal cortex, basal ganglia, response inhibition, working memory, impulse control
Neuropsychiatric research needs models of stopping
Many psychiatric disorders involve problems with controlling urges. These problems include urges for movement (as in Tourette’s syndrome, and some forms of Attention Deficit Hyperactivity Disorder), compulsive urges (e.g for hand-washing or hair-pulling as in Obsessive Compulsive Disorder or Trichotillomania), and craving for drugs and gambling. A better understanding of the aetiology, risk factors, treatment and outcome for these disorders would be aided by neurocognitive markers that relate to symptoms such as ‘impulsivity’ and are also rooted in neuroscience and genetics (1).
Evaluating cognitive markers requires developing computerized tests in the laboratory that have ‘ecological validity’ for the disorder of interest, but are also amenable to neuroscience approaches in humans. This is a particularly challenging problem for emotion and motivation control. While behavioral paradigms for emotion control do exist (e.g. 2) (and see for review 3, 4), the mapping to neural circuits is very incomplete. The same can be said for the control of motivational urges, especially in humans, although there have been advances in understanding, for example, how craving may be resisted (e.g. 5). Thus, much research has focused on the easier question about the stopping of motor responses, where behavior can be better operationalized and where the target of the stopping (the motor system) is better understood. Here, ‘stopping’ refers to the behavioral outcome when subject halts an incipient response tendency. Behavioral stopping has its counterpart in a psychological stopping process, and this may be implemented in the brain by a set of functions/circuits including inhibitory control, attentional monitoring/detection and working memory.
It is acknowledged that the stopping of motor responses, no matter how sophisticated the model, will only be relevant for impulse control some of the time. Even when the focus is on disorders of movement such as Tourette’s Syndrome, it is likely that successful real-world control relies on strategies such as anxiety reduction, mindfulness and cognitive-behavioral training (e.g. 6) as much as or probably more than a voluntary, top-down, process of movement control. Nevertheless, mechanisms for the top-down stopping of initiated response tendencies are important too.
This article reviews the cognitive neuroscience of stopping. It begins by focusing on paradigms in which subjects stop a response outright when instructed by a signal. This is referred to as ‘reactive stopping’’. While clearly useful as a beginning point for mapping the neural architecture of cognitive control, it is argued that reactive stopping is limited as a model. Instead, behavioral paradigms are advocated that model how a subject prepares to stop an upcoming response tendency (i.e. ‘proactive stopping’). Proactive stopping is developed according to the goals of the subject, rather than being simply triggered reactively by an external signal. Moreover proactive stopping allows the inhibitory control to be more selective. As will be shown, this bears stronger face validity to the problems faced by psychiatric patients.
The cognitive neuroscience of reactive stopping
Behavioral paradigms
Many experimental paradigms exist for studying how people – and, in some cases, experimental animals – control their response tendencies. These include Stop signal, Go/NoGo, Anti-Saccade, Flanker, Stroop, Simon, Wisconsin Card Sort, Continuous Performance, Reversal learning, and many others. These all require control over a prepotent response tendency. Here we consider the first three in brief detail.
The stop signal test requires people to stop an already initiated response (7). On each trial, the subject is presented with a Go signal, such as: press the left button for a leftward pointing arrow, or the right button for a rightward pointing arrow. On a minority of trials, a Stop signal is presented after the Go signal. The subject is instructed to respond as fast as possible on Go trials, and to do his/her best to stop the response when the Stop signal occurs (Figure 1A). If the delay between Go and Stop signals is short, the subject is more likely to stop; whereas if the delay is long then the subject is less likely to stop. Using information about the probability of stopping at various delays, and reaction time on non-stop signal (i.e. Go) trials, it is possible to calculate the internal speed of stopping, i.e. stop signal reaction time (SSRT) (8).
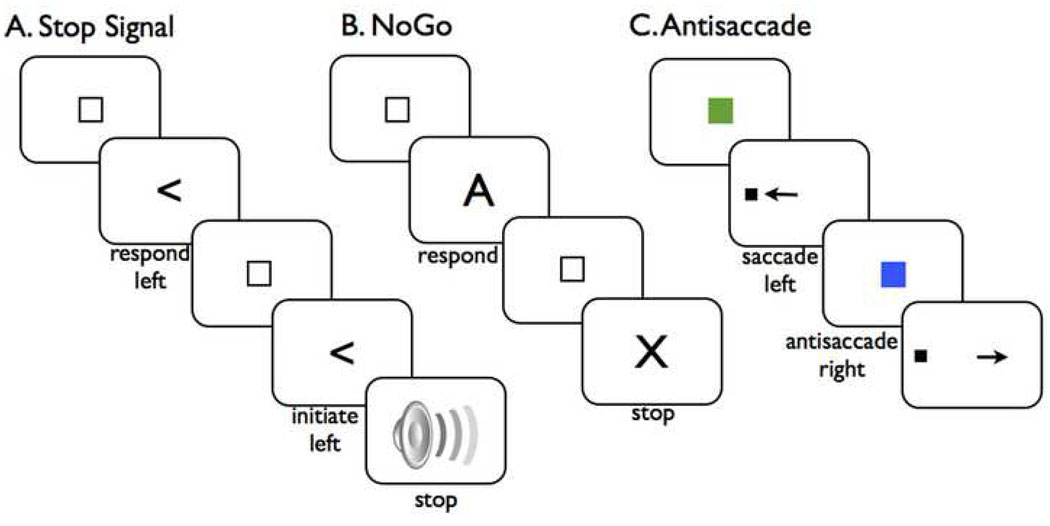
Figure 1.
Three behavioral paradigms for measuring stopping. A. The stop signal test. A ready signal (small box) is presented followed by a Go signal (left-pointing arrow). The subject initiates and executes a left button press (Go trial). The next trial begins in the same way, however a stop signal (auditory) is presented at a delay (e.g. 200 ms) after the Go signal. The subject stops the response (stop trial). B. The Go/NoGo test. Typically this is done with a stream of letters. The subject responds to all except the letter 'X'. C. The antisaccade test. The central eye fixation signal is a colored box. Here, green means 'make a saccade' in the direction of the upcoming target, while blue means 'make a saccade in the opposite direction to the upcoming target'. On the antisaccade trial shown above, the target triggers an automatic saccade to the left, but the subject over-rides this to move her eyes to the right.
In a typical Go/NoGo paradigm the subject is presented with a stream of letter stimuli, and is required to quickly respond to all letters except the letter ‘X’ (Figure 1B). Thus the stop signal and Go/NoGo paradigms are different in several respects. In the stop signal paradigm the experimenter has tight control over when Go and Stop processes begin on each trial, while in the Go/NoGo paradigm one cannot be sure when the Go and stop processes begin. In Go/NoGo tests with rapid stimulus presentation and a low probability of NoGos, the Go process could be ‘preactivated’ on each trial, and therefore stopping on a NoGo trial may be similar to stopping on a stop trial in a stop signal paradigm. But in other Go/NoGo studies with lower prepotency, successful stopping may be more about deciding not to go than countermanding an initiated response. By contrast, in the stop signal paradigm, the response is already underway (on that particular trial) when the control signal occurs. Hence, the control is unlikely to reflect mere restraint of the movement plan (9), or the generation of an alternative plan (i.e. no response) or the activation of antagonist muscles; instead, the control needs to be targeted at those parts of the motor system that are already activated (10, 11).
Another paradigm which also requires stopping a response that is already underway is the antisaccade test (reviewed by 12) (Figure 1C). On each trial a cue indicates whether the trial will require a saccadic eye movement to an upcoming spatial target or an antisaccade (i.e. eye movement in the opposite direction of the target). When the target occurs a reflexive eye movement is generated (visible in increased firing of neurons in the eye movement circuitry); thus correct performance on antisaccade trials requires inhibiting the reflexive tendency and generating a new saccade.
While the level of prepotency in stop signal and antisaccade tasks may supercede that in other paradigms (such as Go/NoGo, Eriksen Flanker, and response/task switching) these other paradigms often also require rapid action control. Consistent with this, fMRI activation for Stop signal, NoGo, antisaccade and response switching/reversing reveals partly overlapping circuits (e.g. 13, 14–20) (see Figure 2A for some examples). Thus evidence from some of these other paradigms will also be considered when motivating a model of the neural circuitry underlying stopping.
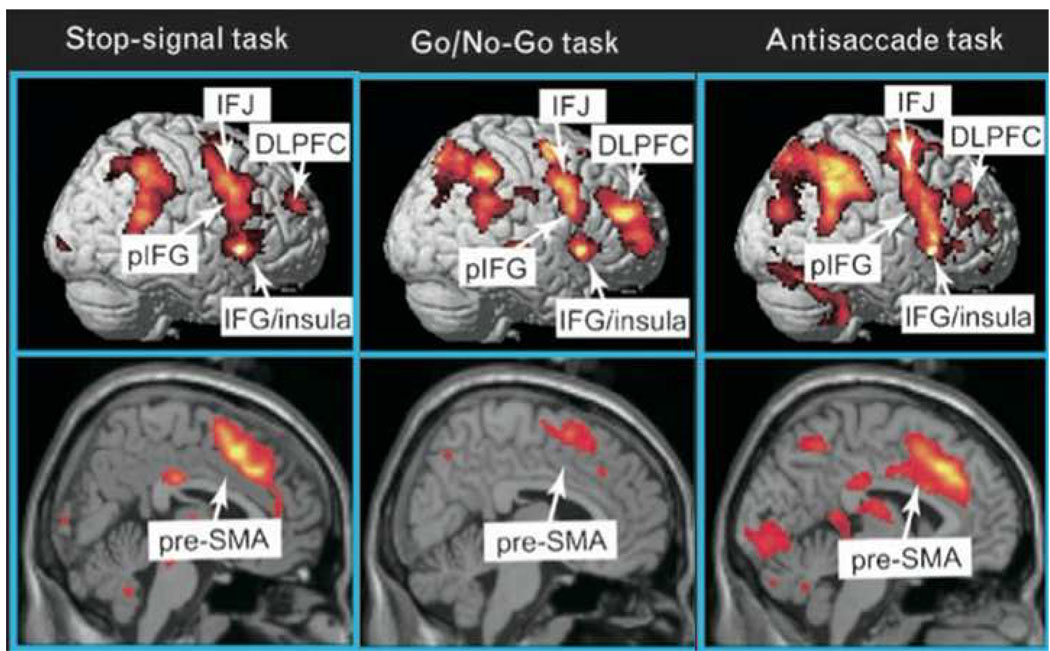
Figure 2.
Functional MRI studies of stop signal and related paradigms. The ‘stopping network’ in the cortex is activated by different control tasks and is predominantly right-lateralized. It includes the presupplementary motor area (preSMA) and the right inferior frontal cortex (rIFC). Right IFC activity is broadly distributed and may reflect an inferior frontal junction (IFJ) component, a more ventral posterior inferior frontal (pIFG) (putatively implementing inhibitory control) and an insula region of unknown function. Maps of the activation during performance of go/no-go, stop-signal and antisaccade tasks were revealed by contrasting no-go vs. frequent-go, stop vs. go, and antisaccade vs. baseline-saccade trials, respectively, see (16) for further details. Copyright permission requested.
Overview of neural systems for reactive stopping
The neural systems underlying reactive stopping in the stop signal paradigm have been reviewed elsewhere (16, 21–24). Here some of the key data are summarized, especially concerning humans, and evidence is updated in light of recent developments. In brief: Sensory information about the stop signal is quickly relayed to the prefrontal cortex, where the stopping command is presumably generated. Two broad regions of the prefrontal cortex are apparently critical for stopping behavior – the right inferior frontal cortex (rIFC), and the dorsomedial frontal cortex (especially the presupplementary motor area, preSMA). These two regions appear to work together to send a stop command to intercept the Go process, via the basal ganglia (Figure 3). The consequence could be suppression of basal ganglia output with downstream inhibitory effects on the primary motor cortex (M1).
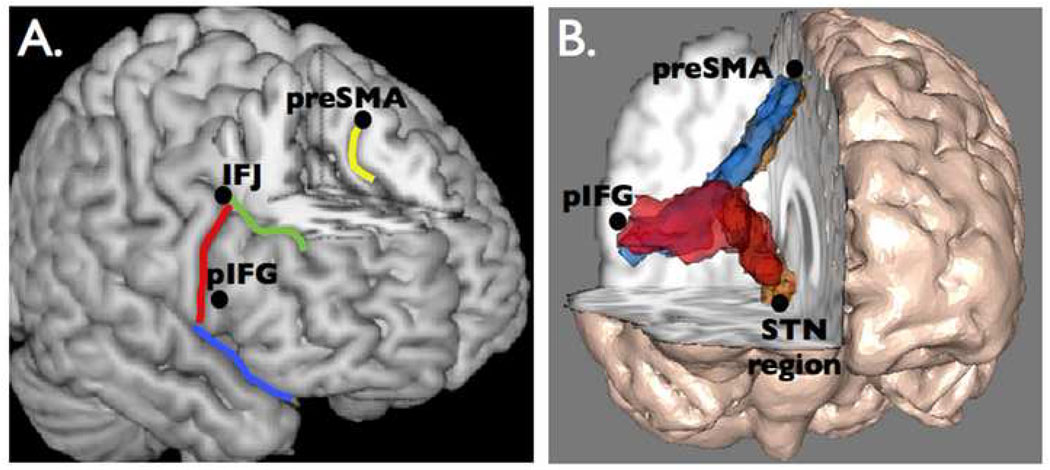
Figure 3.
The brain network for reactive stopping. A. Regions that are critical for stopping in the standard stop signal paradigm. Two regions within the inferior frontal cortex (IFC) are the inferior frontal junction (IFJ) and the posterior inferior frontal gyrus (pIFG). The presupplementary motor area (preSMA) is in the medial surface. B. White-matter tractography using diffusion tensor imaging reveals a three-way network in the right hemisphere between nodes that are critical for stopping action (31). Copyright permission requested.
In many task and real-world scenarios, this ‘stopping network’ is probably highly integrated with valuational and mnemonic functions in other sectors of the prefrontal cortex, such as orbital frontal, and dorsolateral sectors, in both the right and left hemispheres; however, it appears that the standard (and simple) stop task may not require the integrity of these other sectors. This is perhaps because it represents a relatively ‘pure’ version of inhibitory control.
The right inferior frontal cortex
The right inferior frontal cortex (rIFC) referred to here corresponds to areas of lateral prefrontal cortex that are anterior to the precentral sulcus and inferior to the inferior frontal sulcus (Figure 3A). This encompasses pars triangularis, pars opercularis, and some of pars orbitalis. This is coextensive with Brodmann Areas 44, 45 and 47. In one study, damage to the rIFC in humans impaired stopping, whereas left hemisphere damage did not (25) (also see 26) [but see (27) for evidence that left frontal damage can affect Go/NoGo performance]. The importance of the rIFC for stopping in the stop signal task has been confirmed by three studies with Transcranial Magnetic Stimulation (TMS) using the ‘virtual lesion’ approach (i.e. disruptive stimulation followed by behavioral testing) (28–30).
While the rIFC is evidently important for the behavioral act of stopping, its functional role remains to be clarified: It might be critical for inhibitory control itself – for example by projecting directly, or indirectly, to the basal ganglia to block the incipient response (31, 32); and/or it may be critical for attentional monitoring/detection of the stop signal (33–35). A recent study used TMS to directly test the inhibitory vs. attention accounts (30). TMS was used to disrupt the rIFC posterior inferior frontal gyrus (pIFG) region and, in a different session, a more dorsal IFC region known as the inferior frontal junction (IFJ) (Figure 3A). While disruption of both regions affected the speed of stopping (SSRT), it did so by affecting different processes. The results suggested that the right IFJ implements attentional detection while the more ventral sector of rIFC (the posterior inferior frontal gyrus region) implements the inhibitory control. This is highly consistent with a recent fMRI study (36). Thus, different sectors of right IFC could implement both attentional monitoring/detection and inhibitory control functions. This makes sense on the view that a subject needs to detect a signal in the environment in order to stop motor output. Thus a putative right ventral fronto-parietal circuit braker for stimulus driven attention (37) could be closely coupled with a mechanism for rapid inhibitory control.
Other recent evidence supports an inhibitory control function for the rIFC. Electrocorticography recordings from the surface of the brain revealed activity increases in the inferior frontal gyrus at around 150 to 300 ms following the stop signal, especially in the beta frequency band (18 to 30Hz) (32). Notably, this beta band response was greater for successful than failed stop trials. The timing of the response is consistent with an inhibitory control function that occurs within the time-scale of the behavioral stopping process (SSRT). The observation of increased activity in the beta band is consistent with the possibility of long-range functional coupling with the basal ganglia to implement the stop (discussed below).
Other recent evidence for the importance of the rIFC for inhibitory control comes from studies with a paired-pulse TMS paradigm. In this technique, one coil was held over the rIFC and another over the hand area of primary motor cortex (M1). On each trial there was either an M1 pulse alone (with the motor evoked potential recorded from the hand muscles with electromyography) or an M1 pulse preceded by a rIFC pulse (with a very short delay, e.g. 8 ms). If the rIFC has an inhibitory effect on M1 then the size of the motor evoked potential recorded from the hand should be smaller when rIFC stimulation precedes M1, than when there is M1 stimulation alone. This is what was found in two studies in which subjects needed to cancel an initiated action in favor of making another (13, 38). These results are very difficult to reconcile with an attention-only account of rIFC function.
In monkeys, lesions, microstimulation and recording with the related Go/NoGo task all point to the importance of a possibly homologous region (the inferior frontal convexity) for inhibiting motor responses (39–42). These results complement a classic finding that macroelectrode stimulation of the right inferior frontal gyrus produces motor arrest (43).
Taken together, these studies clearly show that the rIFC’s role in behavioral stopping cannot simply be interpreted in terms of an attentional function. Instead, the literature continues to support the proposition that the rIFC implements an inhibitory control function, likely in addition to attentional monitoring/detection (which could be a function of the more dorsal IFJ region in IFC).
Below it will be shown that reactive stopping likely ‘targets’ the basal ganglia, specifically the subthalamic nucleus. However, under some circumstances stopping could be implemented via inputs to the striatum, and perhaps directly to M1 (38). The pathway used may relate to whether action control is purely reactive to an environmental change, or proactive as with anticipating the need for control.
The dorsomedial frontal cortex
Many studies in humans (lesion, TMS and fMRI) and monkeys (recording and stimulation) also point to a role for the dorsomedial frontal cortex, especially the preSMA region, in stopping behavioral responses in the stop signal, Go/NoGo and other, related, behavioral paradigms (14, 35, 44–49) (also see reviews by 21, 50, 51). The preSMA is a region of dorsomedial frontal cortex. It is in the medial wall of the superior frontal gyrus, dorsal to the anterior cingulate, and anterior to the supplementary motor area proper (Figure 3).
Connectivity studies, using tract tracing in the monkey and diffusion tensor imaging in humans, show that the preSMA is connected with the rIFC and also with the basal ganglia input nuclei – the striatum and subthalamic nucleus (31, 52, 53) (Figure 3B). The findings about the functional importance of both preSMA and rIFC, and their structural connectivity, complements classic macrostimulation studies in humans describing this ‘anterior SMA’ region as a ‘negative motor area’ – a region at which stimulation produces arrest of manual movements and speech (43, 54).
Yet the precise functional role of preSMA and its relation to rIFC in these forms of behavioral control is still unclear. A recent recording study in monkeys performing a manual stop signal task concluded that neurons in the preSMA/SMA may represent the motivation for specification actions rather than controlling whether or not the movement is made (11). Moreover, preSMA activation is evident in fMRI studies examiningg preparation to stop rather than stopping reactive, suggesting it may be more ‘set’ related (55, 56).
There have been numerous conceptual accounts for the functional role of the preSMA including ‘selecting superordinate sets of action-selection rules’ (57), motivation (11), conflict resolution/monitoring (14, 58) and modulating response thresholds (59). These accounts might predict that preSMA generates a control signal and rIFC implements the inhibitory control. Indeed a recent study using paired pulse TMS found evidence for this (38). When a TMS pulse was delivered over preSMA, the inhibitory effect on M1 was observed at 125 ms after a signal to behaviorally control action; whereas when a TMS pulse was delivered over rIFC the inhibitory effect on M1 was observed at 175 ms. However, a study using Granger Causality analysis of fMRI data came to the reverse conclusion – i.e. that rIFC precedes the preSMA (35). Yet such causality methods are not well-validated with fMRI data. Indeed a recent combined intracranial recording/fMRI study in rodents concluded that, because hemodynamics varied so much between regions, the question of the relative timing of recruitment of regions was unaswerable with fMRI functional connectivity (60).
The subthalamic nucleus
The subthalamic nucleus (STN) input structure of the basal ganglia is a good candidate for a neural system for stopping action. First, the STN receives direct input (no interventing synapses) from the cortical foci reviewed above, viz. preSMA and rIFC (31, 52). This means that the cortical regions could quickly activate the STN via a so-called ‘hyperdirect pathway’ (61) with cortical-STN effects occuring in less than 10 ms (62, 63). Second, the STN broadly excites the globus pallidus pars interna which increases the neural inhibition on thalamocortical output. Thus it has been conjectured that a massively connected STN leads to ‘widespread pulses’ that could inhibit basal ganglia output and the motor system generally (64). This broad effect of the STN on basal ganglia output has motivated the view that the hyperdirect pathway is recruited as part of movement preparation to briefly ‘clear’ the response system so that the appropriate movement can then be made through direct pathway selection (65).
Functional/behavioral studies also point to the STN as important for stopping. In a high resolution fMRI study, activation of the STN region was found on successful stop trials (66) (also see 67). Modulation of the STN with deep brain stimulation in patients with Parkinson’s disease, affects SSRT, NoGo comission error rates and antisaccade performance, although not always in consistent directions (68–72). A single unit recording study found neurons in monkey STN that increased their response on both switch and NoGo trials (73). In a rodent study, lesions to the STN produced a generalized stopping impairment for the stop signal task (74) (and see review by 75). A local field potential recording study from the STN in human patients showed increases in the beta frequency band for NoGo compared to Go trials (76). This is especially interesting insofar as enhancement of beta band power was observed in the right IFC for stop trials (32) (see above). Taking these results together, it is possible that inhibitory control is mediated via a right IFC – preSMA – STN structurally-connected functional circuit operating in the beta band (~16 Hz). Consistent with this possiblility, simultaneous cortical and STN recording studies in humans have shown ‘entrainment’ of STN neurons by the cortex in the beta band (77).
Other, indirect, evidence for the importance of the STN in stopping comes from the observation that when reactive stopping occurs it has global effects on the motor system. For example, when subjects stopped a thumb movement there was significant, below baseline, suppression of corticomotor excitability of the tibia muscles of the leg (78). As the leg was not relevant for task performance, this implies there is a brain mechanism for stopping that has global effects on the motor system (such as the STN with its massive output to the GPi). Similarly, behavioral studies have shown that stopping one effector leads to long delays when continuing with another (79, 80), also consistent with a global stop command.
The striatum
Another way that reactive stopping could be implemented is via inputs to the striatum. This is a fronto-striatal system for control rather than a fronto-subthalamic one.
For the stop signal task, fMRI studies do show striatal activation (e.g. 31, 66). But this could reflect the slower speed of the Go process on Stop than Go trials (66), or feedback concerning a successful outcome, or preparation for stopping. Consistent with the last, an fMRI study which examined activation on Go trials in a stop signal task found a parametric increase of striatal activation the more stopping was anticipated (81). This points to the possible importance of the striatum for proactive rather than reactive stopping, and will be further discussed below. Other studies have observed that patients with basal ganglia damage are slow to stop their responses (26), but that could relate to generalized damage (including to the STN). Similarly, patients with Parkinson’s disease are slow to stop (82) but that is also likely to reflect alterations in general basal ganglia function. Lesions to the medial striatum in rodents did lead to overall longer SSRT, but the results were complex with better stopping at earlier delays, increased Go RT and increased omissions errors (83). Two stop signal studies of patients with manifest Huntington’s disease did not reveal any deficit in stopping (84), yet at that stage of the disease up to 50% of the striatum has been lost.
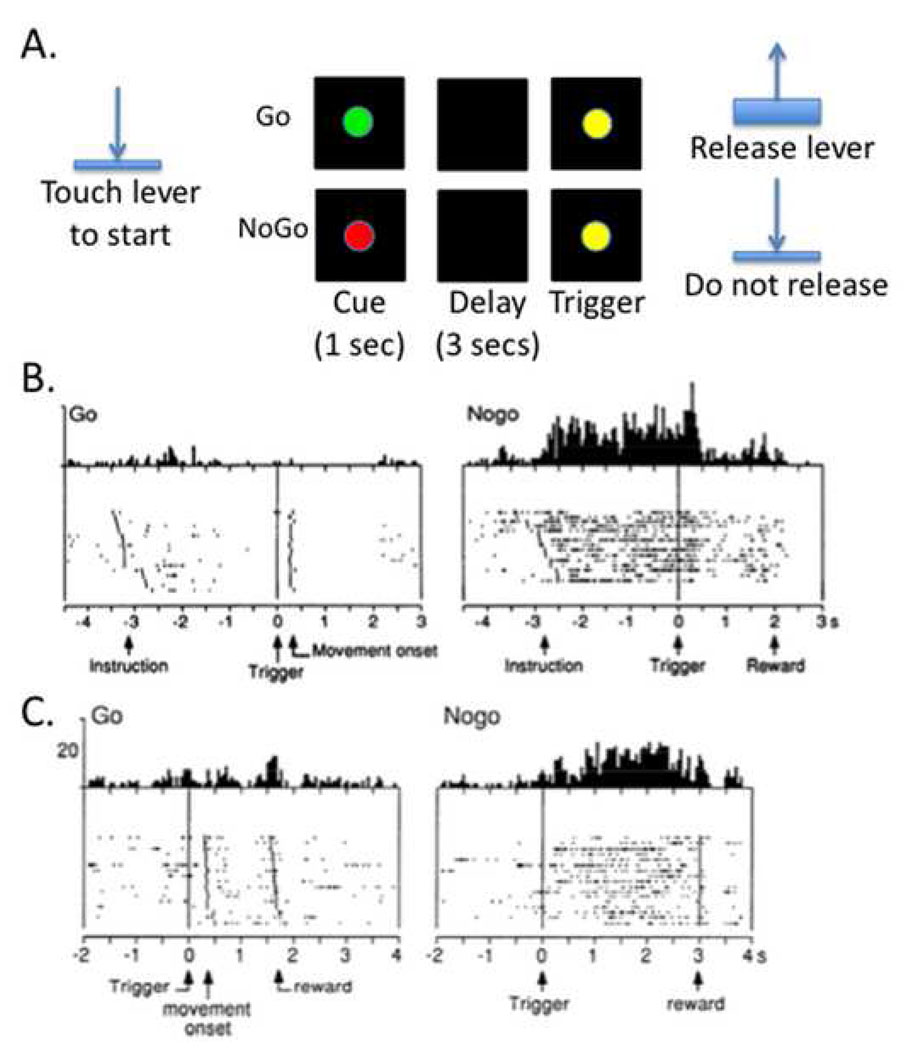
The striatum has been implicated in other paradigms such as Go/NoGo and antisaccade tests. For example, many functional and structural MRI studies point to a fronto-striatal ‘circuit’ underlying response inhibition in the Go/NoGo paradigm (85–87). In a neurophysiological experiment, striatal activity was recorded while monkey performed a Go/NoGo task (88) (Figure 4A). Each trial began with the animal holding down a lever. A red (NoGo) or Green (Go) stimulus then appeared. The animal had to wait several seconds for a subsequent trigger stimulus before movement (Go) or continued non-movement (NoGo) led to reward. Two important types of neural response were observed on NoGo trials: ‘preparatory’ activity between the NoGo cue and the trigger stimulus (Figure 4B); and sustained activity after the trigger stimulus (Figure 4C). These two kinds of neural responses suggest that the striatum could be important for preparing to stop a response (under working memory) and then implementing inhibitory control over a sustained period (perhaps selectively, see below). These findings are complemented by studies showing that neurons in the caudate nucleus are active on antisaccade trials (89) and that microstimulation of caudate neurons improves antisaccade performance (90). These latter two studies are informative because these antisaccade conditions require selectivity – the subject has to inhibit the reflexive saccade to the target and issue a new antisaccade. Below it will be argued that the striatum may be especially important for selective rather than global stopping.
Figure 4.
Neurophysiological recording from the striatum shows the signature of proactive inhibitory control. A. Monkeys were studied with a symmetrically reinforced delayed Go/NoGo task (88). The trial began with a lever press. A Go or NoGo cue was then presented. After an interval of 2.5 to 3.5 secs, a trigger stimulus was presented, requiring the animal to either release the lever or not release it to get reward. B. Some neurons in the striatum showed a pattern of sustained activity between the NoGo instrution and the trigger stimulus (perhaps involved in preparing to inhibit movement) C. Other neurons showed sustained activity between the trigger stimulus and the reward (perhaps implementing the movement inhibition itself). Copyright permission requested.
Taken together, these studies do point to the importance of the striatum for stopping. However the scenarios in which the striatum is engaged seem more related to proactive and/or selective control rather than reactive and global stopping.
The primary motor cortex
The primary motor cortex is the last cortical site before movement commands descend the corticospinal tract. The pyramidal cells in layer 5 that generate the corticospinal volleys are embedded in a network of local connections including many GABAergic inhibitory interneurons. Generating a movement requires driving the pyramidal cells as well as removing the GABAergic inhibition (91).
In behavioral stop signal or Go/NoGo studies, the impending response on Go trials can be ‘visualized’ as a ramping up of corticomotor excitability, measured using TMS of the primary motor cortex and concurrent electromyography (92, 93). The impending response is also evident in desynchronization in the alpha/beta frequency band of local field potential recordings from electrocorticography (32, 94). Stopping the initiated response also has observable effects on M1 (reviewed by 95). TMS studies using a paired-pulse protocol reveal the signature of increased GABAergic inhibition of the M1 effector representation on stop or NoGo trials (92, 93, 96). Futher, electrocorticography of M1 shows a reduction of alpha/beta desynchronization (i.e. a relative increase of synchronization) on stop trials (32).
In standard stop paradigms it appears as if reactive stopping leads to a ‘global’ effect on the motor system. As we saw above, a TMS study with the stop signal paradigm showed that stopping a finger movement was associated with a suppression of the task-irrelevant leg (78) (also see 92, 96). This could be the ‘TMS signature’ of a stopping command generated by inputs to the STN with global downstream effects on M1.
Summary
Reactive stopping depends on a fronto-basal-ganglia network in the right hemisphere. The network includes the preSMA, the IFC, the basal ganglia and M1. Within the IFC, two regions seem important – the inferior frontal junction (IFJ) and the posterior inferior frontal gyrus (pIFG). When a stop signal occurs, the IFJ may implement an attentional detection function, while the pIFG may implement inhibitory control. The pIFG may implement inhibitory control via inputs to the basal ganglia. The relative functional roles of the preSMA vs. pIFG in reactive stopping are not yet clear. Some evidence suggests the preSMA is recruited before the pIFG; so it may be involved in setting up and/or triggering the IFG response. Further research is needed.
Reactive stopping has global effects on the motor system. This may be because it uses the fast hyperdirect pathway via the STN, with broad effects (Figure 6A–C). The striatum may be more important for preparing to stop, and for stopping selectively, than stopping reactively with global effects. However, as we will see below, reactive stopping may also be selective, and the striatum could be implicated in this form.
Figure 6.
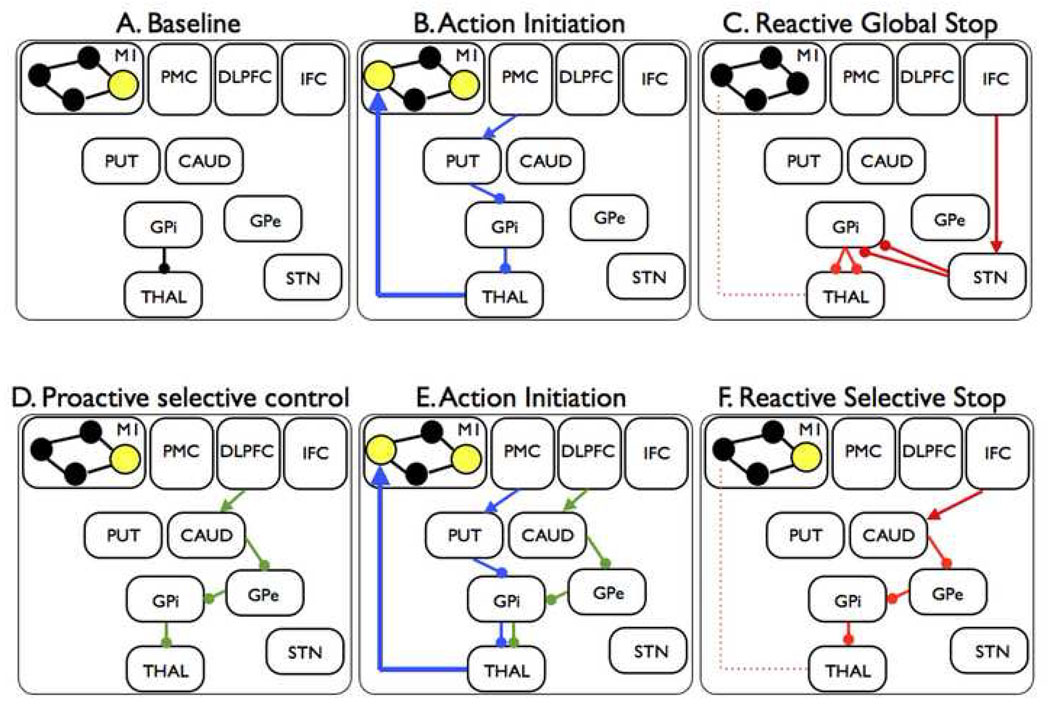
Hypothetical fronto-basal-ganglia circuits for global and selective stopping. A. When the subject's hand is at rest, the GPi is tonically inhibiting thalamocortical output to hand representations so that these are only weakly active (small filled circles). By contrast, one M1 representation, for example for the speech system, is strongly active (large yellow-filled circle). B. The subject initiates a hand movement using the direct pathway. The PMC activates the putamen, the putamen inhibits the GPi, this removes inhibition from the thalamus and increases drive to the hand area of M1. C. The IFC sends input to the STN via the hyperdirect pathway. The STN has a broad effect on GPi, leading to global suppression of thalamocortical programs, including hand and speech systems. D. Proactive selective control may be set up via the indirect pathway. The DLPFC activates a specific channel of the caudate, the caudate inhibits a specific channel of the GPe, the GPe inhibits a specific channel of the GPi (directly or via the STN) and inhibition of a particular thalamocortical channel is prepared (but perhaps not triggered until stopping is needed). E. Action initiation occurs as for B. above, except it occurs with the proactive selective control system activated. This could lead to slower response emission. F. The indirect pathway may be triggered by the IFC when a stop signal occurs. This leads to suppression of one, but not all, representations in M1. M1 = primary motor cortex; PMC = premotor cortex; DLPFC = dorsolateral prefrontal cortex; IFC = inferior frontal cortex; PUT = putamen; CAUD = caudate; GPi = globus pallidus pars interna; GPe = globus pallidus pars externa; STN = subthalamic nucleus; THAL = thalamus.
This relatively simple right hemisphere network may be all that is required for reactive stopping in standard stop signal, Go/NoGo and other paradigms. However, when stopping is complicated by adding conditional rules (‘Stop if this happens but not if that’), or by the need to prepare to stop, or by the need to stop selectively, then this basic network may be augmented by other brain systems, as will be seen below.
The limitations of reactive stopping as a model of control
Reactive stopping is a useful endophenotype for psychiatry
Clearly much has been learned about the neural architecture of reactive stopping. The convergent findings from different methods and species have motivated the stop signal task and related paradigms as endophenotypes for psychiatric disorders (reviewed by 21, 23, 97, 98, 99). To take some examples, many studies in patients have shown case-control increases in SSRT, as well as functional activation or structural integrity differences within regions such as the rIFC and dorsomedial frontal cortex. These differences are seen in methamphetamine addicts, cocaine addicts and people who abuse alcohol, as well as in ADHD, OCD (including trichotillomania) and eating disorders (see above for reviews). In ADHD, for example, meta-analysis shows that the SSRT effect size is one of the biggest for executive function tasks (100).
Although reactive stopping in these paradigms is generated in artificial laboratory conditions, it evidently has relevance to these real world problems. How can this be? Three explanations are considered. First, a common symptom in psychiatric disorders is impulsivity – and one form of impulsivity relates to motor disinhibition (101). Accordingly, someone with poor reactive stopping ability who feels a motoric urge that is inappropriate may not suppress that urge very well. Second, reactive stopping in the motor domain may use overlapping circuitry with stopping in non-motor domains, such as emotion and motivational control. Good control of emotion and motivation, even by means of a rapid and global mechanism, could be useful. Consider that an emotionally-salient stimulus could trigger a chain of neural and bodily events that may cascade and be self-sustaining – e.g. increased heart-rate could exacerbate one’s anxiety. Having a mechanism to rapidly interrupt the chain could be of high psychiatric relevance [Note: thanks to Josh Berke for making this point]. While a review of the neural circuitry underlying emotional and motivational stopping is beyond the current scope it is interesting to note that there is evidence for overlapping motor and autonomic stopping systems insofar as stopping a motor response has concurent effects on heart-rate (102, 103). It is also important to recognize that the basal-ganglia have different processing circuits with similar organizational principles. All of the striatum, the STN and the pallidum have different sectors for sensorimotor, associative (cognitive) and limbic processing (104). Thus, in the same way that prefrontal input could lead to fast motor stopping via the basal ganglia it could also lead to fast limbic control. A third explanation for the relevance of reactive motor stopping to psychiatry is that reactive stopping taps into brain regions such as the rIFC and preSMA that have much more general purpose functions in decision-making and intelligent behavior (105). Accordingly, hypoplasia or damage to these regions could affect many cognitive domains. This will contribute to psychiatric problems, just as it relates to poor reactive stopping.
When do we stop reactively in the real world?
Reactive stopping may be important in everyday life. One example is preventing oneself from stepping into the street when the light changes color. Another is in sports requiring fast action control, such as stopping and switching movements in response to changing environmental signals. Reactive stopping may even have evolved in a context when stopping and freezing was a key requirement to avoid danger. Yet, the number of scenarios requiring fast stopping, and especially stopping that has global effects on the motor system, is probably limited. Moreover, with the possible exceptions of motor disinhibition and the interrupting-the-chain example (see above) these scenarios bear limited relation to the kind of control problems in impulse control disorders. It seems doubtful that a child with a premonitory urge to tic is using a brain mechanism that reactively stops responses in a global fashion. Tic suppression, if it involves top-down control at all, is likely to be extended over time, and also selectively targeted at a particular urge. The same goes for the control of urges in the context of substance abuse, such as cigarette smoking. Thus, a brain mechanism for rapid, stimulus-driven, reactive stopping of response tendencies, with global effects, seems limited as a model of control. It would be better to enrich the model of stopping so it captures core cognitive processes that are more related to real world demands.
Stopping reactively vs. holding your horses?
One possibility is that the neural architecture for reactive stopping could be recruited during decision making to prevent incorrect response output. This is different from reactive stopping because the control is partial – the response is kept at bay until the decision is made. One theory of decision-making proposes that, in the case of conflict between response tendencies, a control system is used to ‘hold the horses’ on motor output until the decision is made (106). This could prevent impulsive (overhasty) decisions. Evidence that such a mechanism might depend on stopping-related circuitry came from a study in patients with Parkinson’s disease (107). On each trial patients were presented with high or low conflict decisions. It was found that modulation of the STN with deep brain stimulation changed the error rate in the high conflict situation. Apart from ‘hold-your-horses’, this kind of mechanism has also been referred to as ‘braking’ (64), ‘proactive control’ (108), and ‘conflict-induced slowing’ (31). Thus, within a particular trial, the presence of conflict could require a hold-your-horses mechanism to temporarily with-hold response emission rather than to stop it reactively and completely.
Holding your horses is still spur of the moment
Yet the above example still requires control in the spur of the moment. The control may not require reactive stopping, but it requires a punctate process to withhold responding for a fraction of a second while a decision is made. This may somewhat extend the range of real-word scenarios that could require such a mechanism, but not by very much. When a trying-to-abstain nicotine addict is confronted with a stimulus-driven urge to reach for a cigarette, the urge/action control is probably not applied in a one-off punctate fashion. It is likely applied tonically, or at least repeatedly over time, but presumably not in a way that has global effects, as that would interfere with ongoing thought/action/feeling. Similar observations apply for the example of trying to control the urge to tic. Thus, the success of such forms of control seems to depend on keeping one’s goals in mind and using them to target a particular tendency. Insofar as cognitive neuroscience can develop translational behavioral models for impulse control, and insofar as models of motor response control will fit the bill, it appears that such models would be enriched by at least two additional requirements: a) proactivity or advance preparation, and b) selectivity, or control that is targeted at particular response tendencies. The remainder of this article focuses on paradigms of stopping which address these criteria.
Proactive inhibitory control – preparing to stop
Reactive stopping requires completely countermanding the initiated response. By contrast, hold-your-horses is a hypothesized mechanism by which subjects put a ‘brake’ on response tendencies when conflict is detected. Another type of control is referred to here as ‘proactive inhibitory control’. This involves a preparatory step before the response tendency is triggered. This can occur trial-by-trial in response to control cues (109), or at the level of blocks of trials (110) or in a strategic sense when accuracy is favored over speed (59). For example, behavioral performance can be compared in blocks of mixed Go and NoGo trials compared to pure blocks of Go trials. The behavioral manifestation of proactive inhibitory control is that response times are slower. The neural basis of proactive inhibitory control has been investigated with several paradigms (e.g. 108, 111, 112). Here we consider research with stop signal and Go/NoGo tests.
One study addressed proactive control using a conditional stop paradigm (56). Subjects were given a rule that if they initiated (for example) a right button response and a stop signal occurred then they would have to stop (critical direction), but if they initiated a left button response and a stop signal occurred they could ignore it (noncritical direction). Thus as soon as the Go (choice) signal occurs, and it is the critical direction, subjects can potentially use proactive control to prepare to stop. It was found that RT was longer for Go critical than noncritical trials and those subjects who slowed more were able to stop more quickly. Using fMRI it was found that a network for ‘reactive stopping’ (i.e. rIFC, preSMA, and the STN region), was more activated the greater the degree of slowing on Go critical trials. These results thus suggest that the brain network for reactive stopping could be ‘prepared’ in advance, i.e. control is proactive. Similar findings of proactive activation of the ‘stopping network’ – including some or all of the preSMA, rIFC and the STN region – have been reported for variants of the stop signal task (81, 109, 113), and the Go/NoGo task (114). Notably, in some of these studies there was also DLPFC and striatal activation. This likely reflects the increased working memory demands when preparation is needed. The striatal activation could also reflect use of an indirect fronto-basal-gangla pathway for proactive selective stopping. This will be considered below.
Other evidence for the neural systems underlying proactive control came from a study examining STN stimulation in humans (68). They key behavioral index was the difference in RT for mixed blocks of Go and Nogo vs. pure blocks of Go trials. When the patients were On stimulation, this slowing (or braking) effect was found to be smaller and rIFC activation was less than when patients Off stimulation. Thus STN-stimulation may have altered the ‘stopping network’, leading to a poorer ability to apply proactive inhibitory control. These findings could possibly explain the increased impulsivity that comes with STN-DBS in some patients (115).
Considered together, these studies suggest that regions important for reactive stopping, including the preSMA, the rIFC, and the STN, are also activated in situations in which NoGo or Stop signals are anticipated. Since recruitment of this stopping network is also often accompanied by slowing of response emission (55, 56, 81, 113) (also see 116, 117) the possibility exists that the stopping network can act as a ‘brake’ on motor output (without stopping it completely). Further, if the ‘stopping network’ is preactivated by preparing to stop, then stopping should be quicker when it is needed. Two studies have shown that this is in fact the case (56, 109).
Is proactive inhibitory control global or selective? The examples above probably relate mainly to the global case, chiefly because selectivity is not required. For example, if Go trials require a choice response, and a subject slows down in a run of mixed Go and Stop trials, then the slowing down presumably reflects a general effect on response tendencies rather than on a particular response tendency. Indeed, some computational theories of the speed-accuracy trade-off propose that increased accuracy can be achieved by modulating cortical input to the STN (reviewed by 59). However, proactive selective inhibitory control may also be possible, as seen in examples below.
Summary
The brain network that is used for reactive stopping – the preSMA, right IFC and the STN – may also be used to prepare to stop. The behavioral consequence of proactive inhibitory control is that subjects slow down, and if stopping is required, they may stop more quickly. It is not yet clear whether proactive inhibitory control for these sorts of paradigms has global or selective effects on the motor system.
Selective inhibitory control
Distinguishing selective from global mechanisms for stopping
We saw that the STN is involved in both stop signal and NoGo paradigms. We also saw that the STN may lead to widespread pulses that could inhibit basal ganglia output generally. Behavioral studies and TMS studies with the stop signal paradigm are consistent with the idea that such global suppression has functional consequences in the motor system. Other evidence, reviewed above, points to a role for the STN in ‘hold your horses’ and also in proactive inhibitory control. Yet, a widespread pulse from the STN appears unsuitable for situations in which the subject is required to stop selectively.
It is important to distinguish selective stopping in the behavioral sense from the mechanistic sense. Behaviorally selective stopping could be achieved by stopping one response and continuing to make another – yet this could be implemented with a global stop mechanism followed by a restart of the new response. Rather than this, is mechanistically selective stopping possible?
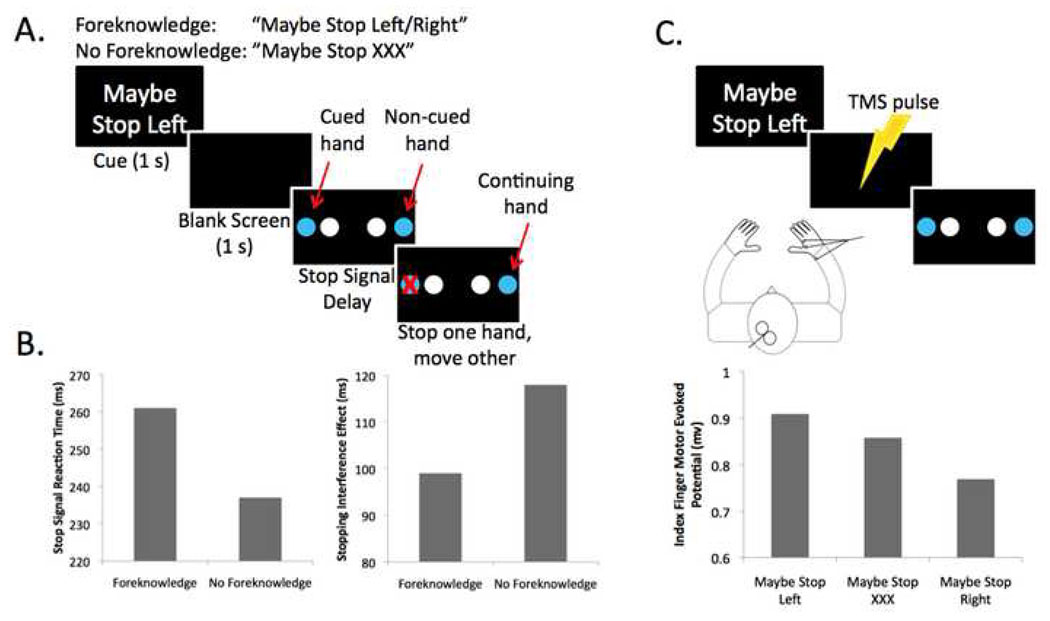
We tried to dissociate mechanistically global and selective stopping with a novel version of the stop signal paradigm (79) (Figure 5A). On each trial participants initiated a coupled response with fingers of both hands, and then, when a stop signal occurred, they tried to stop one response while continuing with the other one. This design allows a measurement of the selectivity of the stopping in terms of the degree of interference that is produced in the alternative (non-stopped) response – we refer to this as the ‘stopping interference effect’. We compared a condition where a stopping goal was provided of which response(s) may need to be stopped compared to a condition where no stopping goal was provided. To do this, we presented a cue with the stopping goal (“Maybe Stop Left” or “Maybe Stop Right”) or a cue without a specific stopping goal (“Maybe Stop XXX”). When a stopping goal was provided the stopping interference effect was reduced while stop signal reaction time was increased (Figure 5B). Thus, when the stopping goal was provided reactive stopping was more selective, but stopping was also slower. The slower stopping may relate to use of the so-called ‘indirect pathway’ of the basal ganglia which has more synapses than the hyper-direct pathway.
Figure 5.
Behavioral and TMS studies of selective stopping. A. The selective stopping task has stopping goal and no stopping goal conditions. Each trial begins with a cue providing a stopping goal (foreknowledge) or none (no foreknowledge). This was followed by a blank screen. A Go stimulus was then presented, requiring the subject to initiate a bimanual response with index fingers or middle fingers of each hand. On a minority of trials, a visual stop signal (red ‘X’) occurred and the subject tried to stop the indicated hand, while continuing with the other hand. B. When a stopping goal is provided (foreknowledge), the speed of stopping, SSRT, is longer, while the degree of slowing of the continuing hand is less (79). Taken together this pattern of data suggests that stopping without a stopping goal (or with less information about what to stop) may recruit a mechanism with more global effects, but which is also quicker. C. For the same paradigm, TMS was delivered to left M1 with electromyography recorded from the right hand. The level of corticomotor excitability was significantly less when subjects anticipated they would have to stop the right hand – consistent with the possibility of proactive selective inhibitory control (126).
Another paradigm in which mechanistically selective stopping may occur is the antisaccade test (where a reflexive saccade must be stopped while a new saccade is generated). In this task, there is simply not enough time to stop all response tendencies and then initiate a new one. Mechanistically selective stopping could also be used in other paradigms such as the Eriksen Flanker, and Simon tests, but the evidence for this is less clear.
Selective stopping via the indirect pathway of the basal ganglia
Mechanistically selective stopping may be implemented by the indirect pathway of the basal ganglia. One form of this is a projection from the striatum to the GPe and then to the GPi (118). The termination pattern of striatal neurons onto the GPi, and from GPe to GPi has a very focused effect (unlike, for example the effect of the STN on the GPi which is very diffuse). Thus the striatum – GPe – GPi pathway offers a means to selectively control a particular response tendency, consistent with many conceptualizations (65, 119). A puzzle however is that the other (more standard) form of the indirect pathway is a projection from the striatum to the GPe to the STN and only then to the GPi – and, notably, this standard form itself includes the STN. If the STN sends a widespread pulse to the GPi this would appear to be ill-suited to selective stopping. It is possible that the standard pathway is not used in the circumstance when selective stopping is required, or that it corresponds to a more specific set of STN neurons with more specific effects on GPi than does the cortico-STN-GPi pathway (for review of these complex issues about the indirect pathway of the basal ganglia see 120). Regardless of the precise way in which selective stopping may be implemented in the basal ganglia, circuitry considerations motivate the indirect pathway via the striatum as a more selective control mechanism than the cortico-STN one.
To reprise some functional evidence for the importance of the striatum for selective control: In one study, neurophysiological recordings were made from the caudate nucleus during an antisaccade task (89). This showed that some neurons specifically increased their firing rates for antisaccades, but not for prosacades. It was postulated that the suppression of the eye movement on antisaccade trials was due to activation of the indirect pathway of the basal ganglia, with suppressive effects on the superior colliculus. For a similar result using microstimulation in the caudate see (90). These findings make the prediction that lesions to the indirect pathway of the basal ganglia should lead to saccade suppression deficits. One way this idea can be tested in humans is to study patients with early stage Huntington’s disease as they may primarily have lost striatal-pallidal projections in the indirect pathway (121, 122). Indeed such patients have striking deficts on antisaccade trials (123, 124).
Top-down fronto-striatal input for proactive selective inhibitory set
What determines whether one stops using a putative global/hyperdirect pathway vs. a more selective one? One factor may be the amount of information one has about what to stop. Another factor could be whether one is required to use that information. In a typical stopping experiment, the stop signal is infrequent, and it appears after the response has been initiated. Thus, stopping is an emergency. The easiest thing for a subject to do would be to use the putative hyperdirect pathway. If this does have a global effect on the motor system that won’t matter. However in circumstances where the subject has more time or when selectivity of stopping is a key performance requirement, the selective stopping pathway could be used.
Using the selective stopping system may be especially likely if the selective stopping pathway has been primed. Such ‘priming’ could correspond to a prefrontally-mediated imposition of inhibitory set over the striatum. For this top-down biasing to occur the subject must have specific information in working memory of what to stop and the subject must use this information. This concept of ‘top down inhibitory set’ complements earlier conceptions of the functional role of the striatum in ‘response set’. For example, Robbins and Brown (125) wrote: “The striatum functions at an early stage of response selection to constrain, or weight, potential response tendencies. We refer to this process as ‘response set’ which we have previously defined as the prior assignment of the probability of selection from the repertoire of available responses”.
Here the notion of ‘response set’ is extended to ‘inhibitory set’. Accordingly, when particular response tendencies may need to be controlled, the striatum could function to weight, or constrain, potential tendencies – not just by ‘priming’ those response channels that may need to be activated, but by suppressing those channels which might need to be stopped.
We studied how such stopping goals are set up and how they are proactively deployed to target specific response tendencies using TMS and concurrent electromyography (126) (Figure 5C). TMS has the great advantage that it can reveal changes in the motor system before any behavior is initated. As before, we used a design in which subjects were given a stopping goal (cue) “Maybe Stop Left” or “Maybe Stop Right” followed by a Go stimulus (move two hands together), followed sometimes by a stop signal (requiring them to stop one hand and to continue with the other). We delivered TMS over left M1 and recorded motor evoked potentials from the right hand. Importantly, we measured the excitability of the right hand in the interval between the cue (stopping goal) and the Go response. We found that corticomotor excitability for the right hand was reduced when the cue indicated it might need to be stopped (‘Maybe Stop Right’) compared to when it would not need to be stopped. This shows that having a goal of what response may need to be stopped in the future is accompanied by applying advance control onto a specific motor representation.
A speculative model of proactive selective inhibitory set
At a neural systems level, proactive and selective inhibitory set may be instantiated by a fronto-striatal circuit (Figure 6D–F). The evidence implicating the striatum has been reviewed above. Regarding the frontal cortex, the key region may be the dorsolateral PFC (DPFC) – specifically the middle frontal gyrus in humans. What is the evidence for this?
First, the DLPFC is key for working memory (reviewed by 127, 128–130). Second, stopping goals are a form of working memory – and consistent with this, there is DLPFC activation in stop signal or Go/NoGo paradigms with increased working memory load (56, 109, 131). There is also preparatory set activity in DLPFC in an antisaccade task (132) (also see 133), and pharmacological manipulation of this region (with noradrenergic antagonists) impairs response inhibition (134) just as it does working memory (135). Third, monkey tract tracing and human diffusion tensor imaging show that the DLPFC is connected with the head of the caudate via a so-called “associative” fronto-striatal-pallidal-thalamic loop (136, 137).
It is proposed that the DLPFC – caudate frontostriatal circuit is used to selectively stop via the indirect pathway. Specifically, the subject’s goal of what to stop may be implemented, at the neural level, in a signal from the DLPFC, which is sent to the striatum to inhibit the GPe, which then removes inhibition from the GPi (via the STN or directly) and which finally increases inhibition of particular cortical response representations (e.g. in M1). In situations in which the subject prepares to stop in the future, this top down inhibitory set could be established over the indirect pathway without actually being implemented (in the sense of affecting motor output) (Figure 6D). This would correspond to a cortical bias over the striatum (top down inhibitory set). Subsequently, a change in the environment could trigger the inhibitory control, so that it does affect particular response initiation/execution, in a selective way (Figure 6F).
This conception of a cortical bias followed by a trigger is motivated by a fronto-basal-ganglia model of eye movement initation (138). It is also motivated by striatal recording studies showing that NoGo striatal responses occur in both a preparatory phase and a trigger phase (Figure 4) (88). This system for top down selective inhibitory set could be implemented to reactively stop a movement, or it could be done in partial mode (leading to selectively slower response emission without cancelation) (Figure 6E).
Although this model of how the stopping goal is used to prepare to stop selectively is speculative, it makes testable predictions about the locus and timing of activity in PFC, striatum, pallidum, STN and M1 during specific behavioral conditions. For example, the model predicts that mechanistically selective stopping will activate the indirect pathway (including the striatum) more than does standard (global) stopping. The model also predicts that whereas standard stopping requires the integrity of a relatively simple cortical network (i.e. preSMA and rIFC), proactive inhibitory control (especially with a selectivity requirement) will additionally recruit DLPFC. In studies of lesion patients one might find that while damage to DLPFC does not affect standard (global) stopping (c.f. 25), it does affect selective stopping because the stopping goal will be disrupted (139).
Summary
If selective stopping is required by the behavioral paradigm then subjects may be able to use a selective mechanism to do it. This selective mechanism could engage the indirect pathway of the basal ganglia, rather than the hyperdirect pathway that is putatively used in the standard case. Proactive selective stopping may also be possible if subjects use stopping goals to prepare the indirect pathway system in advance. This proactive selective control may be setup via an influence of DLPFC over the caudate nucleus, external pallidum and so forth. Thus, a proactive inhibitory set could be used partially (when selective slowing is required) or it could be triggered completely.
Conclusions and Further Questions
Cognitive neuroscience has made progress with behavioral paradigms that require reactive stopping. Accumulating evidence from many research groups clearly points to the critical importance of right IFC, the dorsomedial frontal cortex (esp. preSMA) and the basal ganglia, with downstream effects on M1 (16, 21–24). The identification of this network is leading to efforts that characterize its subcomponents. For example: What are the relative roles of different nodes in the network such as the preSMA vs. the right IFC (38), and the STN vs. the striatum (59)? How do attention and inhibitory control functions map to the network (30, 49)? The cognitive neuroscience advances in this area have also provided some useful endophenotypes for psychiatric research in terms of candidate behavior, brain regions and genetics (21, 98, 99, 140).
Notwithstanding these fruitful developments several considerations suggest reactive stopping is limited as a model for control in both everyday life and psychiatric disorders. First, there are scenarios in which a rapid, punctate, stopping process appear ill-suited. For example, when someone has to tonically control their urge to tic. Second, whereas reactive stopping appears to have global effects on the motor system, many scenarios require selectivity. Third, in everyday life, control is specified according to one’s goals, which are monitored over seconds, minutes or longer, and periodicaly retrieved from long-term memory in particular contexts. Thus, the control must be setup in advance, extended across time and it must be targeted endogenously rather than exogenously at particular tendencies as these emerge. This article motivates a model that meets these two additional requirements by including proactivity (i.e. advance preparation) and selectivity (i.e. control that is targeted at particular response tendencies). Adding these two features creates several kind of stopping paradigms whose features are summarized in Table 1.
Table 1.
Types of control and stopping mechanism discussed in the article. BG circuit = basal ganglia; SSRT = stop signal reaction time; WM = working memory
| Type of Control | |||
|---|---|---|---|
| Reactive | Proactive | ||
| Stopping Mechanism | Global |
BG circuit: Hyperdirect Behavior: Fast SRT/ High interference WM: low Example paradigms standard stop signal some types of Go/NoGo |
BG circuit: Hyperdirect Behavior: Slowed RT when Going (with global effects) WM: high Example paradigms conditional stop signal mixed Go/NoGo vs. pure Go |
| Selective |
BG circuit: Indirect Behavior: Slower SSRT/ Lower interference WM: high Example paradigms Selective stop signal Antisaccade? Ericksen? Simon? |
BG circuit: Indirect Behavior: Slowed RT when Going (selective effects) WM: very high Example paradigms selective stop signal |
|
It appears that some forms of proactivity, such as preparing to stop in stop signal or Go/NoGo paradigms, or favoring accuracy over speed, engage the same brain network that is used for reactive stopping (viz. the preSMA, right IFC and the STN) – but in a partial mode (leading to response slowing) (56, 59, 109). Further studies are needed to verify these findings, and to understand the relative roles of the STN vs. the striatum.
Other research suggests that mechanistically selective stopping is possible. When subjects are given a stopping goal and they use this to prepare to stop a particular response tendency, they can stop one response and continue making another with very little interference (79, 126). Further research is required to examine the neural basis of selective stopping. It is predicted that it will enagage the striatum and the indirect pathway, and it is predicted that it is made possible by a top-down influence from DLPFC. This creates a proactive selective inhibitory set. When reactive selective stopping is needed it could be implemented by the same cortical regions that are important for standard reactive stopping, but with a striatal rather than STN target.
The research summarized here has been focused on motor response control. However, there are fronto-basal-ganglia circuits with a highly similar organization for limbic control (104, 141–143). It is likely that the circuitry principles that govern proactive and selective inhibitory control will also extend to the limbic domain too. For example, top down inhibitory set could be used to implement stopping goals for motivation. The circuitry for this could be highly similar to that for implementing stopping goals for action. Such implementation could occur via the (limbic) indirect pathway, including the ventral striatum/ventral-pallidum, or via the hyperdirect pathway, including the ventral-medial sector of the STN (144). Testing this idea requires developing behavioral paradigms for proactive and selective control of motivation.
Overall, recent findings motivate a richer model of how people control their inappropriate response tendencies. The model provides greater insight into why control could fail in psychiatric or neurological disorders. This may happen for several different reasons, for example because people cannot maintain their stopping goals, or because they cannot implement the inhibitory set, or because they cannot trigger inhibitory control when it is needed. As argued here, these functions may be dissociated to different brain systems. Validating this could have important implications for classifying patients according to different symptoms and for determining treatment.
Acknowledgements
Thanks to Frederick Verbruggen for discussion of some of the ideas expressed here, to Josh Berke, Amy Arnsten and the anonymous reviewers for helpful comments on the manuscript, and to Bill Lanouette for proof-reading. Funding is gratefully received from the Alfred P Sloan Foundation, NIH NIDA Grant DA026452 and NSF Grant 0921168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
The author reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 2.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33:613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piacentini JC, Chang SW. Behavioral treatments for tic suppression: habit reversal training. Adv Neurol. 2006;99:227–233. [PubMed] [Google Scholar]
- 7.Logan GD, Cowan WB. On the Ability to Inhibit Thought and Action: A Theory of an Act of Control. Psych Rev. 1984;91:295–327. [Google Scholar]
- 8.Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci. 2008 doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2007;35:229–238. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- 10.De Jong R, Coles MGH, Logan GD, Gratton G. In Search of the Point of No Return: The Control of Response Processes. JEP-HPP. 1990;16:164–182. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- 11.Scangos KW, Stuphorn V. Medial frontal cortex motivates but does not control movement initiation in the countermanding task. J Neurosci. 2010;30:1968–1982. doi: 10.1523/JNEUROSCI.4509-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- 13.Buch ER, Mars RB, Boorman ED, Rushworth MFS. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci. 2010:1395–1401. doi: 10.1523/JNEUROSCI.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- 15.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chikazoe J Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr Opin Psychiatry. doi: 10.1097/YCO.0b013e3283387a9f. [DOI] [PubMed] [Google Scholar]
- 17.Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 18.Swainson R, Cunnington R, Jackson GM, Rorden C, Peters A, Morris PG, et al. Cognitive control mechanisms revealed by ERP and fMRI: Evidence from repeated task-set switching. J Cognit Neurosci. 2003;15:785–799. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- 19.Heinen SJ, Rowland J, Lee BT, Wade AR. An oculomotor decision process revealed by functional magnetic resonance imaging. J Neurosci. 2006;26:13515–13522. doi: 10.1523/JNEUROSCI.4243-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgson T, Chamberlain M, Parris B, James M, Gutowski N, Husain M, et al. The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain. 2007 doi: 10.1093/brain/awm064. [DOI] [PubMed] [Google Scholar]
- 21.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 24.Schall JD, Stuphorn V, Brown JW. Monitoring and control of action by the frontal lobes. Neuron. 2002;36:309–322. doi: 10.1016/s0896-6273(02)00964-9. [DOI] [PubMed] [Google Scholar]
- 25.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 26.Rieger M, Gauggel S, Burmeister K. Inhibition of ongoing responses following frontal, nonfrontal, and basal ganglia lesions. Neuropsychology. 2003;17:272–282. doi: 10.1037/0894-4105.17.2.272. [DOI] [PubMed] [Google Scholar]
- 27.Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, et al. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J Neurophysiol. 2007 doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- 29.Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, et al. Executive "brake failure" following deactivation of human frontal lobe. J Cogn Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 30.Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1001957107. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, et al. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for response inhibition, attentional capture, error processing. Proc Natl Acad Sci U S A. 107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, et al. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- 37.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 38.Neubert F, Mars RB, Buch ER, Olivier E, Rushworth MFS. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1000674107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakagami M, Tsutsui K, Lauwereyns J, Koizumi M, Kobayashi S, Hikosaka O. A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. J Neurosci. 2001;21:4801–4808. doi: 10.1523/JNEUROSCI.21-13-04801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki K, Gemba H, Tsujimoto T. Suppression of Visually Initiated Hand Movement by Stimulation of the Prefrontal Cortex in the Monkey. Brain Research. 1989;495:100–107. doi: 10.1016/0006-8993(89)91222-5. [DOI] [PubMed] [Google Scholar]
- 41.Iversen SD, Mishkin M. Perseverative Interference in Monkeys Following Selective Lesions of the Inferior Prefrontal Convexivity. Exp Brain Res. 1970;11 doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 42.Hasegawa RP, Peterson BW, Goldberg ME. Prefrontal neurons coding suppression of specific saccades. Neuron. 2004;43:415–425. doi: 10.1016/j.neuron.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Luders H, Lesser RP, Dinner DS, Morris HH, Wyllie E, Godoy J. Localization of cortical function: new information from extraoperative monitoring of patients with epilepsy. Epilepsia. 1988;29 Suppl 2:56–65. doi: 10.1111/j.1528-1157.1988.tb05799.x. [DOI] [PubMed] [Google Scholar]
- 44.Floden D, Stuss DT. Inhibitory Control is Slowed in Patients with Right Superior Medial Frontal Damage. Journal Cognitive Neuroscience. 2006;18:1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- 45.Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of Focal Frontal Lesions on Response Inhibition. Cereb Cortex. 2006 doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- 46.Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36 Suppl 2:155–163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mars RB, Klein MC, Neubert FX, Olivier E, Buch ER, Boorman ED, et al. Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J Neurosci. 2009;29:6926–6931. doi: 10.1523/JNEUROSCI.1396-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America. 2010 doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mostofsky SH, Simmonds DJ. Response Inhibition and Response Selection: Two Sides of the Same Coin. J Cogn Neurosci. 2008 doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 51.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 52.Inase M, Tokuno H, Nambu A, Akazawa T, Takada M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain Res. 1999;833:191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- 53.Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, et al. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chikazoe J, Jimura K, Hirose S, Yamashita K-i, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci. 2009:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with Restraint: What Are the Neurocognitive Mechanisms? J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 59.Bogacz R, Wagenmakers E-J, Forstmann BU, Nieuwenhuis S. The neural basis of the speed-accuracy tradeoff. Trends in Neurosciences. 2010:10–16. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 60.David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, et al. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 2008;6:2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 62.Magill PJ, Sharott A, Bevan MD, Brown P, Bolam JP. Synchronous unit activity and local field potentials evoked in the subthalamic nucleus by cortical stimulation. J Neurophysiol. 2004;92:700–714. doi: 10.1152/jn.00134.2004. [DOI] [PubMed] [Google Scholar]
- 63.Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the corticosubthalamic circuits. J Neurosci. 1998;18:9539–9546. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillies AJ, Willshaw DJ. A massively connected subthalamic nucleus leads to the generation of widespread pulses. Proceedings. 1998;265:2101–2109. doi: 10.1098/rspb.1998.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mink JW. The Basal Ganglia: Focused Selection and Inhibition of Competing Motor Programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 66.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li CS, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ballanger B, van Eimeren T, Moro E, Lozano AM, Hamani C, Boulinguez P, et al. Stimulation of the subthalamic nucleus and impulsivity: release your horses. Annals of Neurology. 2009:817–824. doi: 10.1002/ana.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62:1110–1114. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- 70.Ray NJ, Jenkinson N, Brittain J, Holland P, Joint C, Nandi D, et al. The role of the subthalamic nucleus in response inhibition: Evidence from deep brain stimulation for Parkinson's disease. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 71.van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson's disease. J Cogn Neurosci. 2006;18:626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- 72.Yugeta A, Terao Y, Fukuda H, Hikosaka O, Yokochi F, Okiyama R, et al. Effects of STN stimulation on the initiation, inhibition of saccade in Parkinson disease. Neurology. 74:743–748. doi: 10.1212/WNL.0b013e3181d31e0b. [DOI] [PubMed] [Google Scholar]
- 73.Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-Signal Reaction-Time Task Performance: Role of Prefrontal Cortex and Subthalamic Nucleus. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- 75.Eagle DM, Baunez C. Is there an inhibitory-response-control system in rat? Evidence from anatomical pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, et al. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- 77.Sharott A, Gulberti A, Hamel W, Koeppen JA, Zittel S, Hidding U, et al. Society for Neuroscience. Chicago: 2009. Entrainment of STN neurons by cortical beta oscillations in Parkinson's disease. pp Abstract 567.515/EE551. [Google Scholar]
- 78.Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, et al. Suppression of human cortico-motoneuronal excitability during the Stop-signal task. Clin Neurophysiol. 2009;120:1717–1723. doi: 10.1016/j.clinph.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 79.Aron AR, Verbruggen F. Stop the presses: dissociating a selective from a global mechanism for stopping. Psychol Sci. 2008;19:1146–1153. doi: 10.1111/j.1467-9280.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- 80.Coxon JP, Stinear CM, Byblow W. Selective inhibition of movement. J Neurophysiol. 2007 doi: 10.1152/jn.01284.2006. [DOI] [PubMed] [Google Scholar]
- 81.Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp. 2005;25:336–344. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gauggel S, Rieger M, Feghoff TA. Inhibition of ongoing responses in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:539–544. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- 84.Aron AR. Unpublished PhD dissertation. Cambridge University; 2003. The neural basis of inhibitory mechanisms in executive function. [Google Scholar]
- 85.Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom NE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cog Neuro. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 86.Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 87.Kelly AMC, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur J Neurosci. 2004:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 88.Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol. 1992;68:945–960. doi: 10.1152/jn.1992.68.3.945. [DOI] [PubMed] [Google Scholar]
- 89.Ford KA, Everling S. Neural activity in primate caudate nucleus associated with pro- and antisaccades. Journal of Neurophysiology. 2009:2334–2341. doi: 10.1152/jn.00125.2009. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe M, Munoz DP. Saccade suppression by electrical microstimulation in monkey caudate nucleus. J Neurosci. 2010:2700–2709. doi: 10.1523/JNEUROSCI.5011-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- 92.Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol. 2006 doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- 93.van den Wildenburg W, Burle B, Vidal F. Mechanism and Dynamics of Cortical Motor Inhibition. Journal of Cognitive Neuroscience. 2008 doi: 10.1162/jocn.2009.21248. [DOI] [PubMed] [Google Scholar]
- 94.Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 2005;26:347–355. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 95.Stinear CM, Coxon JP, Byblow WD. Primary motor cortex and movement prevention: where Stop meets Go. Neurosci Biobehav Rev. 2009;33:662–673. doi: 10.1016/j.neubiorev.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 96.Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol. 2002;88:333–338. doi: 10.1152/jn.2002.88.1.333. [DOI] [PubMed] [Google Scholar]
- 97.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 98.Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW. CNTRICS Final Task Selection: Executive Control. Schizophr Bull. 2009;35:115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- 100.Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 101.Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- 102.Jennings JR, Brock K, Vandermolen MW, Somsen RJM. On the Synchrony of Stopping Motor-Responses and Delaying Heartbeats. Journal of Experimental Psychology-Human Perception and Performance. 1992;18:422–436. doi: 10.1037//0096-1523.18.2.422. [DOI] [PubMed] [Google Scholar]
- 103.Jennings JR, van der Molen MW, Tanase C. Preparing hearts and minds: cardiac slowing and a cortical inhibitory network. Psychophysiology. 2009;46:1170–1178. doi: 10.1111/j.1469-8986.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 104.Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 105.Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Frank M. Hold you horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006 doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: Impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 108.Jaffard M, Longcamp M, Velay J, Anton J, Roth M, Nazarian B, et al. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage. 2008:1196–1206. doi: 10.1016/j.neuroimage.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 109.Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci. 2009;29:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. J Exp Psychol Hum Percept Perform. 2009;35:835–854. doi: 10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, et al. Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci U S A. 2008;105:17538–17542. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boulinguez P, Ballanger B, Granjon L, Benraiss A. The paradoxical effect of warning on reaction time: demonstrating proactive response inhibition with event-related potentials. Clin Neurophysiol. 2009:730–737. doi: 10.1016/j.clinph.2009.02.167. [DOI] [PubMed] [Google Scholar]
- 113.Zandbelt B, Van Buuren M, Gladwin TE, Hoogendam R, Kahn S, Vink M. Society for Neuroscience. Washington DC: 2008. Brain regions involved in response inhibition are also activated during anticipation of inhibition. [Google Scholar]
- 114.Hester RL, Murphy K, Foxe JJ, Foxe DM, Javitt DC, Garavan H. Predicting success: patterns of cortical activation and deactivation prior to response inhibition. J Cogn Neurosci. 2004;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- 115.Voon V, Kubu C, Krack P, Houeto JL, Troster AI. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord. 2006;21 Suppl 14:305–327. doi: 10.1002/mds.20963. [DOI] [PubMed] [Google Scholar]
- 116.van Gaal S, Ridderinkhof KR, Scholte HS, Lamme VA. Unconscious activation of the prefrontal no-go network. J Neurosci. 30:4143–4150. doi: 10.1523/JNEUROSCI.2992-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Gaal S, Ridderinkhof KR, Fahrenfort JJ, Scholte HS, Lamme VA. Frontal cortex mediates unconsciously triggered inhibitory control. J Neurosci. 2008;28:8053–8062. doi: 10.1523/JNEUROSCI.1278-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bolam JP, Smith Y. The striatum and the globus pallidus send convergent synaptic inputs onto single cells in the entopeduncular nucleus of the rat: a double anterograde labelling study combined with postembedding immunocytochemistry for GABA. J Comp Neurol. 1992;321:456–476. doi: 10.1002/cne.903210312. [DOI] [PubMed] [Google Scholar]
- 119.Penney JB, Jr, Young AB. Speculations on the functional anatomy of basal ganglia disorders. Annu Rev Neurosci. 1983;6:73–94. doi: 10.1146/annurev.ne.06.030183.000445. [DOI] [PubMed] [Google Scholar]
- 120.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 121.Albin RL, Reiner A, Anderson KD, Dure LSt, Handelin B, Balfour R, et al. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington's disease. Ann Neurol. 1992;31:425–430. doi: 10.1002/ana.410310412. [DOI] [PubMed] [Google Scholar]
- 122.Crossman AR, Mitchell IJ, Sambrook MA, Jackson A. Chorea and myoclonus in the monkey induced by gamma-aminobutyric acid antagonism in the lentiform complex. The site of drug action and a hypothesis for the neural mechanisms of chorea. Brain. 1988;111(Pt 5):1211–1233. doi: 10.1093/brain/111.5.1211. [DOI] [PubMed] [Google Scholar]
- 123.Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS. Saccades in Huntington's disease: initiation defects and distractibility. Neurology. 1987;37:364–370. doi: 10.1212/wnl.37.3.364. [DOI] [PubMed] [Google Scholar]
- 124.Lasker AG, Zee DS. Ocular motor abnormalities in Huntington's disease. Vision Res. 1997;37:3639–3645. doi: 10.1016/S0042-6989(96)00169-1. [DOI] [PubMed] [Google Scholar]
- 125.Robbins TW, Brown VJ. The Role of the Striatum in the Mental Chronometry of Action: A Theoretical Review. Rev Neurosci. 1990;2:181–213. doi: 10.1515/REVNEURO.1990.2.4.181. [DOI] [PubMed] [Google Scholar]
- 126.Claffey M, Sheldon S, Stinear C, Verbruggen F, Aron A. Having a goal to stop action is associated with advance control of specific motor representations. Neuropsychologia. 2010;48:541–548. doi: 10.1016/j.neuropsychologia.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139:51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 128.Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Progress in Brain Research. 1990 doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- 129.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 130.Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- 131.Mostofsky SH, Schafer JGB, Abrams MT, Goldberg MC, Flower AA, Boyce A, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Cognitive Brain Research. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 132.Johnston K, Everling S. Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. J Neurosci. 2006;26:12471–12478. doi: 10.1523/JNEUROSCI.4101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 134.Ma CL, Qi XL, Peng JY, Li BM. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha 2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- 135.Li BM, Mao ZM, Wang M, Mei ZT. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 136.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 137.Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- 138.Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- 139.Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- 140.Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2008;147:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- 141.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 142.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behaviour by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 144.Mallet L, Schupbach M, N'Diaye K, Remy P, Bardinet E, Czernecki V, et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci U S A. 2007;104:10661–10666. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]