Abstract
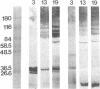
Pleural tuberculosis constitutes a human model of local protective immunity to mycobacterial infection as the disease is usually self-limited and recurrent pleurisy is rare. To identify potentially protective antigens of Mycobacterium tuberculosis, 37 human pleural fluid B cell clones were established using EBV and their supernatants assayed by ELISA and Western blot for antibody reactivity with M. tuberculosis sonicate and culture filtrate. One antibody identified 29,000, 31,000, and 33,000 bands in culture filtrate, and 31,000, 33,000, and 47,000 bands in sonicate; its species reactivity by ELISA was limited to M. tuberculosis. Eight antibodies identified a 31,000 band in culture filtrate and a 68,000 band in M. tuberculosis sonicate, suggesting recognition of a secreted antigen. The species crossreactivity of these eight antibodies extended to M. avium. Six antibodies identified multiple bands and had crossreactivity that included M. avium and M. kansasii. There was no reactivity with recombinant M. tuberculosis 65,000 antigen. Tuberculous pleurisy may prove useful in the identification of potentially protective mycobacterial antigens, particularly those secreted during active infection, and thus accessible to the human immune response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Smith I., Grange J. M., Ratliff T. L., Steele J., Rook G. A. The secreted antigens of Mycobacterium tuberculosis and their relationship to those recognized by the available antibodies. J Gen Microbiol. 1988 Feb;134(2):531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- Atlaw T., Kozbor D., Roder J. C. Human monoclonal antibodies against Mycobacterium leprae. Infect Immun. 1985 Jul;49(1):104–110. doi: 10.1128/iai.49.1.104-110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Mistry S. D., Cooper C. L., Pirmez C., Rea T. H., Modlin R. L. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989 Feb 15;142(4):1114–1119. [PubMed] [Google Scholar]
- Berger H. W., Mejia E. Tuberculous pleurisy. Chest. 1973 Jan;63(1):88–92. doi: 10.1378/chest.63.1.88. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Olds G. R. Demonstration of a shared epitope among mycobacterial antigens using a monoclonal antibody. Clin Exp Immunol. 1985 May;60(2):249–258. [PMC free article] [PubMed] [Google Scholar]
- EDWARDS P. Q., EDWADS L. B. Story of the tuberculin test from an epidemiologic viewpoint. Am Rev Respir Dis. 1960 Jan;81(1):1–47. [PubMed] [Google Scholar]
- Ellner J. J. Pleural fluid and peripheral blood lymphocyte function in tuberculosis. Ann Intern Med. 1978 Dec;89(6):932–933. doi: 10.7326/0003-4819-89-6-932. [DOI] [PubMed] [Google Scholar]
- Emanuel D., Gold J., Colacino J., Lopez C., Hammerling U. A human monoclonal antibody to cytomegalovirus (CMV). J Immunol. 1984 Oct;133(4):2202–2205. [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., Kleinhenz M. E., Wallis R. S., Ellner J. J. Increased interleukin-1 production and monocyte suppressor cell activity associated with human tuberculosis. Am Rev Respir Dis. 1986 Jan;133(1):73–77. doi: 10.1164/arrd.1986.133.1.73. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Okuda Y., Fukukawa T., Tsuyuguchi I. In vitro tuberculin reactivity of lymphocytes from patients with tuberculous pleurisy. Infect Immun. 1982 Feb;35(2):402–409. doi: 10.1128/iai.35.2.402-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., Tsuyuguchi I. Frequency of tuberculin-reactive T-lymphocytes in pleural fluid and blood from patients with tuberculous pleurisy. Chest. 1986 Apr;89(4):530–532. doi: 10.1378/chest.89.4.530. [DOI] [PubMed] [Google Scholar]
- Gillis T. P., Miller R. A., Young D. B., Khanolkar S. R., Buchanan T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun. 1985 Aug;49(2):371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding B., Inghirami G., Peters E., Hoffman T., Balow J. E., Tsokos G. C. In vitro generated human monoclonal trinitrophenyl-specific B cell lines. Evidence that human and murine anti-trinitrophenyl monoclonal antibodies cross-react with Escherichia coli beta-galactosidase. J Immunol. 1987 Dec 15;139(12):4061–4066. [PubMed] [Google Scholar]
- Husson R. N., Young R. A. Genes for the major protein antigens of Mycobacterium tuberculosis: the etiologic agents of tuberculosis and leprosy share an immunodominant antigen. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1679–1683. doi: 10.1073/pnas.84.6.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Kleinhenz M. E., Ellner J. J., Spagnuolo P. J., Daniel T. M. Suppression of lymphocyte responses by tuberculous plasma and mycobacterial arabinogalactan. Monocyte dependence and indomethacin reversibility. J Clin Invest. 1981 Jul;68(1):153–162. doi: 10.1172/JCI110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozbor D., Lagarde A. E., Roder J. C. Human hybridomas constructed with antigen-specific Epstein-Barr virus-transformed cell lines. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6651–6655. doi: 10.1073/pnas.79.21.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Nash D. R., Douglass J. E. Anergy in active pulmonary tuberculosis. A comparison between positive and negative reactors and an evaluation of 5 TU and 250 TU skin test doses. Chest. 1980 Jan;77(1):32–37. doi: 10.1378/chest.77.1.32. [DOI] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Husson R., Young R. A., Godal T. Human T cell clones recognize two abundant Mycobacterium tuberculosis protein antigens expressed in Escherichia coli. J Immunol. 1987 Feb 1;138(3):927–931. [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPER W. H., WARING J. J. Primary serofibrinous pleural effusion in military personnel. Am Rev Tuberc. 1955 May;71(5):616–634. doi: 10.1164/artpd.1955.71.5.616. [DOI] [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986 Feb;51(2):718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G. A., Balbi B., Manca F. Tuberculous pleural effusions. Evidence for selective presence of PPD-specific T-lymphocytes at site of inflammation in the early phase of the infection. Am Rev Respir Dis. 1987 Sep;136(3):575–579. doi: 10.1164/ajrccm/136.3.575. [DOI] [PubMed] [Google Scholar]
- Shimokata K., Kawachi H., Kishimoto H., Maeda F., Ito Y. Local cellular immunity in tuberculous pleurisy. Am Rev Respir Dis. 1982 Nov;126(5):822–824. doi: 10.1164/arrd.1982.126.5.822. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka H., Kiyotani K., Matsuo Y. Purification and antigenic specificity of alpha protein (Yoneda and Fukui) from Mycobacterium tuberculosis and Mycobacterium intracellulare. Hiroshima J Med Sci. 1983 Mar;32(1):1–8. [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Yamamura Y., Misaki A., Azuma I. Chemical and immunological studies on polysaccharide antigens of mycobacteria, nocardia and corynebacteria. Bull Int Union Tuberc. 1972 Feb;47:181–191. [PubMed] [Google Scholar]
- Yoneda M., Fukui Y. Isolation, purification, and characterization of extracellular antigens of Mycobacterium tuberculosis. Am Rev Respir Dis. 1965 Dec;92(6):9–18. doi: 10.1164/arrd.1965.92.6P2.9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]