Abstract
The membrane origin of autophagosomes has long been a mystery and it may involve multiple sources. In this punctum, we discuss our recent finding that the plasma membrane contributes to the formation of pre-autophagic structures via clathrin-mediated endocytosis. Our study suggests that Atg16L1 interacts with clathrin heavy-chain/AP2 and is also localized on vesicles (positive for clathrin or cholera toxin B) close to the plasma membrane. Live-cell imaging studies revealed that the plasma membrane contributes to Atg16L1-positive structures and that this process and autophagosome formation are impaired by knockdowns of genes regulating clathrin-mediated endocytosis.
Key words: autophagy, plasma membrane, endocytosis, phagophore, origin
Where do autophagosomes get their membrane from? Although the field of autophagy has grown tremendously since its discovery a few decades ago, the origin(s) of the membranes that contribute to autophagosome biogenesis has been a mystery among autophagy researchers until recently. Mammalian autophagosomes are formed randomly throughout the cytoplasm via a process that involves elongation and fusion of phagophores to form double-membraned autophagosomes. This process involves two ubiquitin-like conjugation systems: conjugation of Atg12 to Atg5 that later forms a macromolecular complex with Atg16L1, and conjugation of phosphatidylethanolamine (PE) with Atg8/LC3-I. The Atg12-Atg5-Atg16L1 complex is targeted to the preautophagic structures, which then acquire Atg8. Atg12-Atg5-Atg16L1 dissociates from completed autophagosomes, while LC3-PE (LC3-II) is associated both with pre-autophagic structures and completed autophagosomes.
Some recent studies have explored the contribution of membranes from different organelles supporting the general idea that autophagosomes derive membranes from pre-existing organelles. It is quite possible that there may be multiple membrane sources involved. A few groups have revisited the hypothesis that the endoplasmic reticulum (ER) may be one of the membrane donors. High-resolution 2D electron microscopy (EM) and 3D EM-tomography studies have revealed connections between the ER and the growing autophagosomes. Whether the ER contributes to general autophagy or a specific form of autophagy, reticulophagy, remains to be determined. In addition, it has not been shown if ER membrane is required for autophagosome formation. Recently another study has reported that autophagosomes receive lipids from the outer mitochondrial membrane, but only under starvation conditions, again fueling the multiple-membrane source hypothesis.
We have now found evidence for plasma membrane contribution to pre-autophagic structures via endocytosis. Unlike the previous studies, which have focused on LC3- positive structures, we looked specifically at the Atg5-, Atg12- and Atg16-positive pre-autophagic structures, an idea that stemmed from our finding that clathrin heavy-chain immunoprecipitates with Atg16L1. We think that this interaction is partly mediated by the adaptor protein AP2, since knockdown of AP2 decreases the clathrin heavy-chain-Atg16L1 interaction. Immunogold EM also shows clathrin localization on Atg16L1-labeled vesicles close to the plasma membrane.
These findings led us to test whether knockdown of proteins involved in clathrin-mediated endocytosis affected Atg16L1-positive pre-autophagic structures. Indeed, knockdown of key proteins in the clathrin-mediated endocytic pathway results in a decrease in the formation of Atg16L1-positive structures both under basal or autophagy-induced conditions (starvation or trehalose treatment). This correlates with a decrease in the number of LC3-labeled autophagosomes. When we directly analyzed vesicle fusion by livecell microscopy, we observed that vesicles endocytosed from the plasma membrane fuse to the Atg16L1-positive vesicles close to the plasma membrane. This was confirmed by immuno-EM when we found cholera toxin B-labeling (used to label plasma membrane that is subsequently internalized by endocytosis) on Atg16L1-vesicles. We noticed that overexpression of an Atg16L1 mutant that does not bind clathrin heavy-chain does not form Atg16L1-vesicular structures in the way we see with wild-type Atg16L1, suggesting that the binding of Atg16L1 to AP2/clathrin is required for the subsequent formation of the Atg16L1 vesicles.
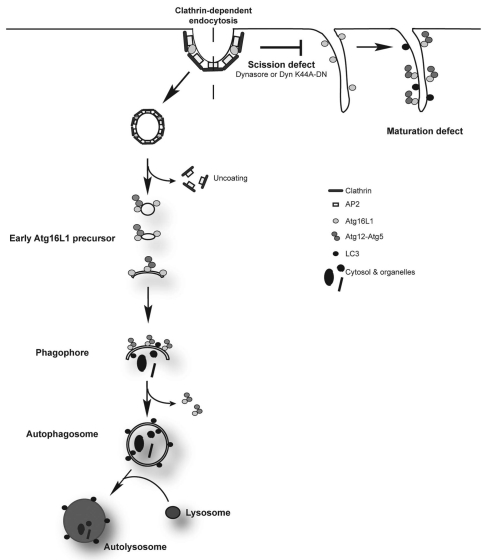
When we blocked endocytic vesicle scission (using both genetic and chemical inhibitors) we found that Atg16L1 strongly immunoprecipitates with clathrin-heavy chain probably due to the accumulation of clathrin-Atg16L1 structures at the plasma membrane that failed to pinch off. This was strongly supported by our fluorescence microscopy and immuno-EM studies that showed what we predicted—accumulation of Atg16L1 at the plasma membrane. This suggests that Atg16L1 in a complex with AP2/clathrin is targeted to the plasma membrane and subsequently internalized as Atg16L1-positive structures. Thus, our data strongly suggest that plasma membrane contributes to early autophagic precursors that subsequently mature to form phagophores (Fig. 1).
Figure 1.
Plasma membrane contributes to the formation of early autophagic precursors. Previous studies show that delivery of fully formed autophagosomes to lysosomes requires fusion of such autophagosomes with early or late endosomes to form amphisomes, which are Atg16L1-negative, LC3-positive and are also positive for endosomal markers. We show that blocking clathrin-mediated endocytosis inhibits formation of Atg16L1-positive structures that mature to form phagophores and later autophagosomes. These Atg16L1-vesicles are positive for other early autophagosomal markers like Atg5 and Atg12, but are negative for early endosomal markers like EEA1, suggesting that they are high up in the autophagosome biogenesis cascade. Inhibition of dynamin with Dynsasore or the use of a dominant negative K44A mutant blocks scission and results in Atg16L1 accumulation on the plasma membrane, suggesting that endosomal scission is critical for this process.
Although previous studies suggest that completely formed autophagosomes need to fuse with early or late endosomes in order for subsequent autophagosomelysosome fusion to occur, they did not look at the formation of pre-autophagic structures. Our study shows that active endocytosis is required both for the formation of autophagosomes, when very early endocytic intermediates immediately pinching off the plasma membrane (not early endosomes) fuse with Atg16L1-positive structures to form phagophores, and also for maturation of autophagosomes when early or late endosomes fuse with Atg16L1-negative but LC3-positive autophagosomes to form amphisomes. Since blocking clathrin-mediated endocytosis does not completely abrogate autophagosome formation, we believe that other endocytic pathways may have a similar role. Depending on the cell type or the physiological conditions, the contributions from the different endocytic pathways may vary accordingly. It will be interesting to know if the endocytic pathway continuously delivers membrane for early steps in autophagy as the preautophagic structures grow and mature to form autophagosomes, deriving membrane from other sources.
Acknowledgements
We are grateful for funding from a Wellcome Trust Senior Fellowship (D.C.R.) and an MRC Programme grant.
Punctum to: Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12:747–757. doi: 10.1038/ncb2078.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13428