Abstract
Accumulating evidence strongly suggests that autophagy, which is induced by endoplasmic reticulum (ER) stress in adipocytes, may play an important role in obesity-induced insulin resistance and type 2 diabetes. Obesity induces ER stress in mouse adipose tissue, which correlates with reduced adiponectin levels. In 3T3-L1 adipocytes, induction of ER stress is sufficient to promote autophagy-dependent adiponectin degradation. In contrast, suppressing ER stress increases adiponectin levels in 3T3-L1 adipocytes and alleviates high fat diet-induced adiponectin downregulation in mice. The ER stress-induced adiponectin downregulation can also be suppressed by overexpression of DsbA-L, a newly identified protein involved in promoting adiponectin multimerization and stability. Taken together, our results show that ER stress-induced autophagy provides an important mechanism underlying obesity-induced adiponectin downregulation in adipocytes. In addition, increasing the expression levels of DsbA-L could be an effective approach to improve adiponectin biosynthesis and stability, thus improving insulin sensitivity in cells and in vivo.
Key words: obesity, ER stress, autophagy, adipokine, DsbA-L, adiponectin
Obesity, which is caused by imbalanced energy homeostasis such as excess nutrient input and/or reduction in physical activity, is associated with insulin resistance and various metabolic diseases such as type 2 diabetes. While the mechanisms underlying obesity-induced insulin resistance remain to be fully elucidated, recent studies suggest that ER stress may play a critical role in this process.
The ER is the place responsible for the synthesis, assembly, and/or modification of transmembrane and secreted proteins. Under pathophysiological nutrient excess conditions such as obesity, the ER becomes stressed in many of the metabolically relevant tissues such as adipose tissue, liver and the pancreas β cells, leading to the activation of a coping system termed the unfolded protein response. In response to ER stress, the ER stress sensors IRE1 (the inositol-requiring ER-to-nucleus signal kinase 1), PERK (protein kinase-like ER kinase) and ATF6 (activating transcription factor 6) are activated, resulting in a series of downstream events such as reducing translation and increasing transcription of ER chaperones to ensure that normal cell function and viability are maintained. The UPR also activates the ER-associated degradation (ERAD) system so that misfolded/unfolded proteins are translocated from the ER lumen to the cytosol for subsequent degradation by the ubiquitin proteosome pathway or the autophagy-dependent pathway.
Autophagy is a cellular defense mechanism that plays an important role in recycling substance and energy for cell survival. During autophagy, cytoplasmic constituents are sequestered into double-membrane vesicles (autophagosomes) that subsequently fuse with lysosomes for degradation. Recent studies strongly suggest that autophagy, which is induced by ER stress in adipocytes, may play an important role in obesity-induced insulin resistance and type 2 diabetes. Consistent with this, disruption of the autophagy pathway by targeted deletion of the Atg7 gene in adipose tissue protects mice from high-fat diet-induced obesity and insulin resistance, suggesting that activation of the autophagy-mediated pathway could be a mechanism for obesity-induced insulin resistance. However, deletion of the Atg7 gene in β-cells impairs glucose tolerance with reduced insulin secretion. Thus, the cellular consequences of autophagy may depend on the cell type and stimulus.
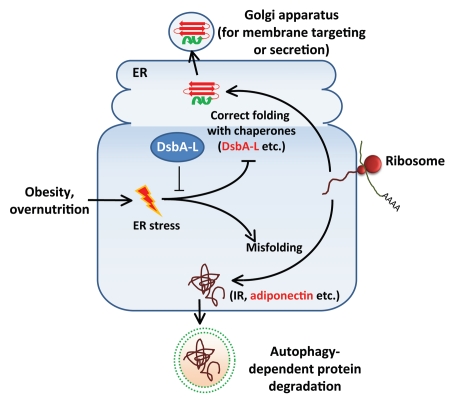
More than functioning as an energy storage depot, the adipose tissue has now been well recognized as an important endocrine organ. Adipocytes synthesize and secrete many of the important adipokines involved in the regulation of energy homeostasis, including adiponectin. Adiponectin is an insulin sensitizer with anti-insulin resistance and antiinflammatory properties. While it is well documented that the cellular and serum levels of adiponectin are negatively correlated with obesity, the precise underlying mechanisms remain unclear. Obesity leads to a low-grade chronic inflammatory state accompanied by increased production of proinflammatory cytokines such as TNFα and IL-6, which reduce adiponectin gene expression. Obesity also increases ER stress and attenuates adiponectin promoter activity. An interesting finding made in this study is that inducing ER stress is sufficient to reduce adiponectin levels in 3T3-L1 adipocytes, suggesting a causative role of ER stress in downregulating the protein levels of adiponectin. Consistent with this, inhibiting ER stress partially restores adiponectin levels in db/db mice and diet-induced obese mice. The ER stress-induced adiponectin downregulation appears to be mediated by an autophagy-dependent mechanism (Fig. 1). Consistent with this, ER stress stimulates adiponectin colocalization with autophagosomes. In addition, treating the ER-stressed 3T3-L1 cells with the autophagy inhibitor 3-MA, but not the proteasome inhibitor MG-132, greatly rescues adiponectin levels.
Figure 1.
A model for the role of DsbA-L in obesity/ER stress-induced adiponectin downregulation. Obesity induces ER stress in adipocytes, leading to increased adiponectin misfolding and subsequent degradation by the autophagy-dependent pathway. DsbA-L suppresses ER stress and facilitates adiponectin assembly and secretion.
Adiponectin is synthesized as a single polypeptide of 30 kDa, which is then assembled in the ER into primarily three species: trimer, hexamer and high molecular weight (HMW) multimer. Impairment in adiponectin multimerization affects both secretion and function of this adipokine and is associated with diabetes and hypoadiponectinemia. Adiponectin multimerization is greatly facilitated by a recently identified adiponectin-interacting protein named disulfide-bond A oxidoreductase-like protein or DsbA-L. DsbA-L is expressed in various mouse tissues such as liver, kidney, pancreas and heart. However, the highest expression of this protein is detected in adipose tissue, where adiponectin is synthesized and secreted. The cellular levels of DsbA-L are significantly reduced in adipose tissues of obese mice and human subjects, suggesting that reduced DsbA-L levels may contribute to increased adiponectin misfolding in the ER and subsequent degradation of this adipokine by the autophagy-dependent pathway. In agreement with this, the expression levels of DsbA-L are reduced in ER stressed 3T3-L1 adipocytes. In addition, overexpression of DsbA-L prevents ER stress-induced and autophagy-dependent downregulation of adiponectin.
Our study demonstrates that autophagy plays a critical role in ER stress-induced adiponectin degradation in adipocytes, thus providing a mechanism underlying obesity-induced adiponectin downregulation. However, inhibition of autophagy suppresses adiponectin degradation but does not rescue insulin sensitivity, suggesting an adaptive role of autophagy in response to ER stress-induced insulin resistance. In contrast, overexpression of DsbA-L protects ER stress-induced adiponectin downregulation and improves adiponectin and insulin signaling in cells. Since the cellular levels of DsbA-L are stimulated by the PPARγ agonist rosiglutazone, an insulin-sensitizing drug, it is conceivable that increasing the expression levels of DsbA-L could be a promising approach to protect cells from obesity-induced ER stress and improve insulin sensitivity.
Acknowledgement
This work was supported by NIH RO1 grants DK76902.
Punctum to: Zhou L, Liu M, Zhang J, Chen H, Dong LQ, Liu F. DsbA-L Alleviates Endoplasmic Reticulum Stress-induced Adiponectin Downregulation. Diabetes. 2010 doi: 10.2337/db10-0412. In press.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13478