Abstract
In a recent study, we reported in vivo evidence of early and sustained alterations of autophagy markers in a novel knock-in mouse model of Huntington disease (HD). The novel model is derived from selective breeding of HdhQ150 knock-in mice to generate mice with ∼200 CAG/polyglutamine repeats (HdhQ200). HdhQ200 knock-in mice exhibit an accelerated and more robust motor phenotype than the parent line with detectable abnormalities at 50 weeks and substantial impairments at 80 weeks. Heterozygous HdhQ200 knock-in mice accumulate htt aggregates as cytoplasmic aggregation foci (AF) as early as 9 weeks of age followed by striatal neuronal intranuclear inclusions (NIIs) by 20 weeks. By 40 weeks, striatal AF are perinuclear and immunoreactive for ubiquitin and the autophagosome marker LC3. Increased LC3-II protein expression is noted at 9 weeks and sustained throughout the disease course, and is paralleled by increased expression of p62. Early and sustained expression of autophagy-related proteins in this genetically precise mouse model of HD suggests that alteration of autophagic flux is an important and early component of neuronal response to polyglutamine expanded huntingtin.
Key words: Huntington disease, huntingtin, polyglutamine, autophagy, neurodegeneration
The pathogenic role of misfolded and aggregated polyglutamine (polyQ) proteins has increased interest in defining the role of protein quality control pathways in HD and other polyQ diseases. Much of the work to date has been carried out with in vitro, invertebrate or transgenic murine models of HD. Knock-in models have the theoretical advantage of examining the effects of mutant htt (mhtt) in the context of physiological gene regulation, but knock-in models characterized to date have had relatively mild phenotypes. Given the relationship between CAG/polyQ repeat length and age of onset in polyQ diseases, generation of knock-in models with very long CAG/polyglutamine repeats may produce more robust phenotypes and facilitate analysis of disease mechanisms. We evaluated a knockin HD model with ∼200 repeats generated by selective breeding of the HdhQ150 line, which has ∼150 repeats.
Heterozygous HdhQ200 mice show progressive motor impairments on a balance beam task starting at 50 weeks, months earlier than the age at which these abnormalities appear in HdhQ150 mice. These motor deficits are followed at 60 weeks by progressive gait deficits demonstrated by footprint analysis. Severe motor deficits at 80 weeks are paralleled by striatal and cortical astrogliosis, and an ∼50% reduction in striatal dopamine receptors. At 80 weeks, stereological analysis reveals no striatal neuron loss, thus it is likely that the behavioral abnormalities of HdhQ200 mice reflect neuronal dysfunction rather than overt neurodegeneration. Striatal neurons of 80-week old HdhQ200 heterozygous mice contain many, often large, cytoplasmic vacuoles. This motor phenotype is even more aggressive in homozygous HdhQ200 mice, with marked impairments by 40 weeks of age.
We applied a sensitive histochemical method employing biotinylated polyQ peptide to demonstrate early cytoplasmic deposition of mhtt protein aggregates in neurons. These aggregation foci (AF) were present in striatal neurons as early as 9 weeks, well before neuronal intranuclear inclusions (NIIs) appear. This observation is consistent with the detection of cytoplasmic aggregates in HD brains, as postmortem brains with early grade disease contain more neuropil aggregates than NIIs. Initially expressed diffusely in the cytoplasm, AF shift to a perinuclear localization by 40 weeks of age. Perinuclear AF in striatal neurons colocalize with the autophagosome marker LC3, and the development of AF is paralleled by increased expression in LC3 immunoreactive cytoplasmic puncta, suggesting increased expression of autophagosomes and association of AF with autophagosomes. The perinuclear localization of AF in 40-week old mice and their absence from the nucleus are consistent with association with autophagosomes and/or lysosomes. Consistent with our morphological results, western blotting indicates early and persistent elevation of LC3-II and p62 in HdhQ200 heterozygous mouse brain. Elevated LC3-II and p62 levels are detected as early as 9 weeks and remain elevated until 80 weeks.
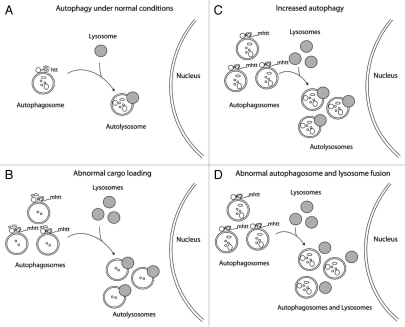
Is this alteration in autophagic markers a reflection of increased autophagy flux or a result of an autophagy block? LC3-II is uniquely associated with autophagosomes, and increased expression of LC3-II and LC3 immunoreactive vesicles often is interpreted as evidence of increased autophagic flux. The protein p62 is another key actor in autophagy; this ubiquitin-binding protein is thought to mediate the entry of abnormal proteins, including mhtt, into autophagosomes by binding LC3-II. Increased p62 expression, however, is commonly interpreted as evidence of reduced autophagic flux. Jointly increased expression of LC3-II and p62 could reflect a downstream block in autophagic flux or result from a recently described cargo-loading abnormality associated with mhtt. Direct measurements of autophagic flux in vivo are needed to determine whether our findings in HdhQ200 mice result from increased autophagy, a cargo loading defect, or a block in the pathway downstream of autophagosome formation (see Fig. 2). While changes in autophagy are often assumed to be a response to mhtt, there are also suggestions that htt has important normal functions in protein quality control. Alteration of these hypothesized normal functions also could drive changes in autophagy markers.
Clearly, much remains to be learned about autophagy and other aspects of protein quality control in polyQ and other neurodegenerative proteinopathies. HdhQ200 mice, with their early and sustained abnormalities in this important protein quality control pathway, are likely to be a useful tool in sorting out the complexities of protein quality control in these diseases.
Figure 1.
A schematic indicating autophagosome and lysosome formation under (A) normal conditions in the presence of wild-type htt or (B–D) in the presence of mhtt. Three possible consequences of mhtt expression in neurons are (B) inefficient cargo loading of autophagosomes; (C) increased normal autophagic flux in response to mhtt, resulting in an increased number of autophagosomes, lysosomes and autolysosomes; and (D) increased number of autophagosomes and lysosomes when turnover is blocked distally, for example, by diminished fusion of autophagosomes and lysosomes.
Acknowledgements
This work was supported by a VA Merit Review grant (R.L.A.); the National Institutes of Health [R21 NS059537 (R.L.A.), R21 NS059647 (P.D.), RO1 NS038712 (H.L.P.), and T32 NS007222 (M.Y.H.)].
Abbreviations
- htt
huntingtin
Punctum to: Heng MY, Duong DK, Albin RL, Tallaksen-Greene SJ, Hunter JM, Lesort MJ, Osmand A, Paulson HL, Detloff PJ. Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet. 2010;19:3702–3720. doi: 10.1093/hmg/ddq285.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13617