Abstract
Background
Although breast cancer risk is greater in users of estrogen–progestin than estrogen-only formulations of menopausal hormonal therapy, reports on their effects have been somewhat inconsistent. We investigated whether the timing of these therapies affected breast cancer incidence.
Methods
A total of 1 129 025 postmenopausal UK women provided prospective information on hormonal therapy use and other factors relevant for breast cancer risk. We used Cox regression to estimate adjusted relative risks (RRs) of breast cancer in hormonal therapy users vs never users and calculated standardized incidence rates. All statistical tests were two-sided.
Results
During 4.05 million woman-years of follow-up, 15 759 incident breast cancers occurred, with 7107 in current users of hormonal therapy. Breast cancer incidence was increased in current users of hormonal therapy, returning to that of never users a few years after use had ceased. The relative risks for breast cancer in current users were greater if hormonal therapy was begun before or soon after menopause than after a longer gap (Pheterogeneity < .001, for both estrogen-only and estrogen-progestin formulations). Among current users of estrogen-only formulations, there was little or no increase in risk if use began 5 years or more after menopause (RR = 1.05, 95% confidence interval [CI] = 0.89 to 1.24), but risk was statistically significantly increased if use began before or less than 5 years after menopause (RR = 1.43, 95% CI = 1.35 to 1.51). A similar pattern was observed among current users of estrogen–progestin formulations (RR = 1.53, 95% CI = 1.38 to 1.70, and RR = 2.04, 95% CI = 1.95 to 2.14, respectively). At 50–59 years of age, annual standardized incidence rates for breast cancer were 0.30% (95% CI = 0.29% to 0.31%) among never users of hormone therapy and 0.43% (95% CI = 0.42% to 0.45%) and 0.61% (95% CI = 0.59% to 0.64%), respectively, among current users of estrogen-only and estrogen–progestin formulations who began use less than 5 years after menopause.
Conclusions
There was substantial heterogeneity in breast cancer risk among current users of hormonal therapy. Risks were greater among users of estrogen–progestin than estrogen-only formulations and if hormonal therapy started at around the time of menopause than later.
CONTEXT AND CAVEATS
Prior knowledge
Breast cancer risk has generally been found to be greater in users of estrogen–progestin than estrogen-only formulations of menopausal hormonal therapy.
Study design
Data on use of hormonal therapy from a large prospective study of postmenopausal women in the United Kingdom were used to investigate the incidence and risk of breast cancer according to various aspects of hormonal therapy use.
Contribution
There was substantial heterogeneity in breast cancer risk among current users of both types of hormonal therapy. Starting hormonal therapy before or soon after the start of menopause was associated with greater risk of breast cancer than starting it later.
Implications
The timing of the start of hormone therapy relative to that of menopause appears to be an important modifier of associated risk of breast cancer.
Limitations
Information on use of hormonal therapy was reported on average 1.4 years before breast cancers were diagnosed, and so there could have been some misclassification of the use of hormone therapy among both breast cancer patients and the population at risk, which would slightly dilute estimates of relative risk.
From the Editors
There is extensive evidence that breast cancer incidence is increased in current users of menopausal hormone therapy and that the risk returns to that of never users of hormonal therapy soon after use ceases (1–4). Virtually all published reports that have compared the effects of different types of hormonal therapy found greater increases in breast cancer risk with use of estrogen–progestin than with use of estrogen-only formulations [see (4), which summarized results from 22 studies]. Results from some studies in the United States (4–6), including the Women’s Health Initiative trial, found little or no increase in breast cancer risk with use of estrogen-only preparations. However, about 90% of the women in the estrogen-only arm of this trial were randomly assigned to hormonal therapy more than 5 years after their menopause, and the Women's Health Initiative investigators recently reported that breast cancer risk was greater if hormonal therapy use began less than 5 years after menopause than after a longer gap, both for estrogen-only and for estrogen–progestin preparations (6). We investigated whether breast cancer incidence varied by the timing of use of different types of hormonal therapy in a large prospective study in the United Kingdom.
Participants and Methods
Data Collection and Follow-up
A total of 1.3 million women who were invited for screening by the Breast Screening Programme of the National Health Service were recruited into the Million Women Study from May 1, 1996, through December 31, 2001. They completed a baseline questionnaire approximately a week before they were screened, which asked about sociodemographic and other factors, including the use of hormonal therapy (7). Participants were resurveyed approximately 3 years after recruitment to update information on hormonal therapy use, menopausal status, and other factors. Study details, including the questionnaires used, can be viewed at www.millionwomenstudy.org. All participants gave written consent to take part in the study, and the Oxford and Anglia Multi-Centre Research Ethics Committee approved the study.
Every study participant had a unique National Health Service number and was followed via record linkage (using that number and other personal details) to data held by the National Health Service Central Registers. These registers routinely collect nationwide information on cancer registrations and deaths [which are coded by use of the International Classification of Diseases, Tenth Revision (ICD-10) (8)]. The registers of the National Health Service regularly provided study investigators with precoded data on cause-specific incident cancers and deaths, and the date when each was registered. Information on tumor characteristics was obtained from cancer registry and screening clinic data, as well as from pathology reports and questionnaire data (9).
Statistical Analyses
The endpoint for these analyses was breast cancer (ICD-10 codes C50 or D05). We excluded women with any type of invasive cancer (except nonmelanoma skin cancer [ICD-10 code C44]) or with in situ breast cancer [ICD-10 code D05] that was registered before recruitment (19 729 with breast cancer) and women with unknown hormonal therapy use (247 with breast cancer). Analyses were further restricted to postmenopausal women (defined as those who reported that they had had a natural menopause or a bilateral oophorectomy or those who were aged 53 years or older with an unknown age at menopause [ie, those who started hormonal therapy or had a hysterectomy before their natural menopause; sensitivity analyses were done to assess the effect of including such women]). Woman-years were calculated from the date of recruitment (or, if not postmenopausal at recruitment, from the date when they completed a subsequent questionnaire and reported that they were postmenopausal or when they reached their 53rd birthday) up to the date of cancer registration, the date of death, 48 months after the last study questionnaire was completed, or December 31, 2002, whichever came first. Censoring at 48 months after the time of last contact was done to minimize misclassification of variables over time and the additional censoring in 2002 was done because many UK women ceased hormonal therapy use after the 2002 publication of the first results from the Women's Health Initiative trial (10,11).
The postmenopausal women were classified as being current, past, or never users of hormonal therapy at the time of last contact. This classification was done initially by use of information reported on the recruitment questionnaire, with woman-years contributed to the appropriate category up to the date that they completed the second study questionnaire, when they were reclassified by use of the updated information provided. (The second questionnaire was mailed to participants approximately 3 years after they completed the first questionnaire. By the end of 2002, the censoring date for these analyses, two-thirds of the participants had been mailed the second questionnaire and the response was 65%.) Current users of hormonal therapy were classified further by the type of hormonal therapy last used, their age at first use of hormonal therapy, the interval between menopause and first use of hormonal therapy, and the total duration of hormonal therapy use. In analyses with respect to duration of hormonal therapy use, women were classified by the duration reported at last contact and, for those diagnosed with breast cancer, estimates were made of the actual average duration of current use at diagnosis. For past users of hormonal therapy at the time of last contact, their time since last use was estimated, by assuming that it increased by 1 year for every year of follow-up.
Cox regression models, which used attained age as the underlying time variable, were applied to estimate relative risks (RRs) and the associated variances for various aspects of hormonal therapy use; the STATA computing package (STATA Corp, Texas, 2007, release 10.1) was used for all analyses. Analyses were also stratified by age at recruitment (<52, 53–55, 56–58, 59–61, 62–64, or ≥65 years) and adjusted for region of residence (10 areas covered by 10 cancer registries, East Anglia, North West [Manchester and Lancashire], North West [Mersey], Northern and Yorkshire, Oxford, Scotland, South West, Thames, Trent, West), by quintiles of socioeconomic group within the study population, as described previously (7), age at menopause (<42, 43–47, 48–52, or ≥53 years), parity and age at birth of first child (nulliparous or 1–2 or ≥3 children, cross-classified by age at birth of first child <25 or ≥25 years), body mass index (<25, 25–29, or ≥30 kg/m2), and alcohol consumption (<10 or ≥10 g/d). For these analyses, it should be noted that a body mass index of less than 25 kg/m2 was referred to as lean or not overweight, that of 25 kg/m2 or more was referred to as overweight, and that of 30 kg/m2 or more was referred to as obese. Those participants with missing or unknown values were assigned to a separate category for each variable. Sensitivity analyses were done to assess the effect of additional adjustments, and stratified analyses were also done to assess any deviation from proportional hazards.
Whenever two groups were compared, relative risks and 95% confidence intervals (CIs) are presented. However, when more than two groups were compared, variances were estimated by treating the relative risks as floating absolute risks, yielding floated confidence intervals (fCIs) (12). This method enabled valid comparisons between any two groups, even if neither was the baseline; it did not alter the relative risks but slightly reduced the variances attributed to relative risks that were not defined as 1.0. Consequently, comparison between any two groups, as in the text, must take the variance of each into account (13). Standardized breast cancer incidence rates per 100 women aged 50–59 years per year were calculated by taking incidence rates in never users as the standard and standardizing by age, region, socioeconomic group, age at birth of first child, parity, and alcohol consumption. All statistical tests were two-sided.
Results
These analyses included 1 129 025 postmenopausal women who provided prospective information on hormonal therapy use and other factors relevant for breast cancer risk. Their average age at entry into the study was 56.6 years (SD = 4.8 years). At the time of last contact, 615 753 (55%) were ever users of hormonal therapy and 394 697 (35%) were current users. During 4.05 million woman-years of follow-up, 15 759 incident breast cancers occurred, 9632 (61%) in ever users and 7107 (45%) in current users of hormonal therapy. The breast cancers were diagnosed an average of 1.4 years after the time of last contact. Current, past, and never users of hormonal therapy did not differ materially by sociodemographic and other factors relevant for breast cancer (Table 1).
Table 1.
Characteristics of the study population and details of follow-up, by last reported use of hormonal therapy (HT)
| Characteristic | Current HT (n = 394 697) | Past HT (n = 221 056) | Never HT (n = 513 272) |
| At recruitment | |||
| Mean age, y (SD) | 55.1 (4.3) | 56.7 (4.4) | 57.7 (5.0) |
| Socioeconomic status, % in upper third | 34 | 34 | 32 |
| Mean parity, No. of children (SD) | 2.1 (1.2) | 2.2 (1.2) | 2.1 (1.3) |
| Mean age at birth of first child (for parous women), y (SD) | 23.5 (4.3) | 24.6 (4.2) | 24.5 (4.3) |
| Mean body mass index, kg/m2 (SD) | 25.7 (4.4) | 26.5 (4.6) | 26.5 (4.9) |
| Strenuous physical activity, % >once a week | 40 | 39 | 37 |
| Mean alcohol consumption, g/wk (SD) | 48 (55) | 44 (53) | 37 (50) |
| % Current smoker | 23 | 21 | 20 |
| Follow-up for breast cancer | |||
| Woman-years of follow-up for incidence per 1000 women | 1418.8 | 749.3 | 1885.6 |
| Total No. of incident breast cancers | 7107 | 2525 | 6127 |
We had previously reported on the association between use of hormone therapy and breast cancer risk in this cohort (7). In this report, we updated information both on menopause and on use of hormone therapy over time, so more postmenopausal women are included this analysis (1 129 025 vs 828 923 previously), and a greater proportion had ever used hormone therapy (55% vs 53% previously). Follow-up has been extended, and there are more incident breast cancers in these analyses (15 759 vs 7140 previously). Because exposure data were updated in this analysis, the average time between breast cancer diagnosis and the last recorded use of hormone therapy was only 1.4 years, whereas it had been 2.6 years previously, and so misclassification of exposure in this analysis was less likely.
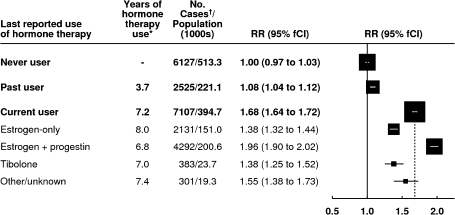
Our initial comparison was of breast cancer risk in current and past users of hormone therapy vs that in never users (Figure 1). The adjusted relative risks of breast cancer were statistically significantly increased, both in current and in past users (Figure 1, P < .001 for each comparison vs never users). We then compared breast cancer risk among current users by the type of hormonal therapy used and found a statistically significant variation in risk across the types (Pheterogeneity < .001); the greatest excess risk was among current users of estrogen–progestin hormonal therapy, but risk was also statistically significantly increased in users of estrogen-only preparations and of tibolone (a synthetic steroid with estrogenic, progestogenic, and androgenic activities that is licensed in Europe but not in the United States) (P < .001 for each of the three formulations compared with never users).
Figure 1.
Risk of breast cancer, by use of hormone therapy. Relative risks (RRs) were calculated, taking never users of hormone therapy as the comparison group (RR = 1.0), stratifying by age, and adjusting by region of residence, socioeconomic status, age at menopause, body mass index, age at birth of first child, parity, and alcohol consumption. Relative risks (and their floated confidence intervals [fCIs]) are represented by squares and lines, with the area of every square being inversely proportional to the variance of the logarithm of the relative risk. This presentation thus provides an appropriate indication of the amount of statistical information involved. The dotted line represents the relative risk for all current users compared with never users. * = Estimated average total duration of use of hormone therapy at the time of diagnosis of breast cancer. †= Cases denote women with breast cancer.
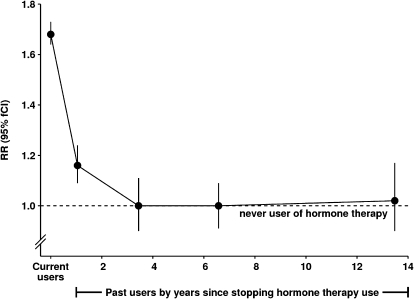
Although the risk of breast cancer was statistically significantly increased among past users of hormonal therapy (Figure 1), incidence rates declined rapidly after use ceased (Figure 2). In the first 2 years after hormonal therapy use had ceased, breast cancer risk was still slightly, but statistically significantly, increased (RR = 1.16, 95% CI = 1.08 to 1.24; P < .001, on the basis of the 1003 exposed women who developed breast cancer in this analysis). Subsequently, and up to 14 years after hormonal therapy use had ceased, the risk in past users of hormonal therapy remained similar to that of never users (RR = 0.99, 95% CI = 0.93 to 1.05, on the basis of 1098 exposed women who developed breast cancer).
Figure 2.
Risk of breast cancer, in current users and in past users by time since stopping hormone therapy. Relative risks (RRs) were calculated by taking never users of hormone therapy as the comparison group (RR = 1.0), stratifying by age, and adjusting by region of residence, socioeconomic status, age at menopause, body mass index, age at birth of first child, parity, and alcohol consumption. Relative risks (and their floated confidence intervals [fCIs]) are represented by circles and lines. The dotted line represents the relative risk for all never users. It should be noted that, for current users, time since last use is effectively zero.
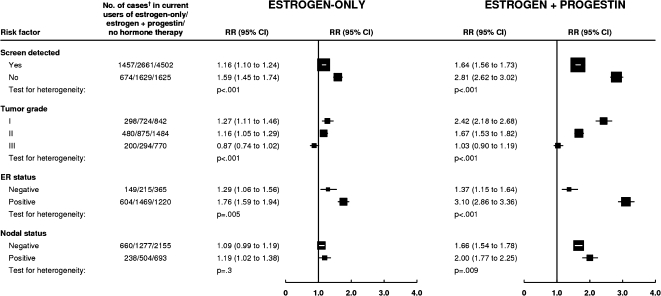
The relative risks of breast cancer among current users of estrogen-only and of estrogen–progestin formulations, the two most commonly used types of hormonal therapy were considerably lower for screen-detected than for non–screen-detected breast cancers (Pheterogeneity < .001, for each type) (Figure 3). Women included in these analyses were screened an average of 7.7 days after they completed the recruitment questionnaire. Breast cancers diagnosed in the first 4 months after recruitment should include virtually all breast cancers found at screening soon after the baseline questionnaire was completed. Among users of the two most commonly used types of hormone therapy, the relative risks for breast cancer were substantially lower in the first 4 months after recruitment than subsequently (for current users of estrogen-only therapy, RR = 1.19, 95% CI = 1.09 to 1.30 in the first 4 months and RR = 1.50, 95% CI = 1.41 to 1.60 subsequently; and for current users of estrogen–progestin hormonal therapy, the corresponding relative risks were RR = 1.41, 95% CI = 1.31 to 1.52 and 2.32, 95% CI = 2.20 to 2.44; Pheterogeneity < .001, for each type). There were also large differences in the hormonal therapy–associated risks for tumors that were estrogen receptor positive compared with those that were estrogen receptor negative and for low-grade compared with high-grade disease (Pheterogeneity ≤ .005, for each hormonal therapy type) (Figure 3). Users of estrogen–progestin formulations were also more likely than never users to have tumors involving the lymph nodes than localized disease (Pheterogeneity = .009).
Figure 3.
Risk of breast cancer in current users of hormone therapy by tumor characteristics. Relative risks (RRs) were calculated by taking never users of hormone therapy as the comparison group (RR = 1.0), stratifying by age, and adjusting by region of residence, socioeconomic status, age at menopause, body mass index, age at birth of first child, parity, and alcohol consumption. Relative risks (and their confidence intervals [CIs]) are represented by squares and lines, with the area of every square being inversely proportional to the variance of the logarithm of the relative risk. † = Cases denote women with breast cancer.
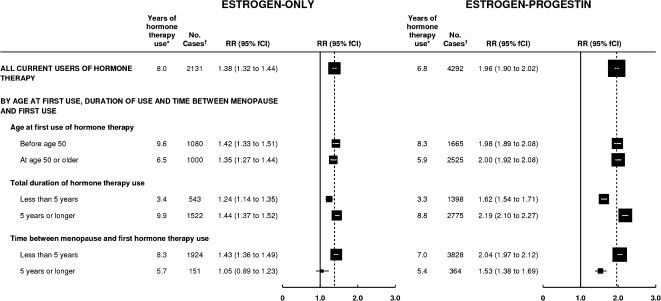
The association between use of the two most commonly used types of hormonal therapy (ie, estrogen-only and estrogen–progestin formulations) and risk for breast cancer was next analyzed by the time when hormone use began and by the duration of its use (Figure 4). For current users of both these formulations, the risk of breast cancer was statistically significantly increased among women who started use of hormonal therapy both before age 50 years and at ages 50 years or older, and there were essentially no differences by age at starting. The risk of breast cancer associated with each type of hormonal therapy was also statistically significantly increased with hormonal therapy use for durations of less than 5 years and of 5 years or more, respectively, but the risk was significantly greater with the longer duration (Pheterogeneity < .001, for each formulation). Among current users of tibolone, the associations were similar to those found for the two more commonly used hormonal therapies (for tibolone users, RR = 1.35, 95% CI = 1.11 to 1.64, for use beginning before age 50 years, vs RR = 1.45, 95% CI = 1.29 to 1.63, for use beginning after age 50 years; RR = 1.20, 95% CI = 1.00 to 1.45, for a total duration of use of <5 years, vs RR = 1.49, 95% CI = 1.32 to 1.69, for a total duration of use of ≥5 years.)
Figure 4.
Risk of breast cancer in current users of estrogen-only and estrogen–progestin hormone therapy by the timing of first use and total duration of use. Relative risks (RRs) were calculated by taking never users of hormone therapy as the comparison group (RR = 1.0, 95% CI = 0.97 to 1.03), stratifying by age, and adjusting by region of residence, socioeconomic status, age at menopause, body mass index, age at birth of first child, parity, and alcohol consumption. Relative risks (and their floated confidence intervals [fCIs]) are represented by squares and lines, with the area of every square is inversely proportional to the variance of the logarithm of the relative risk. * = Estimated average total duration of use of hormone therapy at the time of diagnosis of breast cancer. The dotted line represents the overall relative risk estimates for current users of each type of hormone therapy. † = Cases denote women with breast cancer.
The excess risk of breast cancer in current users was statistically significantly greater if use of hormonal therapy began before or soon after menopause than after a longer gap (Figure 4, Pheterogeneity < .001, both for estrogen-only and for estrogen–progestin formulations). Breast cancer risk was statistically significantly increased in users of estrogen-only hormonal therapy if use began before or less than 5 years after menopause (RR = 1.43, 95% CI = 1.35 to 1.51, P < .001), whereas if such use began 5 years or more after menopause, breast cancer risk was not increased (RR = 1.05, 95% CI = 0.89 to 1.24, P = .6). Breast cancer risk in users of estrogen–progestin hormonal therapy was also statistically significantly greater if use began before or less than 5 years compared with 5 years or more after menopause (RR = 2.04, 95% CI = 1.95 to 2.14, P < .001, and RR = 1.53, 95% CI = 1.38 to 1.70, P < .001, respectively). Corresponding results for tibolone showed a similar pattern but the difference was not statistically significant (RR = 1.49, 95% CI = 1.33 to 1.67, P < .001, and RR = 1.16, 95% CI = 0.92 to 1.47, P = .2).
Additional results on breast cancer risk in relation to the interval between menopause and starting hormonal therapy are shown in Table 2. Breast cancer risk among current users of estrogen-only or estrogen–progestin did not differ statistically significantly by whether women started using hormonal therapy either before or soon after menopause. Most women who had started hormonal therapy in the 5 years after their menopause had begun use almost immediately after the onset of their menopause; in this group, the average time between menopause and starting hormonal therapy was only 0.7 years for users of estrogen-only hormonal therapy and 1.4 years for users of estrogen–progestin hormonal therapy. Among women who started hormonal therapy 5 years or more after menopause, the average time between menopause and starting hormonal therapy was 10.3 years for estrogen-only and 9.3 years for estrogen–progestin formulations. Women who started hormonal therapy before their menopause had, by definition, an unknown age at menopause. To assess how this affected the results, we did sensitivity analyses restricted to women with a known age at menopause (natural or bilateral oophorectomy) who started hormonal therapy after menopause. The risk estimates among these women were similar to those observed among all women (Table 2). We also did sensitivity analyses to assess the effect of not adjusting for age at menopause and found that the estimates were very slightly lower in the unadjusted analysis than the adjusted analysis (eg, among women who started hormonal therapy after a natural menopause or bilateral oophorectomy, the unadjusted vs adjusted values were RR = 1.35 vs RR = 1.40 for estrogen-only use beginning <5 years after menopause and were RR = 0.94 vs RR = 1.08 for estrogen-only use beginning ≥5 years after menopause; and corresponding unadjusted vs adjusted values among users of estrogen–progestin hormonal therapy were RR = 2.06 vs RR = 2.05 and RR = 1.47 vs RR = 1.61, respectively).
Table 2.
Associations between risk of breast cancer among current users of estrogen-only hormonal therapy (HT) and among current users of estrogen–progestin HT, by time between menopause and starting HT*
| Timing of HT use | Estrogen-only HT |
Estrogen–progestin HT | ||
| No. | RR* (95% CI) | No. | RR* (95% CI) | |
| Women who started HT | ||||
| Before menopause | 1091 | 1.49 (1.40 to 1.58) | 2364 | 2.10 (2.00 to 2.20) |
| <5 y after menopause | 833 | 1.36 (1.27 to 1.46) | 1464 | 1.99 (1.89 to 2.10) |
| ≥5 y after menopause | 151 | 1.05 (0.90 to 1.24) | 364 | 1.53 (1.38 to 1.69) |
| Women with a natural menopause or bilateral oophorectomy, who started HT after menopause | ||||
| <5 y after menopause | 663 | 1.40 (1.30 to 1.52) | 1427 | 2.05 (1.94 to 2.17) |
| ≥5 y after menopause | 97 | 1.08 (0.88 to 1.31) | 336 | 1.61 (1.44 to 1.79) |
| Women with duration of HT use of <5 years, who started HT | ||||
| Before or <5 y after menopause | 474 | 1.31 (1.19 to 1.43) | 1206 | 1.76 (1.66 to 1.87) |
| ≥5 y after menopause | 69 | 0.86 (0.68 to 1.09) | 192 | 1.34 (1.16 to 1.54) |
| Women with duration of HT use of ≥5 years, who started HT | ||||
| Before or <5 y after menopause | 1441 | 1.36 (1.29 to 1.43) | 2605 | 2.27 (2.18 to 2.36) |
| ≥5 y after menopause | 81 | 1.09 (0.88 to 1.36) | 170 | 1.65 (1.42 to 1.92) |
Relative risks (RRs) were calculated by taking never users of HT as the comparison group (RR = 1.0) stratifying by age and adjusting by region of residence, socioeconomic status, body mass index, parity, age at first birth, and alcohol consumption; except for analyses by duration of HT use results are also adjusted by age at menopause. Small numbers of women with breast cancer (10 using estrogen-only HT and 19 using estrogen–progestin HT) had missing information on duration of HT use and were not included in analyses relating to duration of use.
The average duration of hormonal therapy use was longer in women who started hormonal therapy less than 5 years after menopause than after a longer gap (Figure 4). However, the greater risk among those who started hormonal therapy at around the time of menopause was consistently observed for both short and long duration use of each hormonal therapy type (Table 2). The relative risk estimates in Table 2 with respect to duration of use were not adjusted by age at menopause because among current users of hormonal therapy of a given age, age at menopause and time from menopause to starting hormonal therapy were completely confounded with duration of hormonal therapy use. Sensitivity analyses of the effect of adjusting for age at menopause (see above) suggested that omission of this variable in the model was unlikely to have materially affected the results.
Among never users of hormonal therapy, the standardized incidence rate for breast cancer at age 50–59 years was 0.30% (95% CI = 0.29% to 0.31%) per year. For users of estrogen-only hormonal therapy aged 50–59 years beginning use before or less than 5 years after menopause, the standardized incidence rate was 0.43% (95% CI = 0.42% to 0.45%), and for such use beginning 5 years or more after menopause, the standardized incidence rate did not differ statistically significantly from that of never users (ie, standardized incidence rate = 0.32%, 95% CI = 0.27% to 0.37%). The corresponding standardized incidence rates for estrogen–progestin hormonal therapy were 0.61% (95% CI = 0.59% to 0.64%) and 0.46% (95% CI = 0.41% to 0.51%).
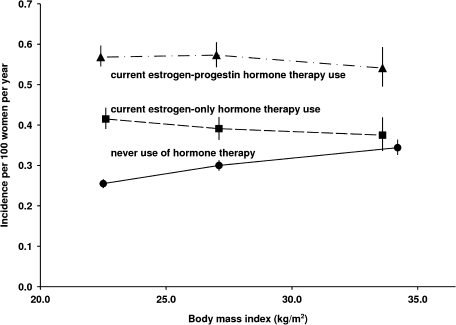
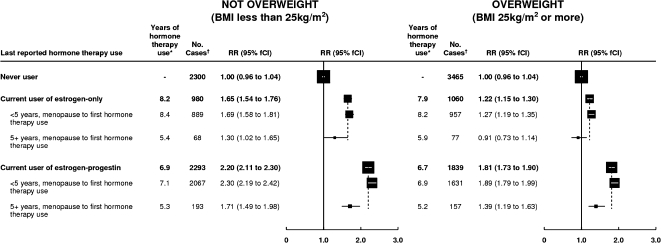
As expected, breast cancer incidence rates among never users of hormone therapy increased with body mass index (Figure 5). However, contrary to the trends among never users of hormonal therapy, standardized incidence rates among current users of hormonal therapy varied little by body mass index (Figure 5). The different relationships between breast cancer incidence and body mass index among never users and among current users mean that the proportionate increase in risks for breast cancer associated with use of hormonal therapy was greater among lean women than among obese women (eg, for current users of estrogen-only hormonal therapy, RR = 1.65, 95% CI = 1.53 to 1.78 among lean women and RR = 1.22, 95% CI = 1.13 to 1.31 among overweight and obese women) (Figure 6). Within subgroups defined by their body mass index, risks of breast cancer were still statistically significantly greater if hormonal therapy use began at around the time of menopause than after a longer gap (Figure 6). Among overweight and obese current users of estrogen-only hormonal therapy, no statistically significant increase in breast cancer risk was observed if hormonal therapy use began 5 years or more after menopause (RR = 0.91, 95% CI = 0.73 to 1.14, P = .4).
Figure 5.
Standardized incidence rates for breast cancer in current users of hormone therapy by the type of hormone therapy used and women’s body mass index. Standardized incidence rates per 100 women aged 50–59 years per year were calculated by taking never users of hormone therapy as the standard and standardizing by age, region of residence, socioeconomic status, age at menopause, age at birth of first child, parity, and alcohol consumption. It should be noted that incidence rates are plotted against the mean body mass index within each subgroup. 95% confidence intervals are shown (error bars).
Figure 6.
Risk of breast cancer in current users estrogen-only and estrogen–progestin hormone therapy, by the timing of first use and women's body mass index. Relative risks (RRs) were calculated by taking never users of hormone therapy as the comparison group (RR = 1.0), stratifying by age, and adjusting by region of residence, socioeconomic status, age at menopause, body mass index, age at birth of first child, parity, and alcohol consumption. Relative risks (and their floated confidence intervals [fCIs]) are represented by squares and lines, with the area of every square is inversely proportional to the variance of the logarithm of the relative risk. * = Estimated average total duration of use of hormone therapy at the time of diagnosis of breast cancer. The dotted line represents the overall relative risk estimates for current users of each type of hormone therapy. † = Cases denote women with breast cancer.
Apart from body mass index, none of the adjustment factors substantially modified the effect of hormonal therapy on breast cancer risk. Additional adjustment by other factors associated with breast cancer risk, including age at menarche, height, and having a first-degree relative with breast cancer, did not alter the main findings. Also use of analyses that stratified rather than adjusted for potential confounders gave very similar results to those in this analysis, indicating that there was little deviation from the assumption of proportional hazards.
Discussion
In this large prospective study, we found substantial and clinically important heterogeneity in the effects of hormonal therapy on breast cancer incidence among postmenopausal women. Among current users of hormonal therapy, there were independent effects of the type of hormonal therapy used, the time between menopause and first use of hormonal therapy, and the duration of hormonal therapy use. Some of these findings confirm what is already well known, for example, that current users of estrogen–progestin preparations are at the greatest risk of breast cancer and that the associated risk increases with duration of use (1–4). We have previously reported (7) such findings in this cohort, but with updated information on hormonal therapy use and menopausal status and extended follow-up, these analyses include more postmenopausal women, a greater number of ever users of hormone therapy, and more incident breast cancers, with a shorter time between last reporting hormone use and the diagnosis of breast cancer. As well we presented new analyses showing that the interval between menopause and initiating hormonal therapy use has an important modifying effect on breast cancer risk.
The Million Women Study includes one in every four UK women who were aged 50–64 years at the time of recruitment. The characteristics of the study population are similar to those of women of a comparable age in the United Kingdom, with slightly greater proportions using hormonal therapy [33% at recruitment vs 31% nationwide (7,11)] and from the upper socioeconomic classes (28% from the top socioeconomic quintile in the recruitment areas) than in the general UK population. All study participants were followed by record linkage to routinely collected national cancer registration and death data. Information on cancer site and date of diagnosis was coded before notification to the study investigators, thus providing nondifferential ascertainment of incident cancers. To minimize differential reporting of exposures, information on hormonal therapy use was collected before any diagnosis of cancer and updated whenever possible during follow-up. [There is excellent agreement between women's reported use of hormonal therapy and general practitioner prescription records (14).]
A limitation of this study is that information on use of hormonal therapy was reported an average of 1.4 years before the breast cancers were diagnosed. Over this period, an estimated 97% of those classified as never users of hormonal therapy would still have been never users, and 88% of those classified as current users would still have been current users. Such residual misclassification of hormonal therapy use would slightly dilute the estimates of relative risk but would not produce spurious associations. Correcting for such misclassification, by use of the regression dilution approach (15), would increase the logarithm of the relative risk among current users of either type by a factor of approximately 1.2.
We found that the risks of breast cancer associated with hormonal therapy were considerably lower for screen-detected breast cancers than for non–screen-detected breast cancers (Figure 3), consistent with findings from meta-analyses (16,17), showing that use of hormonal therapy decreases mammographic sensitivity and specificity. The greater risks for estrogen receptor–positive than estrogen receptor–negative breast cancer have also been reported previously (18), as has a greater risk of lymph node involvement in users of estrogen–progestin therapy (19). It has been suggested that part of the increased hormone therapy–associated risk of breast cancer observed in this study may have resulted from selective recruitment of hormonal therapy users who already had symptoms of breast cancer. If that had happened, there would have been a greater excess of hormonal therapy–associated breast cancer soon after recruitment than subsequently. However, the opposite was found: the risk of breast cancer was lower in the first 4 months after recruitment than afterward (RR = 1.19 vs RR = 1.50, for estrogen-only; and RR = 1.41 vs RR = 2.32, for estrogen–progestin hormonal therapy), largely reflecting the lower hormonal therapy–associated risks observed for screen-detected breast cancers than for non–screen-detected breast cancers.
That hormonal therapy–associated relative risks for breast cancer were attenuated among overweight and obese women has been found in many studies (3,4,9). However, when incidence rates, rather than relative risks, were calculated, it was clear that this apparent attenuation was driven by adiposity-related breast cancer incidence among never users of hormonal therapy. Among never users, but not among users of hormonal therapy, incidence rates for breast cancer increased with increasing adiposity (Figure 5). Hence, the proportionate increase in breast cancer risk among hormone users was smaller among overweight and obese women than among leaner women. Circulating levels of endogenous estrogens increase with increasing body mass index among nonusers of hormonal therapy, and this observation explains most, if not all, of the increased risk of postmenopausal breast cancer with increasing adiposity (20). Because endogenous estrogen levels normally increase with adiposity among postmenopausal women, it seems plausible that hormonal therapy would change women's net exposure to sex hormones to a greater extent among leaner women than among obese women. Indeed, among obese women using estrogen-only hormonal therapy, breast cancer incidence rates did not differ statistically significantly from those of never users (Figure 5).
The epidemiological evidence has consistently shown that breast cancer risk among users of hormonal therapy returns to that of never users soon after use ceases (1–4), as is illustrated in Figure 2. The observed decline in breast cancer incidence rates in the United States and many other countries after 2002 that followed reductions in the prevalence of hormonal therapy use (21,22) provides independent support for the epidemiological data. If use of hormonal therapy merely accelerated the presentation of preexistent breast cancers, incidence rates in past users would be expected to fall well below those of never users. However, no marked reduction in breast cancer risk was observed after hormonal therapy use ceased (Figure 2), indicating that the breast cancers diagnosed while women used hormonal therapy were not just those that would have occurred anyway.
A new finding of this study, which has been little investigated previously, is that the interval between menopause and starting hormonal therapy has a substantial effect on breast cancer risk. A similar finding was first reported by Women's Health Initiative investigators (6), and results from a French study (23) suggested possible similar associations, but only in certain subgroups. In this large study, we found greater risks of breast cancer if hormonal therapy use began either before or soon after menopause than after a longer gap; and this pattern of risk was seen across different types of hormonal therapy, among women who used hormonal therapy for either short or long durations, and also in lean and in overweight and obese women. The findings were similar when various sensitivity analyses were done, such as restricting analyses to women with a bilateral oophorectomy or natural menopause who started hormonal therapy after their menopause.
The substantial heterogeneity in hormonal therapy–associated risks of breast cancer that we found, with independent effects of the type of hormonal therapy used, the interval between menopause and starting hormonal therapy, and the duration of hormonal therapy use could well account for much of the apparent variation in hormonal therapy–associated risks across studies (4). The populations that have been studied vary in terms of the prevalence of obesity and the interval between menopause and starting hormonal therapy. For example, in the Women's Health Initiative randomized trials, some 80% of participants were overweight or obese (5,10) and approximately 90% were randomly assigned to hormonal therapy 5 years or more after their menopause (6). Among users of estrogen-only hormonal therapy, we found little or no increase in the risk of breast cancer among overweight or obese women who started hormonal therapy 5 years or more after menopause (RR = 0.91, 95% CI = 0.73 to 1.14), consistent with findings from the Women's Health Initiative trial (RR = 0.77, 95% CI = 0.59 to 1.01) (10). The corresponding value for users of estrogen–progestin hormonal therapy in our data (RR = 1.39, 95% CI = 1.18 to 1.64) was also consistent with findings from the Women's Health Initiative trial (RR = 1.26, 95% CI = 1.00 to 1.59) (5). Moreover, breast cancer incidence rates in the estrogen–progestin randomized trial were similar to those observed here (0.46% per year in this study for use beginning 5 years or more after menopause and 0.43% per year in the randomized trial vs 0.3% per year in nonusers of hormone therapy both in this study and in the trial). However, breast cancer incidence rates in this study were highest among current users of estrogen–progestogen who began use less than 5 years after menopause (0.61% per year), and no comparable randomized data are available because the large majority of women were randomly assigned to estrogen–progestin more than 5 years after their menopause (6). Hence, the findings from randomized trials of hormonal therapy with respect to breast cancer risk may not apply to women who start using hormonal therapy at around the time of their menopause.
Funding
Public funds from Cancer Research UK and the UK Medical Research Council.
Footnotes
The funders did not influence the conduct of the study or the preparation of this report.
Steering Committee: Emily Banks, Valerie Beral, Ruth English, Jane Green, Julietta Patnick, Richard Peto, Gillian Reeves, Martin Vessey, and Matthew Wallis.
National Health Service Breast Screening Centres collaborating in the Million Women Study (in alphabetical order): Avon, Aylesbury, Barnsley, Basingstoke, Bedfordshire and Hertfordshire, Cambridge and Huntingdon, Chelmsford and Colchester, Chester, Cornwall, Crewe, Cumbria, Doncaster, Dorset, East Berkshire, East Cheshire, East Devon, East of Scotland, East Suffolk, East Sussex, Gateshead, Gloucestershire, Great Yarmouth, Hereford and Worcester, Kent (Canterbury, Rochester, Maidstone), Kings Lynn, Leicestershire, Liverpool, Manchester, Milton Keynes, Newcastle, North Birmingham, North East Scotland, North Lancashire, North Middlesex, North Nottingham, North of Scotland, North Tees, North Yorkshire, Nottingham, Oxford, Portsmouth, Rotherham, Sheffield, Shropshire, Somerset, South Birmingham, South East Scotland, South East Staffordshire, South Derbyshire, South Essex, South Lancashire, South West Scotland, Surrey, Warrington Halton St Helens and Knowsley, Warwickshire Solihull and Coventry, West Berkshire, West Devon, West London, West Suffolk, West Sussex, Wiltshire, Winchester, Wirral, and Wycombe.
Million Women Study Co-ordinating Centre: Simon Abbott, Miranda Armstrong, Krys Baker, Angela Balkwill, Vicky Benson, Valerie Beral, Judith Black, Anna Brown, Diana Bull, Ben Cairns, James Chivenga, Barbara Crossley, Gabriella Czanner, Dave Ewart, Sarah Ewart, Lee Fletcher, Toral Gathani, Laura Gerrard, Adrian Goodill, Jane Green, Isobel Green, Joy Hooley, Sau Wan Kan, Carol Keene, Oksana Kirichek, Nicky Langston, Maria-Jose Luque, Lynn Pank, Kirstin Pirie, Gillian Reeves, Andrew Roddam, Emma Sherman, Moya Simmonds, Elizabeth Spencer, Helena Strange, Sian Sweetland, Alison Timadjer, Sarah Tipper, Joanna Watson, Stephen Williams, and Lucy Wright.
We thank Adrian Goodill for drawing the figures, all the women who participated in the Million Women Study, collaborators from the National Health Service Breast Screening Centres, members of the study co-ordinating centre, and the study steering committee (listed below).
References
- 1.International Agency for Research on Cancer. Monograph on the Evaluation of Carcinogenic Risks to Humans. Hormonal Contraception and Post-Menopausal Hormonal Therapy. Vol 72. Lyon, France: IARC Press; 1999. [Google Scholar]
- 2.International Agency for Research on Cancer. Monograph on the Evaluation of Carcinogenic Risks to Humans. Combined Estrogen/Progestogen Contraceptives and Combined Estrogen/Progestogen Menopausal Therapy. Vol 91. Lyon, France: IARC Press; 2008. [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 women without breast cancer. Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 4.Medicines and Healthcare products Regulatory Agency. UK Public Assessment Report. Hormone-Replacement Therapy: Safety Update. London, UK: MHRA; 2007. http://www.mhra.gov.uk/Safetyinformation/. Accessed April 27, 2009. [Google Scholar]
- 5.The Women's Health Initiative Randomized Controlled Trial. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 6.Prentice RL, Manson JA, Langer, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170(1):12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Million Women Study Collaborators. Breast cancer and hormone replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organisation. International Classification of Diseases. 10th ed. Geneva, Switzerland: World Health Organisation; 1988. [Google Scholar]
- 9.Reeves GK, Travis R, Green J, et al. Incidence of breast-cancer risk and its subtypes in relation to individual and multiple low-penetrance genetic susceptibility loci. JAMA. 2010;304(4):426–434. doi: 10.1001/jama.2010.1042. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Watson J, Wise L, Green J. Prescribing of hormone therapy for menopause, tibolone, and bisphosphonates in women in the UK between 1991 and 2005. Eur J Clin Pharmacol. 2007;63(9):843–849. doi: 10.1007/s00228-007-0320-6. [DOI] [PubMed] [Google Scholar]
- 12.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10(7):1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 13.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: further results. Contraception. 1996;54(4):1S–106S. doi: 10.1016/s0010-7824(15)30002-0. [DOI] [PubMed] [Google Scholar]
- 14.Banks E, Beral V, Cameron R, et al. Agreement between general practice prescription data and self-reported use of hormone replacement therapy and treatment for various illnesses. J Epidemiol Biostat. 2001;6:357–363. doi: 10.1080/13595220152601837. [DOI] [PubMed] [Google Scholar]
- 15.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart-disease. Part 1. Prolonged differences in blood-pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 16.Banks E. Hormone replacement therapy and the sensitivity and specificity of breast cancer screening: a review. J Med Screen. 2001;8:29–35. doi: 10.1136/jms.8.1.29. [DOI] [PubMed] [Google Scholar]
- 17.Chiarelli AM, Kirsh VA, Klar NS, et al. Influence of patterns of hormone replacement therapy use and mammographic density on breast cancer detection. Cancer Epidemiol Biomarkers Prev. 2006;15(1):1856–1862. doi: 10.1158/1055-9965.EPI-06-0290. [DOI] [PubMed] [Google Scholar]
- 18.Althuis MD, Fergenbaum JH, Garcia-Closas M, et al. Etiology of hormone receptor–defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–1568. [PubMed] [Google Scholar]
- 19.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(10):1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endogenous Hormones and Breast Cancer Collaborative Group. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(19):1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 21.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 22.Kumle M. Declining breast cancer incidence and decreased HT use. Lancet. 2008;372(9639):608–610. doi: 10.1016/S0140-6736(08)61255-6. [DOI] [PubMed] [Google Scholar]
- 23.Fournier A, Mesrine S, Boutron-Ruault M-C, Clavel-Chapelon F. Estrogen-progestogen menopausal hormone therapy and breast cancer: does delay from menopause onset to treatment initiation influence risks? J Clin Oncol. 2009;27:5138–5143. doi: 10.1200/JCO.2008.21.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]