Autophagy is a critical survival mechanism that underlies the cellular response to a variety of death stimuli including that of oxidative stress. Known functions of autophagy that may promote cell survival from oxidants include the supply of substrates to maintain energy homeostasis during injury, the removal of damaged organelles that may otherwise trigger apoptosis and the degradation of oxidized proteins that may compromise cellular function. However, whether autophagy prevents cell death from oxidants by any of these mechanisms remains unclear and other novel unidentified functions of autophagy may mediate cell survival. Our recent work examined the function of autophagy in the resistance of hepatocytes to death from oxidative stress.
The function of autophagy was investigated in the hepatocyte model of oxidant stress induced by the superoxide generator menadione. Inhibition of macroautophagy in hepatocytes by a lentiviral genetic knockdown of ATG5 sensitized these cells to death from a normally nontoxic concentration of menadione. Death occurred through a sequence of events that included ATP depletion, mitochondrial death pathway activation leading to cytochrome c release, effector caspase 3 and 7 activation and resultant apoptosis. These findings suggest that macroautophagy has a protective mitochondrial effect either through the removal of damaged mitochondria by mitophagy or by the generation of substrates essential for the maintenance of ATP levels. However, the menadione-induced changes in mitochondria occur downstream of activation of the mitogen-activated protein kinase (MAPK) c-Jun N-terminal kinase (JNK) and its effector substrate c-Jun, a signaling pathway whose overactivation we had previously demonstrated mediates hepatocyte death from menadione. Menadione-induced JNK/c-Jun activation is converted from a transient to a prolonged activation in the absence of macroautophagy. JNK/c-Jun signaling is an upstream initiator of cell death as JNK/c-Jun inhibition blocks mitochondrial ATP depletion, subsequent downstream events and cell death. The mechanism of JNK overactivation in the absence of macroautophagy is unclear and potentially involves known functions of macroautophagy such as the removal of limited numbers of damaged mitochondria that otherwise would trigger JNK activation. Alternatively, the loss of macroautophagy may have modulated MAPK signaling more directly by altering levels of upstream activating kinases, scaffolding proteins or deactivating phosphatases that determine levels of JNK activation. The effects were not specific for JNK, as activation of the protective MAPK extracellular signal-regulated kinase (ERK) 1/2 is also increased by menadione treatment in the absence of macroautophagy. Although further studies must delineate the molecular mechanism, the findings do demonstrate for the first time that macroautophagy functions to downregulate a pro-apoptotic MAPK signaling pathway.
Our investigations examined the function of chaperone-mediated autophagy (CMA) as well as macroautophagy in hepatocyte resistance to oxidant stress. Inhibition of CMA by a genetic knockdown of LAMP-2A also sensitizes cells to death from menadione. Although a simultaneous genetic knockdown of both forms of autophagy could not be established due to lethality, the combination of a pharmacological inhibitor of macroautophagy in cells with a genetic knockdown of CMA leads to a greater amount of menadione-induced cell death than does the inhibition of either form of autophagy alone. The two types of autophagy mediate hepatocyte resistance to oxidant stress by distinct mechanisms. In contrast to findings in cells lacking macroautophagy, inhibition of CMA sensitizes cells to death from menadione without inducing JNK/c-Jun hyperactivation or cellular ATP depletion. CMA limits the chronic accumulation of oxidized proteins in the liver during aging. CMA may promote hepatocyte survival from menadione by removing oxidized proteins that initiate cell death pathways directly or indirectly through the promotion of cellular dysfunction. Aggregated proteins that accumulate in neurodegenerative disorders associated with oxidant stress are likely CMA targets in the brain, but specific protein targets in acute and more generalized oxidant injuries in the liver remain to be delineated. The synergistic increase in cell death from a loss of both macroautophagy and CMA, and their differential effects on JNK activity and cellular ATP levels, demonstrate distinct, non-overlapping functions for the two forms of autophagy in hepatocellular resistance to oxidative stress that need further elucidation.
The findings highlight important differences in autophagic function between hepatocytes and fibroblasts. Our prior investigations in ATG5 knockout murine embryonic fibroblasts (MEFs) revealed that macroautophagy and CMA have redundant functions in these cells during oxidant stress from menadione. MEFs lacking Atg5 and macroautophagy have increased resistance to menadione toxicity because the upregulation of CMA in compensation for the loss of macroautophagy protects the cells from oxidative stress. Thus, CMA is able to duplicate the functions of macroautophagy in MEFs and mediate survival from a menadione challenge. In contrast, although similar crosstalk occurs in hepatocytes that results in increased CMA in ATG5 knockdown cells, hepatocytes are sensitized to death from menadione. These findings indicate that CMA cannot replace the protective function of macroautophagy in hepatocytes as occurs in MEFs. Our study demonstrates that although crosstalk between these two autophagic pathways occurs in hepatocytes, the resultant upregulation of one form of autophagy cannot always replace the function of the impaired pathway. Functional redundancy for protection from oxidant stress does not exist for the pathways of macroautophagy and CMA in hepatocytes. This difference from MEFs may reflect an increased vulnerability of hepatocytes to oxidant stress, a greater dependency of hepatocytes on autophagy for resistance to oxidant stress or fundamental differences in the functions of autophagy in the two cell types.
The present study expands the mechanisms by which autophagy may protect against oxidative stress to include the regulation of MAPK signaling and establishes that macroautophagy and CMA perform distinct functions during hepatocellular oxidative stress (Fig. 1). Hepatic diseases mediated by oxidant stress may be promoted by factors that impair hepatocyte autophagic function such as lipid accumulation or aging. These findings highlight the need to develop additional compounds to increase autophagic function that may serve as therapeutic agents in the prevention or treatment of liver injury.
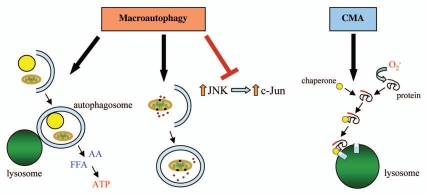
Figure 1.
Mechanisms by which autophagy promotes hepatocyte resistance to oxidative stress. In response to oxidative stress, macroautophagy can serve a protective function through the breakdown of cellular constituents such as lipid droplets and mitochondria to provide free fatty acids (FFA) and amino acids (AA) to maintain ATP levels, the removal of damaged mitochondria that might otherwise release pro-apoptotic factors or the inhibition of the JNK/c-Jun MAPK signaling pathway. CM A has a distinct and equally critical role in hepatocellular resistance to oxidant stress likely by mediating the degradation of oxidized proteins before they can trigger cell dysfunction and death.
Acknowledgements
Supported in part by NIH grants DK044234, DK061498 and AG031782.
Punctum to: Wang Y, Singh R, Xiang Y, Czaja MJ. Macroautophagy and chaperone-mediated autophagy are required for hepatocyte resistance to oxidant stress. Hepatology. 2010;52:266–277. doi: 10.1002/hep.23645.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13885