Abstract
Apoptotic defects endow tumor cells with survival advantages. Such defects allow the cellular stress response to take the path of cytoprotective autophagy, which either precedes or effectively blocks an apoptotic cascade. Inhibition of the cytoprotective autophagic response shifts the cells toward apoptosis, by interfering with an underlying molecular mechanism of cytoprotection. The current study has identified such a mechanism that is centered on the regulation of caspase-8 activity. The study took advantage of Bax-/- Hct116 cells that are TRAIL-resistant despite significant DISC processing of caspase-8, and of the availability of a caspase-8-specific antibody that exclusively detects the caspase-8 large subunit or its processed precursor. Utilizing these biological tools, we investigated the expression pattern and subcellular localization of active caspase-8 in TRAIL-mediated autophagy and in the autophagy-to-apoptosis shift upon autophagy inhibition. Our results suggest that the TRAIL-mediated autophagic response counter-balances the TRAIL-mediated apoptotic response by the continuous sequestration of the large caspase-8 subunit in autophagosomes and its subsequent elimination in lysosomes. The current findings are the first to provide evidence for regulation of caspase activity by autophagy and thus broaden the molecular basis for the observed polarization between autophagy and apoptosis.
Key words: apoptosis, autophagy, caspase-8, lysosome, TRAIL
Introduction
Apoptosis is a form of cell death that is executed by activation of caspases—highly potent and specific enzymes. Because of the need to tightly control this form of cell death, caspase activity needs to be strictly regulated. Caspases are intrinsically regulated by their own structure: They are expressed as weakly active zymogens, which require further cleavages to generate the required subunits for an active enzymatic complex.1,2 Other regulatory mechanisms are mediated by cellular inhibitors of caspases, including cellular FLIP and various inhibitors of apoptosis (IAPs), as well as by IAP antagonists.3,4 Certain IAPs can function as E3 ligases capable of inhibiting caspases by a direct binding, ubiquitination and subsequent proteosomal degradation.4 In addition, post-translational modifications, including phosphorylation and S-nitrosylation, can regulate caspase activity. Presently, the potential involvement of the autophagic degradation system in the regulation of caspase activity has not yet been reported.
Autophagy is an intracellular process in which cytoplasmic materials are transported by double-membraned autophagosomes to lysosomes for degradation.5–7 Whereas basal levels of autophagy ensure the physiological turnover of damaged organelles, a massive accumulation of autophagic vacuoles may represent either an alternative pathway of cell death or an ultimate attempt of the cells to survive by adapting to stress. Accumulating evidence suggests that a regulated autophagic response can provide cells with metabolic substrates to meet their energetic demands under stressful conditions, and thus promote survival. However, the interplay between autophagy and apoptosis is substantially complex and remains unresolved.8,9 In most cases where autophagy was suppressed by genetic KD (knockdown) or KO (knockout) of essential Atg genes, cell death has been accelerated, suggesting a prominent role for autophagy as a pro-survival response.10–17 Autophagy inhibition has been shown to sensitize tumor cells to various treatments, including irradiation,18–20 alkylating agents,21 or arsenic trioxide,22 suggesting that cancer cells can resist chemo- or radiation therapy through autophagy which serves as a mechanism of adaptation to stress. Despite the advances in the characterization of the autophagic response to stress, the molecular mechanisms that allow for the apoptotic sensitization of otherwise stress-resistant tumor cells have not yet been elucidated.
We recently reported that TRAIL-mediated cytoprotective autophagy is induced in apoptosis-deficient tumor cells, and that inhibition of the autophagic response sensitizes the otherwise resistant cells to apoptosis.23,24 Thus, in Bax-/- Hct116 colon carcinoma cells that are resistant to TRAIL-mediated apoptosis, TRAIL signaling mediates a protective autophagic response that takes place despite significant processing of caspase-8 upstream of mitochondrial apoptotic events. In the current study, we investigated the expression pattern and subcellular localization of active caspase-8 in TRAIL-mediated autophagy and in the autophagy-to-apoptosis shift upon autophagy inhibition. Our results suggest that autophagic degradation of a subunit of the active caspase-8 enzyme may serve to cap caspase-8 activity and polarize the two cell-fate determining processes—autophagy and apoptosis—so that only one can prevail at a time.
Results
Inhibition of autophagy induces caspase-8 activity.
Bax deficiency in Hct116 colon carcinoma cells endows these cells with significant resistance to TRAIL-mediated apoptosis.25–27 Instead, TRAIL signaling in these cells mediates a protective autophagic response that takes place despite significant processing of caspase-8 upstream of the mitochondria.24,28 Recently, we reported that autophagic inhibition by either KD of Beclin 1 or Vps34 shifts protective autophagy to apoptosis and that this shift is dependent on caspase-8 activity upstream of the mitochondria. To investigate the possibility that autophagy is involved in regulation of caspase-8 activity, we focused our studies on an assessment of the subcellular localization of processed/active caspase-8. Generation of mature caspase-8 requires several proteolytic steps. The first cleavage of procaspase-8 generates p43 and p12 fragments. The p12 fragment is further processed to yield the p10 small subunit and the p43 fragment is further cleaved to yield the p18 large subunit and the p29/26 N-terminal cleavage product (Fig. S1A). In the numerous immunoblotting experiments that we have performed for caspase-8, we observed that in certain tumor cell lines (including Bax-/- Hct116, RKO, MDA-MB-231, MCF7) only full-length caspase-8, but not the cleaved products, was detected in the cytosolic fraction. Yet, in other tumor cell lines such as WT Jurkat, the cytosolic fraction contained both full-length prodomain caspase-8 and its cleavage products (Fig. 1A). Studies by others also detected the processed caspase-8 fragments mainly in the membranous pellet (MP) and also associated with the mitochondrial outer membrane.29–31 In Bax-/- Hct116 cells treated with TRAIL, the processed caspase-8 fragments were mainly detected in a membranous fraction, which included mitochondria, ER, lysosomes and autophagosomes (Figs. 1A and S1B). In contrast, processed caspase-3 products were mainly detected in the cytosolic fraction (Fig. 1C). The shift from cytoprotective autophagy to apoptosis upon Beclin 1 or Vps34 RNAi was associated with increased processing of the caspase-8 prodomain, processing of caspase-3, cytochrome c release (Fig. 1C) and generation of an Annexin V-positive cell subpopulation (Fig. 1B). However, KD of caspase-8 prevented the exposure of phosphatidylserine (Fig. 1B) and the release of cytochrome c and SMAC from mitochondria to cytoplasm (Fig. 1D). These findings suggest that TRAIL-mediated autophagy keeps the activity of caspase-8 at bay and that autophagy inhibition allows for a significant increase in caspase-8 enzymatic activity.
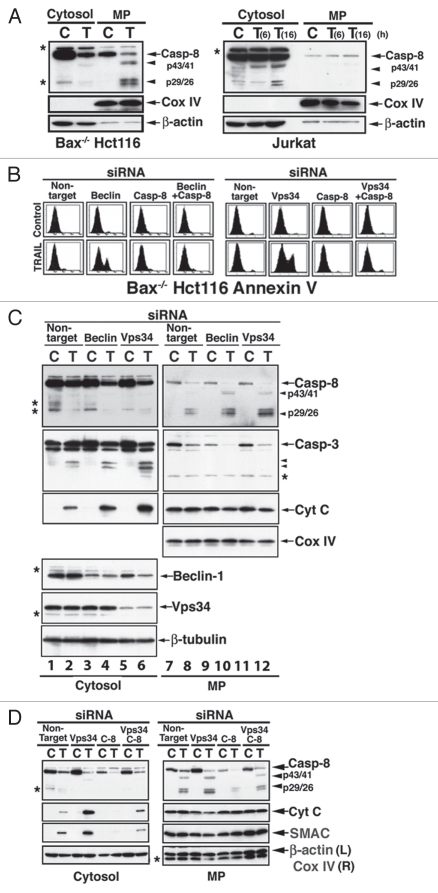
Figure 1.
Caspase-8 requirement for the shift from TRAIL-mediated autophagy to TRAIL-mediated apoptosis. (A) Cell-specific subcellular localization of cleaved caspase-8. Processed fragments of cleaved caspase-8 are associated with a membranous pellet (MP) in TRAIL-treated Bax-/- Hct16 cells (100 ng/ml 6 hr, left part, C = Control; T = TRAIL), but with cytosol in TRAIL-treated Jurkat cells (25 ng/ml, right part). Mitochondrial inner membrane cytochrome c oxidase (Cox) IV and β-actin served as loading controls for their respective subcellular fractions. (B) Inhibition of TRAIL-mediated autophagy induces caspase-8-dependent apoptosis. An autophagy-to-apoptosis shift is induced by Beclin 1 KD (left part) or Vps34 KD (right part), and it is inhibited by concomitant KD of caspase-8. Bax-/- Hct116 cells were treated with the indicated siRNA for 60 hr and then with TRAIL for 6 hr. The cells were assessed by Annexin V flow cytometry. (C) Inhibition of autophagy in TRAIL-treated Bax-/- Hct116 cells is associated with increased processing of caspase-8 and caspase-3, and cytochrome c release. Bax-/- Hct116 cells were treated with the indicated siRNAs and TRAIL as described in (B), and following cytosol and MP separation, the subcellular fractions were assessed by immunoblotting for the expression of the indicated proteins. Please note, the doublet protein bands detected by anti-caspase-8 Ab in control cells (lanes 1 and 3, marked by asterisks) are unidentified, as they do not align with the doublet detected in TRAIL-treated cells (lanes 8, 10 and 12). (D) The release of cytochrome c and SMAC that is induced by a combined treatment of Vps34 siRNA and TRAIL is inhibited by caspase-8 siRNA. Experimental details are described in (A and B).
To further investigate the involvement of caspase-8 in an autophagy-to-apoptosis shift under a different cellular setting, we utilized caspase-3-deficient MCF7 cells that were preselected for cisplatin resistance by multiple treatment cycles with escalating doses of cisplatin.23 Exposure of these apoptosis-deficient cells to a fresh dose of cisplatin induced an autophagic response as assessed by quantitative image cytometry of LC3 and Beclin 1 (Fig. 2A). To this end, we utilized the Cellomics ArrayScan that performs quantitative analyses of fluorescent cell images. The relative mean of LC3 spot fluorescent intensity or of their number per cell were increased in apoptosis-deficient MCF7 cells treated with cisplatin (Fig. 2A, left part). Also, the spot intensity of either cytosolic or nuclear Beclin 1 was increased in response to cisplatin (Fig. 2A, right part). In addition, increased expression of LC3-II was detected in cisplatin-resistant MCF7 cells treated with fresh cisplatin, suberoylanilide hydroxamic acid (SAHA) or TRAIL (Fig. 2B, left part). Furthermore, the presence of the cathepsin inhibitors E64D and pepstatin A enhanced the cisplatin-mediated accumulation of LC3-II, further indicating the occurrence of an autophagic flux (Fig. 2B, right part). Inhibition of cisplatin-mediated autophagy by KD of either Beclin 1 or Vps34 sensitized the otherwise resistant MCF7 cells to cisplatin as determined by Annexin V staining (Fig. 2C, left part) and the increased processing of the caspase-8 prodomain (Fig. 2C, right part). As assessed by immunoblotting, processing/activation of caspase-7 was detected in the treated MCF7 cells, in lieu of their lacking caspase-3 (Fig. 2C, right part). This autophagy-to-apoptosis shift was caspase-8 dependent since it was markedly inhibited by either caspase-8 peptide inhibitors (Fig. 2D, left part) or by caspase-8 RNAi (Fig. 2D, right part). These findings demonstrate that inhibition of autophagy increases the activity of caspase-8 in two different settings of stress-mediated autophagy in apoptosis deficient cells: TRAIL-treated Bax-/- Hct116 cells and cisplatin-treated apoptosis resistant MCF7 cells.
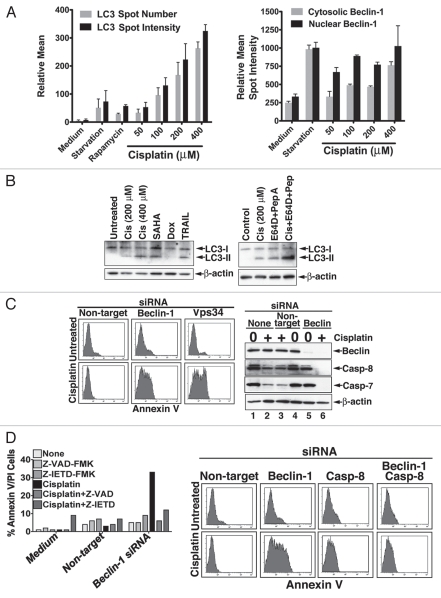
Figure 2.
Sensitization of apoptotic-deficient MCF7 cells to cisplatin by autophagy inhibition is caspase-8-dependent. (A) Quantitation by image cytometry of drug-mediated changes in LC3 (left part) and Beclin 1 (right part) in apoptotic-deficient MCF7 cells. Cisplatin-selected MCF7 cells were treated as indicated for 12 hrs (rapamycin, 2 µM). The cells were then fixed and stained with LC3- and Beclin 1-specific Abs, and fluorescence quantitation was performed by the Cellomics ArrayScan Image Cytometer. The data are means ± SEM of quadruplet determinations of relative spot intensity or spot number per 500 cells counted in each well. Similar results were obtained in at least three independent experiments. (B) Immunoblot analysis for the presence of LC3-II in cisplatin-resistant MCF7 cells treated with cytotoxic drugs. Left part shows induced expression of LC3-II in cisplatin-selected MCF-7 freshly treated with cisplatin, SAHA (2 µM), doxorubicin (100 ng/ml); or TRAIL (100 ng/ml). Right part demonstrates that the cisplatin-mediated increase in LC3-II represents autophagic flux. Cisplatin-selected MCF7 cells were freshly treated with cisplatin (200 µM) in the presence or absence of the cathepsin inhibitors, E64D and pepstatin A. MBL anti-LC3 Ab was used for probing, and β-actin served as a loading control. (C) Inhibition of autophagy by Beclin 1 or Vps34 RNAi reverses the resistance of MCF7 cells to cisplatin. Cisplatin-resistant MCF7 cells were treated with nontarget, Beclin 1 or Vps34 siRNAs for 72 hr and then with cisplatin (200 µM) for 12 hr. The apoptotic response was assessed by Annexin V (left part) and by the level of caspase-8 processing (right part). (D) The cisplatin-sensitization by Beclin 1 siRNA is inhibited in the presence of pharmacological inhibitors of caspases (left part) or by caspase-8 RNAi (right part). The experiments were performed as described in C, and a fresh cisplatin dose was added in the presence of Z-VAD-FMK or A-IETD-FMK (100 µM each). Apoptosis was determined by Annexin V/PI flow cytometry, and one experiment of at least three with similar results is shown. In the right part, cisplatin-selected MCF7 cells were treated as described in (C) with the addition of caspase-8 siRNA. The mild increase in cell death in cells treated only with caspase-8 siRNA may relate to interference with the survival activity of caspase-8.58 Similar results were obtained with siGENOME SMARTpool mixture of four non-overlapping siRNAs from Dharmacon and with three additional independent caspase-8 siRNAs from Invitrogen.
Subcellular localization of cleaved caspase-8 under autophagic or apoptotic conditions.
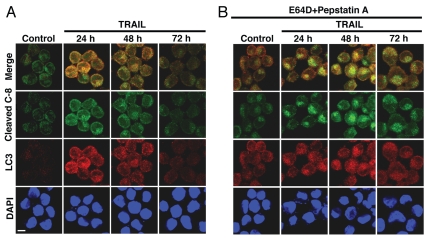
The differential membrane association of caspase-8 and caspase-3 cleaved products in Hct116 cells suggests that these proteins may feature distinct subcellular localizations. To further investigate this issue, we assessed by confocal microscopy potential subcellular localities for cleaved caspase-8 utilizing Ab that is specific to its cleavage site at Asp391, and thus detects only the processed large subunit (p18) and its precursor (p43/41), but not the full-length caspase-8 (Fig. S1A). As expected, the cleaved caspase-8 protein was detected exclusively in TRAIL-treated Bax-/- Hct116 cells, but not in untreated control cells (Fig. 3A). In TRAIL-treated cells, we observed the unexpected co-localization of the cleaved caspase-8 fragment with protein markers of the autophagic/lysosomal system, including LC3 and Beclin 1 (autophagosome/lysosome), LAMP2 (lysosome) and Bap31 (ER, potential site for autophagosome nucleation32). We also detected a weak co-localization with mitochondria, another potential site for autophagosome nucleation33 and activation of caspase-8,31 as well as limited co-localization with the cytoplasmic/cytoskeleton markers, β-tubulin (shown); β-actin (not shown), which may be involved in autophagosome translocation to and fusion with lysosomes.24,34 Remarkably, the observed co-localizations were noticeably enhanced in the presence of the cathepsin inhibitors, E64D and pepstatin A, suggesting potential autophagosomal/lysosomal involvement in the degradation of an active subunit of caspase-8 (Fig. 3A and S2). In TRAIL-sensitive WT Hct116 cells cleaved caspase-8 co-localized with accumulated LC3 only in the few cells that remained TRAIL-resistant, as compared to those demonstrating nuclear fragmentation (Fig. 3B). The loss in LC3 in cells with nuclear fragmentation may relate to their apoptotic level. In contrast to cleaved caspase-8, full-length caspase-8 exhibited markedly reduced co-localization with LC3 (Fig. 3C). Furthermore, cleaved caspase-8 demonstrated a distinct subcellular localization from that of full-length caspase-7 (Fig. 3D), full-length caspase-8 (Fig. S3A) and full-length and cleaved caspase-3 (Fig. S3 and AB), particularly in the presence of lysosomal inhibitors when cleaved caspase-8 mainly accumulated in autophagolysosomes. Thus, procaspase-8 and procaspase-3 did not co-localize with LAMP2 in either control or TRAIL-treated Bax-/- Hct116 cells. Yet, cleaved caspase-8 was mainly co-localized with LAMP2, whereas the majority of cleaved caspase-3 was excluded from lysosomes (Fig. S3).
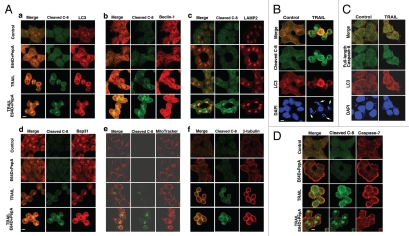
Figure 3.
The co-localization of cleaved caspase-8 subunit with components of the autophagic process increases in the presence of E64D and pepstatin A. (A) Accumulation of cleaved caspase-8 in Bax-/- Hct116 cells treated with TRAIL (100 ng/ml, 5 hr) in the presence of E64D/pepstatin A and its co-localization with LC3 (autophagosome), Beclin 1 (autophagosomal nucleation), LAMP2 (lysosome) and Bap31 (ER), but at a reduced level with MitoTracker or β-tubulin. (B) Co-localization of cleaved caspase-8 with LC3 in the minor fraction of non-apoptotic TRAIL-treated WT Hct116 cells. Cells with fragmented nuclei (major fraction) demonstrate an increase in cleaved caspase-8, but no increase in LC3. Nuclei were stained by DAPI. (C) Partial co-localization of full-length caspase-8 (N-terminus-specific mAb) with LC3 in control or TRAIL-treated Bax-/- Hct116 cells. (D) Differential localization of cleaved caspase-8 and full-length caspase-7 in TRAIL treated Hct116 Bax-/- cells. Scale bars = 40 µm. Please note that in the presence of E64D/PepA, there is an increased distinction between the localization of accumulated cleaved caspase-8 and full-length caspase-7.
In contrast to TRAIL-resistant Hct116 cells, in TRAIL-treated WT Jurkat cells the highly expressed cleaved caspase-8 was spread out all over the cytoplasm (Fig. S4A), with concomitant loss in the detection of Beclin 1 and β-tubulin, two confirmed substrates of caspase-8 (reviewed in refs. 35 and 36 and our unpublished data). In Jurkat cells, TRAIL did not induce the expression of LC3, although some of the cleaved caspase-8 protein co-localized with LAMP2. These findings suggest that depending on the apoptosis-sensitivity of the treated cells, cleaved caspase-8 localized to either the cytosol or to sequestering vesicles such as autophagosomes. In Bax-/- Hct116 cells, co-treatment with TRAIL and inhibition of autophagy by either Beclin 1 KD (Fig. S4B) or Vps34 KD (not shown) resulted in high cytosolic expression of cleaved caspase-8 that was associated with nuclear fragmentation in a similar fashion to that observed for TRAIL-treated WT Jurkat cells.
Lysosomal degradation of cleaved caspase-8 protein.
To investigate the regulation of caspase-8 in TRAIL-resistant cells, we compared the expression levels of caspase-8 in Bax-/- Hct116 treated with either a single or multiple TRAIL doses. In cells treated with a single dose, the level of full-length caspase-8 was reduced as early as 6 hrs after treatment, but resumed 24 hrs later (Fig. 4A, left part). In contrast, successive daily TRAIL applications maintained a continuously low level of prodomain caspase-8 for the duration of the treatments (Fig. 4A, right part). Similar patterns for the reduction of caspase-3 expression levels were observed. As assessed by a long-term clonogenic assay, the Bax-/-Hct116 cells remained TRAIL-resistant even when subjected to a multidose TRAIL treatment regimen (Fig. 4B).
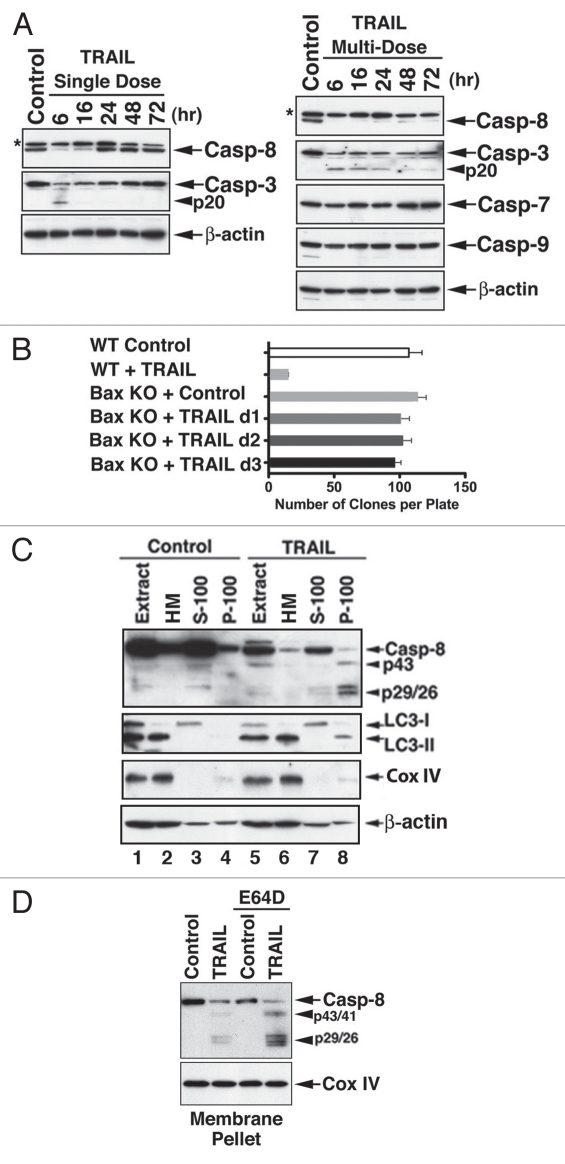
Figure 4.
The clonogenic capability of Bax-/- Hct116 cells is not affected by repeated TRAIL treatments despite continuous processing of caspase-8. (A) processing of caspase-8 in response to a single (left part) or multiple (right part) TRAIL treatments. Bax-/- Hct116 cells were treated once or daily for three days with TRAIL (100 ng/ml). Expression of the various caspases was assessed by immunoblotting. Please note, caspase-3 is processed only into p20, which remains inhibited in the absence of a mitochondrial contribution. The asterisks indicate unidentified protein bands. (B) Clonogenicity of Bax-/- Hct116 cells successively treated with a daily fresh dose of TRAIL (100 ng/ml) for 3 days as compared to WT Hct116 cells treated once with TRAIL (25 ng/ml, 6 hr). Colonies that developed during a 14-day culture in a methylcellulose-based medium in 35-mm plates were counted using an inverted microscope and gridded screen. The data (means ± SE M) were derived from three replicates in one of three experiments with equivalent results. Clonogenic assays were performed 24 hr after the first (d1), second (d2) and third (d3) TRAIL treatments of Bax-/- Hct116 cells. (C) Co-localization of cleaved caspase-8 with P-100 LC3-II during TRAIL-mediated autophagy. Control and TRAIL-treated Bax-/- Hct116 cells were subcellular fractionated to obtain purified mitochondria, S-100 and P-100 fractions. S-100 is regarded as cytosolic fraction, and P-100 as light membrane fraction. While LC3-I was detected in the S-100, LC3-II and cleaved caspase-8 were detected in the P-100 fraction. The loading in this experiment was cell-related, as indicated by the expression levels of Cox IV and β-actin. The presence of β-actin in the P-100 fraction may relate to its reported association with autophagosomes,34 and it serves to demonstrate an equal loading between control P-100 (lane 4) and TRAIL-treated cell P-100 (lane 8). (D) Accumulation of processed caspase-8 fragments in MP fraction of Bax-/- Hct116 cells treated with TRAIL in the presence of E64D.
Because immunoblotting detected the processed fragments of caspase-8 in the MP fraction (Fig. 1) that included both heavy-membrane and light-membrane organelles (Fig. s1B), we subjected the cells to a subcellular fractionation that produces purified mitochondria (with less than 5% microsomal contamination), cytosolic S-100 fraction and its light-membrane pellet fraction P-100. Caspase-8 processed fragments, p43 and p29/26, were detected in co-localization with LC3-II in the P-100 fraction of TRAIL treated Bax-/- Hct116 cells, but not in the mitochondrial fraction (Fig. 4C). LC3-II was also associated with the mitochondrial fraction, potentially marking mitophagy-targeted mitochondria,37 and/or resulting from the use of mitochondrial membrane in autophagic nucleation.38 Interestingly, LC3-II was detected only in P-100 of TRAIL treated cells, but not in control cells. Furthermore, in the presence of E64D, the processed fragments of caspase-8 accumulated in the MP fraction of the TRAIL-treated Bax-/- Hct116 cells (Fig. 4D). These findings represent additional biochemical evidence for the co-localization with LC3-II of processed p43 caspase-8 protein, that upon further processing generates the p18 and p29/26 products. Of note, in Bax-/- Hct116 cells p18 is not detectable by immunoblotting.29
The multidose TRAIL treatment regimen was then utilized to assess the subcellular localization and fate of cleaved caspase-8. As expected, induced expression of cleaved caspase-8 was detected early after each of the TRAIL treatments, but it was considerably diminished 24–30 hr after the last TRAIL treatment. Concomitantly, the expression of punctate LC3 was also increased, with significant co-localization with cleaved caspase-8. The duration of the punctate LC3 expression resembled that of cleaved caspase-8, diminishing as well approximately 24–30 hr post the third TRAIL treatment (Fig. 5A). These findings suggested that the same degradation mechanism is responsible for LC3 and cleaved caspase-8. Indeed, the presence of the cathepsin inhibitors, E64D and pepstatin A, which significantly block lysosomal degradation, maintained the expression levels of either cleaved caspase-8 or punctate LC3 after each of the TRAIL treatments (Fig. 5B).
Figure 5.
Enhanced co-localization of cleaved caspase-8 with LC3-positive punctates in cells treated with TRAIL and cathepsin inhibitors. Bax-/- Hct116 cells were treated daily with TRAIL (A) or with TRAIL and E64D/pepstatin A (B). Cytospins that were prepared at the indicated time points were all stained and assessed by confocal microscopy at the same time. The co-accumulation of cleaved caspase-8 with LC3 is appreciably increased early after TRAIL treatment (24 hr) with a marked reduction in the following days (A); the accumulation of cleaved caspase-8 is most pronounced with less reduction in subsequent days in cells treated with TRAIL and E64D/pepstatin A (B).
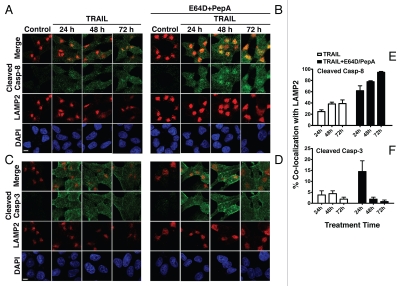
To further confirm the lysosomal role in the degradation of the cleaved caspase-8 protein, we assessed its co-localization with LAMP2 during three successive TRAIL treatments. Co-localization of cleaved caspase-8 with LAMP2 was markedly increased in cells treated with TRAIL in the presence of E64D and pepstatin A, suggesting that in the absence of the inhibitors cleaved caspase-8 is effectively degraded (Fig. 6A, B and E). Since the degradation is an ongoing process and the images represent snapshots of specific time points (each 24 hr after one of three successive TRAIL treatments), it is not surprising that a fraction of cleaved caspase-8 is still detected in the cytosol. As compared to cleaved caspase-8, cleaved caspase-3 demonstrated reduced co-localization with LAMP2, as well as a reduced impact on its expression level by the presence of E64D and pepstatin A (Fig. 6C, D and F). The differential expression patterns of cleaved caspase-8 and cleaved caspase-3 are further depicted in cells treated with a single dose of TRAIL in the presence of E64D and pepstatin A (Fig. S5). The subcellular localization of their active caspase subunits is as distinct as is their response to the marked inhibition of cathepsin activity. Because of its co-localization with lysosomes (LAMP2), lysosomal inhibitors mediate a significant accumulation of cleaved caspase-8, but they have a markedly reduced effect on the expression level of cleaved caspase-3.
Figure 6.
Differential patterns of lysosomal accumulation of cleaved caspase-8 as compared to cleaved caspase-3 in Bax-/- Hct116 cells treated with TRAIL and cathepsin inhibitors. Bax-/- Hct116 cells were treated daily with a fresh dose of TRAIL (100 ng/ml) for three consecutive days in the absence (A and C) or presence (B and D) of E64D/pepstatin A. The staining was performed on adherent cells in chamber slides. Of note, degradation of cleaved caspase-8 (A and B), but not of caspase-3 (C and D), is inhibited by E64D/pepstatin A. Scale bars = 40 µm. (E and F) Quantitation of co-localization with LAMP2 of cleaved caspase-8 (E) and cleaved caspase-3 (F). The co-localization was measured by Metamorph (Molecular Devices, Downingtown, PA) and performed on cells obtained from at least three independent experiments. The data are means ± SE M of 20–25 individually analyzed stained cells per sample.
Discussion
Stress-mediated cytoprotective autophagy develops in apoptosis-deficient tumor cells in response to various forms of stress, including radiation,19,39 cytotoxic drugs,40,41 or activation of a death receptor signaling cascade.28,42 However, this adaptive response may shift to apoptosis or necrosis when the autophagic process is inhibited. The polarization between cytoprotective autophagy and apoptosis or necrosis mandates the existence of tight regulatory process that keeps in check one process while the other prevails. The current study identified a cross-regulation mechanism between these cell fate-determining processes that utilizes the core degradation machinery of autophagy to cap caspase-8 activity.
Previous studies that reported the autophagic association of caspase-8 and FADD mainly focused on the induction of autophagy in the stress response of cells deficient in caspase-8 or harboring a dominant-negative FADD.43–46 Thus, inadequacy in the DISC composition shifts the signaling mechanism toward autophagy that may function in different settings as either a survival46 or a death mechanism.43–45 The mechanism identified in the current study takes place downstream of the DISC, where properly cleaved caspase-8 is selectively eliminated by lysosomal degradation, keeping the apoptotic response at bay.
The research approach took advantage of a Bax-deficient cell line that develops cytoprotective autophagy in response to TRAIL signaling. Bax-/- Hct116 cells maintain their long-term clonogenecity despite effective processing of caspase-8 at the DISC in response to TRAIL. Analysis of active caspase-8 expression patterns and its subcellular distribution led to the conclusion that the majority of the active caspase-8 large subunit or its cleaved precursor is sequestered by autophagosomes and degraded by lysosomes when cytoprotective autophagy prevails. Inhibition of the autophagosome nucleation phase is associated with changes in the expression level and the locality of cleaved caspase-8. Thus, in addition to eliminating misfolded proteins and damaged organelles, the adaptive autophagic response represses a potential caspase-8-mediated apoptotic response by continuous elimination of the caspase-8 large subunit precursor and/or the large subunit itself.
Autophagic repression of caspase-8 appears to be cell-type specific, as in highly TRAIL-sensitive cells the apoptotic response prevails and the high cytosolic levels of active caspase-8 may reflect not only the cause but also the consequence of apoptotic targeting of components of autophagy, as described for Beclin 1, Atg4 and Atg5.47–50 Thus, the sensitivity to caspase-8 may not completely depend on its processing levels, but also on the cell's ability to sequester at least one of the active subunits or its precursor. Although active caspase-8 has been reported to associate with the heavy membrane fraction and particularly mitochondria, we demonstrate that under conditions associated with cytoprotective autophagy, it mainly co-localizes with LC3-II and LAMP2, markers of autophagosomes and autophagolysosomes. Interestingly, full-length caspase-8 as well as other full-length or processed caspases, including caspase-3 and -7 did not demonstrate a significant level of co-localization with LC3 or LAMP2 as detected for cleaved caspase-8.
Although activation of caspase-8 is mainly associated with death receptor signaling cascades, it is also activated downstream of the mitochondria.51,52 In cisplatin-resistant MCF7 cells, a fresh treatment by cisplatin was associated with caspase-8 processing and induction of cytoprotective autophagy. The cisplatin-resistance was abrogated by inhibition of the autophagic nucleation phase by Beclin 1 or Vps34 RNAi. In resemblance to TRAIL-mediated autophagy, the shift from autophagy to apoptosis was caspase-8-dependent, as the KD of other caspases in these cells did not block the apoptotic shift observed with autophagy inhibition. These findings underscore the role of caspase-8 in the shift from autophagy to apoptosis in at least two different biological settings and further indicate the importance of the autophagic degradation of cleaved caspase-8.
It is also interesting to note that relative to its own full-length prodomain containing precursor, as well as other prodomain precursors and active caspases, active caspase-8 appears to be selectively sequestered by autophagosomes. More advances in the field are needed to elucidate the mechanism(s) involved in the observed selectivity.
Materials and Methods
Reagents.
Rabbit Ab specific for cleaved caspase-8 was from Cell Signaling (9496); Anti-caspase-3 Ab from StressGen (AAP-113) was utilized for detection of cleaved subunits and from Cell Signaling Technology (9661) for the prodomain detection; Ab to N-terminal epitopes of caspase-7 was from BD-Biosciences (610812) and from Epitomics for the N-terminal epitopes of caspase-8 (1006-1) and caspase-9 (1084-1); Anti-MAP-LC3 Abs for immunoblotting were from AnaSpec (29783) and from MBL (PD014); anti-MAP-LC3 Ab for immunostaining was from Santa Cruz (sc-28266). Abs specific for LAMP2 (sc-18822), Beclin 1 (sc-11427), β-tubulin (sc-9104) and cytochrome c (sc-13156) were from Santa Cruz. Anti-β-actin Ab (A5316) from Sigma-Aldrich; Anti-SMAC Ab (2954) was from Cell Signaling; Anti-Cox IV Ab (A21347) and Alexa Fluor 488 or 647-conjugated anti-rabbit or anti-mouse Ig were from Invitrogen; Recombinant TRAIL was from PeproTech; E64D from Calbiochem; Pepstatin A and DAPI were from Sigma; Z-VAD FMK and Z-IETD-FMK were from ICN.
Cytosol and membranous pellet fractionation.
Following TRAIL treatment, cytosolic and membranous pellet (MP) fractions were separated using a digitonin-based subcellular fractionation technique as described previously.53 A detailed description is included in the Supplemental data. The cytosolic fraction was enriched in β-actin and LC3-I, whereas the MP fraction was enriched in mitochondria (cytochrome c), ER (Bap31), lysosomes (LAMP2) and autophagosomes (LC3-II) (Fig. S1B).
Mitochondria purification.
To obtain an enriched mitochondrial fraction, Hct116 or Jurkat cells were suspended in mitochondrial buffer (MIB) composed of 0.3 M sucrose, 10 mM MOPS, 1 mM EDTA and 4 mM KH2PO4 pH 7.4 and subjected to Dounce homogenization. The purified mitochondrial fraction was obtained as previously described.54 Mitochondrial fraction purity was approximately 95%, with 5% or less contamination from the microsomal fraction.54–57 The post-mitochondrial fraction was further spun to obtain the S-100 cytosolic fraction and the P-100 light membrane fraction.
RNAi.
Beclin 1, Vps34 and caspase-8 siRNAs as well as the matching nontargeting controls were obtained as siGENOME SMARTpool or ON-TARGET plus SMARTpool siRNAs from Dharmacon. These reagents consist of four distinct RNA oligoduplexes per target or nontarget. Additional individual Beclin 1, Vps34 and caspase-8 siRNAs and their appropriate negative controls were obtained from Invitrogen. RNAi for each gene included three individual Stealth Select siRNAs and matching Stealth negative control. All KD experiments were repeated with at least two distinct siRNAs per target with similar results. Transfection of siRNA was performed with Oligofectamine according to the manufacturer's transfection protocol (Invitrogen).
Confocal microscopy.
Adherent tumor cells were grown and treated on Lab-Tek II chamber slides, whereas cytospins on noncoated slides were made for Jurkat cells. The cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. MitoTracker Deep Red staining was performed with live cells. Images were captured by a confocal Olympus FluoView 1000 system using a 60X oil immersion objective with the companion FV10-ASW1.6 imaging software at 25°C. All compared images were obtained using identical exposure and gain settings. The digital images were split and merged using ImageJ and image contrast and brightness were adjusted with Adobe Photoshop.
Image cytometry.
The Cellomics ArrayScan High-Content Screening (HCS) Reader (Cellomics/Thermo Fisher, Pittsburgh, PA) was utilized to collect information on distribution of fluorescently labeled components in the treated cells. The ArrayScan HCS system is further described in the Supplemental Data.
Western blot analysis.
Proteins in cell lysates, cell extracts and the various subcellular fractions were resolved by SDS-PAGE and transferred to PVDF membranes, as previously described.54,57 Following probing with a specific primary Ab and horseradish peroxidase-conjugated secondary Ab, the protein bands were detected by enhanced chemiluminescence (Pierce).
Cell survival assays.
Clonogenic assays were performed with methylcellulose-based semisolid medium (MethoCult H4230, StemCell Technologies) according to the manufacturer's protocol. In brief, after treatment the cells were washed, suspended in MethoCult medium and cultured in triplicates (300 cells/3 ml) in 35-mm Petri dishes. The cultures were maintained at 37°C in 5% CO2 for 14 days and colonies were counted using an inverted microscope and gridded scoring dishes. Cytofluorometric analyses of apoptosis were performed by co-staining with propidium iodide (PI) and fluorescein isothiocyanate-Annexin V conjugates (Becton-Dickinson).
Acknowledgements
NIH Grants RO1 CA134776 and RO1 CA111786 (H.R.), The Hillman Foundation (H.R.) and The Pittsburgh Foundation (J.H.).
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13038
Supplementary Material
References
- 1.Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]
- 2.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasula SM, Ashwell JD. IAPs: what's in a name? Mol Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell. 2008;15:344–357. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Autophagy. 2007;3:28–31. doi: 10.4161/auto.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine B, Kroemer G. Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell Death Differ. 2009;16:1–2. doi: 10.1038/cdd.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 11.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karantza-Wadsworth V, White E. Role of autophagy in breast cancer. Autophagy. 2007;3:610–613. doi: 10.4161/auto.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathew R, White E. Why sick cells produce tumors: the protective role of autophagy. Autophagy. 2007;3:502–505. doi: 10.4161/auto.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White E. Autophagic cell death unraveled: Pharmacological inhibition of apoptosis and autophagy enables necrosis. Autophagy. 2008;4:399–401. doi: 10.4161/auto.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samaddar JS, Gaddy VT, Duplantier J, Thandavan SP, Shah M, Smith MJ, et al. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol Cancer Ther. 2008;7:2977–2987. doi: 10.1158/1535-7163.MCT-08-0447. [DOI] [PubMed] [Google Scholar]
- 17.Schoenlein PV, Periyasamy-Thandavan S, Samaddar JS, Jackson WH, Barrett JT. Autophagy facilitates the progression of ERalpha-positive breast cancer cells to antiestrogen resistance. Autophagy. 2009;5:400–403. doi: 10.4161/auto.5.3.7784. [DOI] [PubMed] [Google Scholar]
- 18.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 19.Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. 2005;26:1401–1410. [PubMed] [Google Scholar]
- 20.Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007;14:500–510. doi: 10.1038/sj.cdd.4402039. [DOI] [PubMed] [Google Scholar]
- 21.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 22.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–2108. [PubMed] [Google Scholar]
- 23.Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Enhancement of tumor-TRAIL susceptibility by modulation of autophagy. Autophagy. 2008;4:940–943. doi: 10.4161/auto.6769. [DOI] [PubMed] [Google Scholar]
- 24.Han J, Hou W, Goldstein LA, Lu C, Stolz DB, Yin XM, et al. Involvement of Protective Autophagy in TRAIL Resistance of Apoptosis-defective Tumor Cells. J Biol Chem. 2008;283:19665–19677. doi: 10.1074/jbc.M710169200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor—induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 26.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 28.Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Stegh AH, Barnhart BC, Volkland J, Algeciras-Schimnich A, Ke N, Reed JC, et al. Inactivation of caspase-8 on mitochondria of Bcl-xL-expressing MCF7-Fas cells: role for the bifunctional apoptosis regulator protein. J Biol Chem. 2002;277:4351–4360. doi: 10.1074/jbc.M108947200. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, et al. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 33.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, et al. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J. 2010;29:969–980. doi: 10.1038/emboj.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin 1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2009;274:95–100. doi: 10.1016/j.canlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Djavaheri-Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene. 29:1717–1719. doi: 10.1038/onc.2009.519. [DOI] [PubMed] [Google Scholar]
- 37.Kanki T, Wang K, Cao Y, Baba M, Klionsky DJ. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell. 2009;17:98–109. doi: 10.1016/j.devcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vellai T, Klionsky DJ. A second report from the EMBO conference on autophagy: Mechanism, regulation and selectivity of autophagy. Autophagy. 2010;6:197–198. doi: 10.4161/auto.6.1.10819. [DOI] [PubMed] [Google Scholar]
- 39.Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68:1485–1494. doi: 10.1158/0008-5472.CAN-07-0562. [DOI] [PubMed] [Google Scholar]
- 40.Katayama M, Kawaguchi T, Berger MS, Pieper RO. DNA damaging agent-induced autophagy produces a cytoprotective adenosine triphosphate surge in malignant glioma cells. Cell Death Differ. 2007;14:548–558. doi: 10.1038/sj.cdd.4402030. [DOI] [PubMed] [Google Scholar]
- 41.Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corcelle EA, Puustinen P, Jaattela M. Apoptosis and autophagy: Targeting autophagy signalling in cancer cells-‘trick or treats’? FEBS J. 2009;276:6084–6096. doi: 10.1111/j.1742-4658.2009.07332.x. [DOI] [PubMed] [Google Scholar]
- 43.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 44.Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, Luhrs KA, et al. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci USA. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 46.Thorburn J, Moore F, Rao A, Barclay WW, Thomas LR, Grant KW, et al. Selective inactivation of a Fas-associated death domain protein (FADD)-dependent apoptosis and autophagy pathway in immortal epithelial cells. Mol Biol Cell. 2005;16:1189–1199. doi: 10.1091/mbc.E04-10-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17:268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou YC, Hannigan AM, Gorski SM. An executioner caspase regulates autophagy. Autophagy. 2009;5:530–533. doi: 10.4161/auto.5.4.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 50.Betin VM, Lane JD. Caspase cleavage of Atg4D stimulates GABARAP-L1 processing and triggers mitochondrial targeting and apoptosis. J Cell Sci. 2009;122:2554–2566. doi: 10.1242/jcs.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Vries JF, Wammes LJ, Jedema I, van Dreunen L, Nijmeijer BA, Heemskerk MH, et al. Involvement of caspase-8 in chemotherapy-induced apoptosis of patient derived leukemia cell lines independent of the death receptor pathway and downstream from mitochondria. Apoptosis. 2007;12:181–193. doi: 10.1007/s10495-006-0526-6. [DOI] [PubMed] [Google Scholar]
- 52.Ferreira CG, Span SW, Peters GJ, Kruyt FA, Giaccone G. Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Res. 2000;60:7133–7141. [PubMed] [Google Scholar]
- 53.Adrain C, Creagh EM, Martin SJ. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001;20:6627–6636. doi: 10.1093/emboj/20.23.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han J, Goldstein LA, Hou W, Rabinowich H. Functional linkage between NOXA and Bim in mitochondrial apoptotic events. J Biol Chem. 2007;282:16223–16231. doi: 10.1074/jbc.M611186200. [DOI] [PubMed] [Google Scholar]
- 55.Han J, Goldstein LA, Gastman BR, Froelich CJ, Yin XM, Rabinowich H. Degradation of Mcl-1 by granzyme B: implications for Bim-mediated mitochondrial apoptotic events. J Biol Chem. 2004;279:22020–22029. doi: 10.1074/jbc.M313234200. [DOI] [PubMed] [Google Scholar]
- 56.Han J, Goldstein LA, Gastman BR, Rabinovitz A, Rabinowich H. Disruption of Mcl-1.Bim complex in granzyme B-mediated mitochondrial apoptosis. J Biol Chem. 2005;280:16383–16392. doi: 10.1074/jbc.M411377200. [DOI] [PubMed] [Google Scholar]
- 57.Han J, Goldstein LA, Gastman BR, Rabinowich H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem. 2006;281:10153–10163. doi: 10.1074/jbc.M510349200. [DOI] [PubMed] [Google Scholar]
- 58.Yi CH, Yuan J. The Jekyll and Hyde functions of caspases. Dev Cell. 2009;16:21–34. doi: 10.1016/j.devcel.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.