Abstract
T cell receptor activation induces inositol 1,4,5 trisphosphate (IP3)-mediated calcium signaling that is essential for cell metabolism and survival. Moreover, inhibitors of IP3 or pharmacological agents that disrupt calcium homeostasis readily induce autophagy. Using a glucocorticoid-sensitive CD4/CD8 positive T cell line, we found that dexamethasone prevented both IP3-mediated and spontaneous calcium signals within a timeframe that correlated with the induction of autophagy. We determined that this loss in IP3-mediated calcium signaling was dependent upon the downregulation of the Src kinase Fyn at the mRNA and protein level. Because it has previously been shown that Fyn positively regulates IP3-mediated calcium release by phosphorylating Type I IP3 receptors (IP3R1), we investigated the effect of glucocorticoids on IP3R1 phosphorylation at Tyr353. Accordingly, glucocorticoid-mediated downregulation of Fyn prevented IP3R1 phosphorylation at Tyr353. Moreover, selective knockdown of Fyn or treatment with a Src inhibitor also attenuated IP3-mediated calcium release and induced autophagy. Collectively, these data indicate that glucocorticoids promote autophagy by inhibiting IP3-dependent calcium signals. These findings carry important therapeutic implications given the widespread use of dexamethasone as both a chemotherapeutic and immunosuppressive agent.
Key words: autophagy, calcium, Fyn, IP3 receptor, dexamethasone
Introduction
Calcium is a versatile and dynamic 2nd messenger that regulates numerous biological processes including apoptosis and autophagy.1 In lymphocytes, calcium is released from the endoplasmic reticulum (ER) to the cytosol following antigenic stimulation.2 Ligation of T cell receptors induces a signaling cascade that is regulated by several tyrosine kinases and phosphatases. For example, Src family kinases Fyn and Lck regulate calcium release by catalyzing the activation of phospholipase Cγ to generate cellular pools of IP3.3–5 In addition, both Fyn and Lck physically interact with IP3 receptors to positively regulate ER calcium release.6,7 Fyn specifically phosphorylates Type I IP3R (IP3R1) in the IP3-binding domain, thereby increasing its affinity for IP3 and stimulating the release of calcium.8
When calcium is released from the ER, it is transported across the mitochondrial membrane by the uniporter calcium channel.9 The ability of calcium to be taken up by mitochondria is facilitated by the close proximity of the two organelles. Upon entry into the mitochondria, calcium functions as a cofactor by activating enzymes that are required for the generation of ATP.10 This process is essential for T cell development given that positive selection of thymocytes requires IP3-mediated calcium release to generate a sufficient pool of mitochondrial ATP.11 Alternatively, cytosolic calcium that is not taken up by mitochondria can activate the phosphatase calcineurin which subsequently catalyzes the de-phosphorylation of NFAT, a prosurvival transcription factor that regulates proliferative cytokines like IL-2.12,13
Glucocorticoids are immunomodulatory hormones that inhibit IP3-mediated calcium signals and block cell proliferation.14 Because of this they are potent immunosuppressive agents. For example, glucocorticoids block T cell activation by preventing phosphorylation of T cell signaling molecules, such as Fyn, and downstream mitogen activated protein kinases.15,16 The ligand-activated glucocorticoid receptor also inhibits IL-2 synthesis by blocking NFAT and NFκB-dependent transcriptional activation.17–19 Thus, synthetic glucocorticoid derivatives (e.g., prednisone and dexamethasone) are widely used as immunosuppressive agents in virtually all areas of medicine.20 In addition, glucocorticoids have profound cytotoxic effects in immature T cells because of their ability to induce apoptosis.21–24 While this observation led us to investigate the process of glucocorticoid-induced apoptosis for many years, we and others have recently shown that glucocorticoids simultaneously induce macroautophagy (i.e., autophagy) in lymphoid cell lines and primary leukemia cells.25,26 Although, to date, there is little mechanistic insight as to how this process occurs.
In an effort to investigate the mechanism of glucocorticoid-induced autophagy, we hypothesized that the inhibition of IP3-mediated calcium signaling by dexamethasone was responsible for the induction of autophagy. This hypothesis is based, in part, on recent evidence that IP3 antagonizes autophagy and that pharmacological inhibitors or siRNAs against IP3Rs induce autophagy.27,28 By microarray analysis, we discovered that glucocorticoids downregulated the Src kinase Fyn. Decreased expression of Fyn, in turn, prevented IP3R1 phosphorylation at Tyr353 and inhibited cytosolic calcium elevation. Selective knockdown of Fyn also inhibited IP3-mediated calcium release and induced autophagy, leading us to the conclusion that glucocorticoid-induced autophagy occurs, at least in part, because of attenuated calcium signaling.
Results
Glucocorticoids inhibit IP3-mediated calcium signals.
Immature T cells are highly susceptible to the effects of dexamethasone. In this study, we utilized murine WEHI 7.2 cells because they are double positive (CD4+/CD8+) and closely resemble cortical thymocytes. In addition, Bcl-2 protein levels are virtually nondetectable in WEHI 7.2 cells, and thus, Bcl-2 is not a confounding factor when assaying for apoptosis or autophagy.
When WEHI 7.2 cells were incubated with varying concentrations of dexamethasone for 24 hours, we observed that IP3-mediated calcium elevation (induced by anti-CD3 antibody), was markedly attenuated in a dose-responsive fashion (Fig. 1A and B). Anti-CD3 antibody can induce calcium transients or oscillations when administered at high and low concentrations, respectively.29 Calcium oscillations induced by a low concentration of anti-CD3 were also inhibited by dexamethasone, as were spontaneous calcium oscillations that occurred in the absence of ligand stimulation (Fig. 1C–E). While the mechanism has not been fully elucidated, these data complement previous studies that have demonstrated an inhibitory effect of glucocorticoids on IP3-mediated calcium responses in T cells.6,14
Figure 1.
Glucocorticoids inhibit IP3-mediated and spontaneous calcium signals. (A) WEHI 7.2 T cells were pretreated with varying concentrations of dexamethasone for 24 hours and IP3-mediated calcium release was assessed by stimulation with 20 µg/mL (1/50) soluble anti-CD3 antibody (the arrow indicates the time in which antibody was added). Each trace represents the mean from approximately 50 cells; a representative experiment is shown. (B) The mean peak amplitude was calculated for each dexamethasone concentration in (A) and for concentrations of 1 and 1,000 nM, respectively. The asterisk denotes p < 0.05. (C) Cells were pretreated as in (A) and IP3-mediated calcium oscillations (≥3 spikes) were assessed by stimulation with 2 µg/mL (1/500) soluble anti-CD3 antibody. (D) Cells were pretreated with 10 nM dexamethasone for 20 hours and spontaneous calcium oscillations were assessed (i.e., no agonist was added). (E) Quantification of the mean percentage of cells undergoing spontaneous calcium oscillations in the presence of 10 nM dexamethasone from three independent experiments. The asterisk denotes p < 0.05. Error bars represent the S.E.M. Data from (A–C) has been confirmed in multiple independent experiments. Ethanol (0.1%) was used as a vehicle (cntrl) for dexamethasone.
Glucocorticoid-mediated inhibition of calcium signaling is dependent on the Src kinase Fyn.
To better understand how glucocorticoids inhibit IP3-mediated calcium responses, we conducted microarray analysis in dexamethasone-treated WEHI 7.2 and S49A.2 cell lines and in primary thymocytes.30–33 Gene expression analysis revealed that Fyn was one of 207 genes differentially regulated by dexamethasone in all three cell populations, where the mean signal log2 ratio was −0.3375 after 6 to 24 hours of treatment (the negative value indicates gene repression). Fyn was ranked 10th out of 121 genes that were most significantly downregulated in all three model systems. We confirmed that Fyn mRNA levels were downregulated by dexamethasone by real-time qPCR (Fig. 2A). Similar results were obtained when Fyn protein expression was measured by western blotting in WEHI 7.2 cells and the T-ALL line CEMC7 (Fig. 2B–D). In WEHI 7.2 cells, the most dramatic decrease in Fyn expression was observed between 12 and 24 hours of treatment with dexamethasone, which was associated with the inhibition in IP3-mediated calcium signaling reported in Figure 1.
Figure 2.
Dexamethasone downregulates Fyn expression. (A) WEHI 7.2 T cells were treated with varying concentrations of dexamethasone for 24 hours. Fyn mRNA levels were assessed by real-time PCR in triplicate and results were confirmed in independent experiments. (B) Cells were treated as in (A) and Fyn expression was measured by western blotting. (C) Cells were treated with dexamethasone for 3–24 hours and Fyn was measured as in (B). (D) CEMC7 (T-ALL) cells were treated with dexamethasone for 24 or 36 hours and Fyn was measured as in (B). Ethanol (0.1%) was used as a vehicle (cntrl) for dexamethasone. β-actin was used as a loading control in all parts. Data are representative of multiple independent experiments.
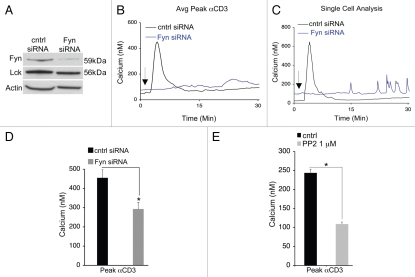
To directly test whether this downregulation of Fyn contributed to glucocorticoid-mediated inhibition of calcium signaling, we selectively targeted Fyn with siRNAs to reduce its expression. siRNAs decreased Fyn expression by greater than 70% but did not significantly affect the level of Lck, indicating that the siRNAs did not have off-target effects on other Src kinases (Fig. 3A). Accordingly, a reduction in Fyn or treatment with the Src inhibitor PP2 was sufficient to reduce IP3-mediated calcium release (Fig. 3B–E). When analyzing calcium responses in single cells (as opposed to a mean population of cells) we observed that the calcium signaling pattern was altered when Fyn levels were decreased (Fig. 3C). Specifically, the transient elevation shown in the control cell population was converted to repetitive oscillations that were significantly lower in amplitude. These responses were delayed relative to control cells, as shown by the latency period following addition of anti-CD3 antibody. Importantly, calcium responses were not affected by stimulation with the SERCA inhibitor thapsigargin, which is an indirect measure of ER luminal calcium (data not shown). Together, these data indicate that dexamethasone-induced downregulation of Fyn is partially responsible for diminished IP3-mediated calcium responses.
Figure 3.
Fyn regulates IP3-mediated calcium responses. (A) WEHI 7.2 T cells were transfected with Fyn siRNAs (or non-targeting control siRNAs) as described in materials and methods. Fyn levels were measured by western blotting 24 hours post-transfection. β-actin was used as a loading control. (B) IP3-mediated calcium release was assessed by stimulation with 13 µg/mL (1/75) soluble anti-CD3 antibody (the arrow indicates the time in which antibody was added). Each trace represents the mean from approximately 50 cells; a representative experiment is shown. (C) A representative single cell trace from (B) to illustrate the oscillations elicited from Fyn knockdown cells. (D) The mean peak amplitude was quantified from Fyn knockdown cells relative to the control in four independent experiments. The asterisk denotes p < 0.05. (E) WEHI 7.2 cells were pretreated with the pan-Src inhibitor PP2 for 1 hour prior to stimulation with anti-CD3 and the mean peak amplitude was quantified. Similar results were obtained in independent experiments. The asterisk denotes p < 0.05.
Downregulation of Fyn inhibits IP3R1 phosphorylation at Tyr353.
Because dexamethasone treatment resulted in the downregulation of Fyn, we hypothesized that this downregulation would prevent Fyn-mediated phosphorylation of IP3R1. It has previously been shown that Fyn phosphorylates IP3R1 at Tyr353, and that this phosphorylation event is required for IP3-mediated calcium release in both B and T lymphocytes.8 We measured phosphorylation of IP3R1 at Tyr353 by western blotting using an antibody specific for this phosphorylation site.8,34 We found that phosphorylation at Tyr353 was decreased after 16–24 hours of treatment with dexamethasone (Fig. 4A and C). Interestingly, dexamethasone increased the total level of IP3R1, which caused the ratio of phosphorylated (Tyr353) to total IP3R1 to be reversed from high to low (Fig. 4B). Although this is the first study demonstrating that dexamethasone attenuates IP3R1 tyrosine phosphorylation, this result is consistent with our recent finding that glucocorticoids upregulate the level of IP3Rs35 (see discussion).
Figure 4.
Dexamethasone inhibits IP3R1 phosphorylation at Tyr353. (A) WEHI 7.2 T cells were treated with dexamethasone for 16 and 24 hours. IP3R1 phosphorylation at Tyr353 was measured by western blotting using an antibody specific for this phosphorylation site. Total levels of IP3R1 were measured simultaneously which were previously shown to be elevated by dexamethasone.35 (B) The ratio of phosphorylated IP3R1 at Tyr353 to total IP3R1 after 24 hours of dexamethasone treatment was quantified by densitometry. (C) The ratio of phosphorylated IP3R1 at Tyr353 to actin was quantified as in (B). Results are from three independent experiments. The asterisk denotes p < 0.05. (D) Cells were transfected with IP3R siRNAs targeting all three subtypes (or nontargeting control siRNAs). IP3-mediated calcium release was assessed by stimulation with 20 µg/mL (1/50) soluble anti-CD3 antibody (the arrow indicates the time in which antibody was added). A representative single cell trace is shown to illustrate the oscillations elicited from IP3R knockdown cells. (E) The mean peak amplitude was quantified from IP3R knockdown cells relative to control cells in four independent experiments. The asterisk denotes p < 0.05. (F) IP3R levels were measured 24 hours post-transfection. β-actin was used as a loading control.
Because dexamethasone downregulates Fyn and subsequently inhibits IP3-mediated calcium signaling, it would be anticipated that knocking down IP3Rs would have a similar effect. As expected, knockdown of IP3Rs with siRNAs partially inhibited IP3-mediated calcium release in a similar manner as in Fyn knockdown cells (Fig. 4D–F). This is further exemplified in Figure 4D which demonstrates that IP3R knockdown cells do not undergo transient calcium elevations, but elicit delayed oscillations that are significantly lower in amplitude and duration.
Glucocorticoid-induced autophagy precedes cell death and apoptosis.
As mentioned previously, glucocorticoids induce autophagy in immature T cell populations, especially when apoptosis is blocked by Bcl-2 overexpression or caspase inhibition.25 Yet, glucocorticoid-induced autophagy, as measured by LC3II accumulation and degradation of p62,36,37 also occurs in wild-type WEHI 7.2 cells that have low levels of Bcl-2 (Fig. 5A–D). Importantly, glucocorticoid treatment results in LC3II accumulation in the presence and absence of lysosomal inhibitors, indicating that autophagic flux has occurred and that our observed increase in LC3II is not due to an aberration in lysosomal degradation (Fig. 5C and D). We also observed that dexamethasone was capable of inhibiting mTOR signaling as shown in Figure 5E where phosphorylation of mTOR substrates S6 kinase and 4EBP1 were reduced. This result suggests that dexamethasone may inhibit mTOR signaling as a result of Fyn downregulation or alternatively, through an independent pathway. In either circumstance, it is likely that mTOR inhibition contributes to the mechanism of glucocorticoid-induced autophagy.
Figure 5.
Glucocorticoid-induced autophagy precedes apoptosis and cell death. (A) p62 protein levels were assessed by western blot analysis in WEHI 7.2 T cells treated with dexamethasone for 8, 16 and 24 hours (note that the lower band is p62). (B) Cells were treated as in (A) and autophagy was measured by assessment of LC3I and II and p62 by western blotting. (C) LC3II levels were assessed by western blotting following treatment with dexamethasone for 24 hours in the presence and absence of lysosomal inhibitors E64d and Pepstatin A (5 and 10 µg/mL, respectively). The part with an asterisk is a lighter exposure of LC3II to better illustrate the difference in levels when cells were incubated with inhibitors. (D) Quantification of LC3II levels relative to β-actin from at least 3 experiments. (E) mTOR activity was assessed by measuring the phosphorylation status of two mTOR substrates S6 kinase and 4EBP1 by western blotting. β-actin was used as a loading control. (F) Glucocorticoid-induced apoptosis was measured by Annexin-V/propidium iodide staining at 16, 24 and 36 hours following treatment with 1 µM dexamethasone. Representative dot-plots are shown. Data are representative of multiple independent experiments. Ethanol (0.1%) was used as a vehicle (cntrl) for dexamethasone.
Finally, we determined that glucocorticoid-induced autophagy occurs prior to the onset of apoptosis (Fig. 5A, B and F). For example, treatment with dexamethasone for 16 hours produces little to no change in cell viability or apoptosis (Fig. 5F). Even after 24 hours of treatment with dexamethasone the vast majority of cells (approximately 70%) are nonapoptotic. Thus, the ability of dexamethasone to inhibit IP3-mediated calcium signals is neither strongly associated with apoptosis nor cell death. In contrast, we found that glucocorticoid-induced autophagy occurs during the timeframe in which Fyn is downregulated and hence IP3R1 phosphorylation and calcium signaling is also inhibited (Figs. 1, 2, 4 and 5).
Downregulation and inhibition of Fyn promotes autophagy.
Based on these results, we predicted that the downregulation of Fyn by dexamethasone and subsequent inhibition of IP3R1 phosphorylation was responsible for the induction of autophagy. To better address this hypothesis, we selectively knocked down Fyn using lentiviral shRNAs; a stable Fyn knockdown was generated to minimize the degree of cellular stress (i.e., cell death) typically associated with transient transfection. The lentiviral knockdown did not decrease Lck levels demonstrating that these shRNAs were highly selective for Fyn (data not shown). As shown in Figure 6A, there was no difference in apoptosis between control cells and those expressing Fyn shRNAs (p = 0.2). However, Fyn knockdown cells had a higher level of autophagy relative to control cells, as shown by an increase in LC3II and decrease in p62 (Fig. 6B and C). Interestingly, mTOR signaling was markedly attenuated in Fyn knockdown cells as determined by levels of phosphorylated S6 kinase and 4EBP1 (Fig. 6D). These results are strikingly similar to that observed after dexamethasone treatment, providing further evidence that Fyn regulates the mTOR pathway. To confirm that our hypothesis was correct, we treated WEHI 7.2 cells with the Src inhibitor PP2. Pan Src inhibition also resulted in the accumulation of LC3II and was accompanied by a decrease in p62 (Fig. 6E and F). Moreover, both PP2 treatment and transduction of Fyn shRNAs resulted in a modest increase of LC3II in the presence of lysosomal inhibitors further demonstrating that autophagic flux is intact when Fyn is downregulated or inhibited (Fig. 6F and G). We speculate that the greater increase in LC3II following PP2 treatment is due to the robust effect of pharmacological inhibition versus transduction with shRNAs or its ability to inhibit multiple Src homologs. All in all, these data support the hypothesis that the downregulation of Fyn by dexamethasone is responsible, in part, for the induction of autophagy.
Figure 6.
Downregulation and inhibition of Fyn promotes autophagy. (A) WEHI 7.2 T cells were stably transduced with vector alone or Fyn shRNAs as described in materials and methods. Apoptosis was assessed by Annexin-V/propidium iodide staining and was quantified by flow cytometry (p = 0.2 from three independent experiments). (B and C) Autophagy was measured by assessment of LC3I and II and p62 by western blotting in cells transduced with empty vector or Fyn shRNAs. Results are representative of three experiments. (D) mTOR activity was assessed in Fyn shRNA-transduced cells by measuring the phosphorylation status of two mTOR substrates S6 kinase and 4EBP1 by western blotting. β-actin was used as a loading control. (E) p62 protein levels were measured by western blotting in cells treated with the pan-Src inhibitor PP2 for 24 hours (10 µM). (F) LC3II levels were assessed by western blotting following treatment with PP2 (20 µM) for 24 hours in the presence and absence of lysosomal inhibitors E64d and Pepstatin A (5 and 10 µg/mL, respectively) to assess autophagic flux. The part with an asterisk is a lighter exposure of LC3II to better illustrate the difference in levels when cells were incubated with inhibitors. Similar results were obtained in three independent experiments for (E and F). β-actin was used as a loading control. (G) Quantification of the mean induction of LC3II relative to β-actin in Fyn shRNA-transduced cells or in cells treated with 10–20 µM PP2. The left y-axis refers to LC3II analysis in the presence of lysosomal inhibitors (purple bars); the right y-axis refers to LC3II analysis in the absence of inhibitors (black bars).
Discussion
In addition to their pro-apoptotic effects in immature lymphocytes, glucocorticoids readily inhibit T cell activation, impede cellular metabolism and block cell proliferation.15,16,38,39 Glucocorticoids also induce autophagy prior to the induction of apoptosis, which we show here, is a downstream consequence of their ability to inhibit IP3-mediated calcium signaling. Our data strongly support the hypothesis that glucocorticoids downregulate Fyn expression in T cells, thereby leading to the inhibition of IP3R1 phosphorylation at Tyr353 and attenuation of ER calcium release. We provide evidence that selectively knocking-down Fyn inhibits IP3-mediated calcium release and this is sufficient for partial induction of autophagy.
Rationale for this work is largely derived from recent studies suggesting that IP3Rs regulate autophagy.27,40 For example, siRNA-mediated knockdown or pharmacological inhibition of IP3Rs stimulates autophagy as documented by the formation of GFP-tagged LC3 aggregates.27 Compounds such as lithium and L-690330 that inhibit inositol-monophosphate also induce autophagy.28,41 Moreover, there is now sufficient evidence that inhibiting cytosolic calcium elevation contributes to autophagy. A recent study showed that L-type calcium channel blockers and calpain inhibitors induce autophagosome formation, suggesting one mechanism by which IP3 antagonizes autophagy is via calcium-dependent activation of calpains.42 Our findings are consistent with the concept that calcium signals negatively regulate autophagy given that glucocorticoids inhibit ER calcium release by downregulating Fyn and preventing IP3R1 phosphorylation.
The work by Kroemer and colleagues also demonstrates that the induction of autophagy following IP3R inhibition is not due to alterations in calcium homeostasis.27 While there is unequivocal evidence that glucocorticoids alter calcium homeostasis in lymphocytes,43–48 our data suggest that this phenomenon is not required for the induction of autophagy. This is supported by the observation that siRNA-mediated knockdown of either Fyn or IP3Rs did not alter thapsigargin-releasable calcium, an indirect measure of the ER calcium pool.35 Therefore, it is likely that glucocorticoid-induced autophagy occurs from the inhibition of IP3-mediated calcium signaling rather than alterations in ER or cytosolic calcium concentration.
The finding that glucocorticoids inhibit IP3R1 phosphorylation partly clarifies our previous observation that prolonged treatment with dexamethasone (e.g., 16–24 hours) upregulates IP3Rs.35 Although the mechanism of IP3R upregulation has not been investigated, the mRNA level was shown to be elevated at 6 to 8 hours following dexamethasone treatment. Somewhat paradoxically, short-term treatment with glucocorticoids (e.g., 60 minutes) decreases IP3R1 expression,6 indicating that glucocorticoids can transiently modulate its expression. We speculate that IP3R1 upregulation at the mRNA and protein level is a secondary response to its acute and rapid downregulation by glucocorticoids. However, until conducting the present study, we were unaware that glucocorticoids decreased IP3R1 phosphorylation at Tyr353, which ultimately regulates IP3-mediated channel activity.8 While we provide evidence that neither Fyn nor IP3R1 directly regulates apoptosis,35 both proteins protect cells from autophagy, presumably by Fyn-mediated phosphorylation of IP3R1.
We anticipate that the inhibition of cytosolic calcium elevation during glucocorticoid treatment promotes autophagy, at least in part, by impeding cellular metabolism. Because of the close spatial relationship between ER and mitochondria, cytosolic calcium is readily taken up by mitochondria to facilitate the generation of ATP during the TCA cycle and electron transport.49,50 In addition, there is a higher metabolic demand for T cells following their activation.10 For example, a recent study suggests that a basal level of autophagy is required for cell proliferation among activated T cells.51 Thus, it is feasible that calcium deprivation in this context stimulates the activity of AMP kinase thereby inhibiting mTOR and inducing autophagy. Calcium/calmodulin-dependent activation of AMP kinase has been previously shown to be important for inhibiting mTOR during the autophagic process.52,53 This hypothesis is consistent with the finding that dexamethasone inhibits downstream components of mTOR signaling such as S6 kinase and 4EBP1 in other cell types,54,55 and also in T lymphocytes. Our data support the concept that Fyn regulates mTOR activity, since lentiviral knockdown of Fyn directly attenuates phosphorylation of mTOR substrates. In fact, siRNA-mediated knockdown of the Src kinase Lyn had a similar effect on mTOR substrates in myeloid leukemia cells.56
Lastly, the induction of autophagy by glucocorticoids and Src inhibitors may carry important therapeutic implications given that both dexamethasone and dasatinib (a potent Src inhibitor) are used to treat hematological malignancies. Recent studies have shown that dasatinib and imatinib induce autophagy in glioblastoma and Philadelphia chromosome positive chronic myelogenous leukemia cells, respectively.57,58 Moreover, the combination of Src inhibitors with agents that prevent autophagy markedly enhanced cell killing of CML stem cells as well as prostate cancer cells in vivo.58,59 Our group recently reported that dasatinib, because of its ability to inhibit Lck, sensitizes chronic lymphocytic leukemia cells to dexamethasone.60 Thus, it is reasonable that these agents when combined with an autophagy inhibitor would further potentiate apoptotic cell death.
Taken together, our data provide novel insight into the mechanism of glucocorticoid-induced autophagy in lymphocytes and further enhance our understanding of how calcium regulates cell survival. These findings carry important therapeutic significance due to the ubiquitous use of synthetic glucocorticoids (e.g., prednisone and dexamethasone) in all areas of clinical medicine.
Methods
Reagents and antibodies.
The following reagents were used in this study: Dexamethasone (Sigma-Aldrich, D4902), Fura-2AM (Invitrogen, F1201), Trizol reagent (Invitrogen, 15596-018), Annexin-V/propidium iodide (Invitrogen, V13241), TaqMan Gold RT-PCR kit (Applied Biosystems, N808-0233), 2X Fast PCR Master Mix (Applied Biosystems, 4352042), thapsigargin (LC Laboratories, T-3250), PP2 (Calbiochem, 529573), Pepstatin (Roche, 1359053) and E64d (Enzo Life Sciences, BML-PI107-00).
The following antibodies were used in this study: Fyn (Santa Cruz Biotechnology, sc-16), Lck (Santa Cruz Biotechnology, sc-433), anti-mouse CD3ε (BD Biosciences, 145-2C11), IP3R3 (BD Biosciences, 610312), β-actin (Sigma-Aldrich, A-5441), p62 (Novus Biologicals, 8878-M03), LC3 (Novus Biologicals, NB100-2220), IP3R1 (Novus Biologicals, NB120-5908), IP3R2 (Novus Biologicals, NB100-2466), phospho-S6 kinase (Thr389) (Cell Signaling Technology, 9205), phospho-4EBP1 (Ser65) (Cell Signaling Technology, 9451), Total S6 kinase (Cell Signaling Technology, 9202), Total 4EBP1 (Cell Signaling Technology, 9452). Anti-phospho IP3R1 (Tyr353) was kindly provided by Andrew Marks (Columbia University).
Cell culture.
WEHI 7.2 cells were cultured in DMEM supplemented with 10% bovine calf serum. CEMC7 cells were cultured in RPMI supplemented with 10% fetal bovine serum. All media were additionally supplemented with 2 mM L-glutamine and 0.1 mM nonessential amino acids.
Western blotting.
Cells were lysed in ice-cold sample buffer or in RIPA buffer containing a protease inhibitor cocktail (Roche) and denatured at 100°C for 10 minutes. Protein lysates were quantified by Bradford assay and the appropriate protein concentration was subjected to SDS-PAGE followed by western blotting. For western blots in which LC3 was measured, PVDF membranes were transferred in CAPS buffer as previously described.25 All other western blots were transferred in Trisglycine buffer, pre-incubated in milk or bovine serum albumin and probed with the appropriate primary antibodies. Bands were visualized by incubating with secondary antibodies conjugated to horseradish peroxidase and exposing to chemiluminescent substrates.
Calcium measurements.
All calcium measurements were obtained by single cell digital imaging. WEHI 7.2 cells were weakly adhered to coverslips (MatTek) coated with poly-L-lysine for at least 1 hour at 37°C. Cells were loaded with the ratiometric dye Fura-2AM and de-esterified in calcium BSS buffer prior to fluorescence microscopy. Cells were imaged using a Zeiss Axiovert S100 microscope and 20X Fluor objective (Carl Zeiss AG) and were excited at alternating wavelengths of 340 and 380 nm every second. A charge-coupled device camera (Hamamatsu Photonics) was used to record the output which was then converted to a digital image using PCI software for Windows. Calcium concentrations were empirically derived using the Grynkiewicz equation as previously described.61
RNA analysis.
Total RNA was isolated using Trizol reagent (Invitrogen) by standard phenol/chloroform methods. RNAs were precipitated in isopropanol, washed in ethanol and solubilized in nuclease-free water prior to quantification by spectrophotometry. For microarray analysis, cRNA was hybridized to Affymetrix Gene Chips as previously described.30 For mRNA quantification by real-time PCR, RNA was reversed transcribed using oligo-dT primers and the TaqMan Gold RT-PCR kit (Applied Biosystems). cDNA generated by reverse transcription was mixed with the appropriate primers and probes (Fyn, Mm01179871_mH; β-actin, 4352933-0711018) and 2X Fast PCR Master Mix (Applied Biosystems), transferred to a 96-well plate and amplified in a 7500 Fast real-time thermal cycler (Applied Biosystems). Samples were quantified relative to the control using β-actin as a reference gene and the 2-ΔΔct method.
RNA interference.
WEHI 7.2 cells were resuspended in serum-free media at a concentration of 5 × 107 cells/mL prior to transfection. Cells were mixed with siRNA on-target SMARTpools (Dharmacon) or a nontargeting control at a concentration of 1 µM (note that the final concentration for the IP3R triple knockdown was 3 µM). Cells were electroporated in a 0.2 cm cuvette using a single 140-V, 10 ms2 wave pulse (Bio-Rad Laboratories) and immediately transferred to pre-warmed serum-containing media. siRNA-transfected cells were incubated for 24 hours prior to analysis. Stable knockdowns were generated by transducing 293T cells with pLKO.1 vectors (Open Biosystems) containing target sequences for Fyn along with vectors pMD2G (encoding env) and pR8.74 (encoding gag and pol) to make viral particles. Virus was collected at 24 and 48 hours post-transduction and incubated with WEHI 7.2 cells in the presence of puromycin.
Cell viability.
Apoptosis and cell viability were assessed by Annexin-V-alexa 488 and propidium iodide staining (Invitrogen). Apoptotic cells are those that are Annexin-V positive while only dead cells are propidium iodide positive. Fluorescence was quantified using an EPICS XL-MCL (Beckman Coulter) flow cytometer. Data analysis was performed using FlowJo 8.8.4 (Tree Star, Inc) and WinList 6.0 (Verity Software House) for Macintosh and Windows, respectively.
Statistical analysis.
A Student's t-test was used to determine statistical significance when appropriate. All statistics were obtained using Microsoft Excel 2004 for Macintosh.
Acknowledgements
This work was supported by NIH grants RO1 CA42755 (C.W.D.), T32 CA059366 (M.W.H.) and T32 GM007250 (J.K.M.). We thank Andrew Marks for generously providing us with antibody to detect phosphorylated IP3R1.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13290
References
- 1.Hanson CJ, Bootman MD, Roderick HL. Cell signalling: IP3 receptors channel calcium into cell death. Curr Biol. 2004;14:933–935. doi: 10.1016/j.cub.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 3.Mustelin T, Tasken K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem J. 2003;371:15–27. doi: 10.1042/BJ20021637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 5.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 6.Harr MW, Rong Y, Bootman MD, Roderick HL, Distelhorst CW. Glucocorticoid-mediated inhibition of Lck modulates the pattern of T cell receptor-induced calcium signals by downregulating inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2009;284:31860–31871. doi: 10.1074/jbc.M109.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayaraman T, Ondrias K, Ondriasova E, Marks AR. Regulation of the inositol 1,4,5-trisphosphate receptor by tyrosine phosphorylation. Science. 1996;272:1492–1494. doi: 10.1126/science.272.5267.1492. [DOI] [PubMed] [Google Scholar]
- 8.Cui J, Matkovich SJ, deSouza N, Li S, Rosemblit N, Marks AR. Regulation of the type 1 inositol 1,4,5-trisphosphate receptor by phosphorylation at tyrosine 353. J Biol Chem. 2004;279:16311–16316. doi: 10.1074/jbc.M400206200. [DOI] [PubMed] [Google Scholar]
- 9.Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhaes PJ, et al. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- 10.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 11.Krauss S, Brand MD, Buttgereit F. Signaling takes a breath—new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001;15:497–502. doi: 10.1016/s1074-7613(01)00205-9. [DOI] [PubMed] [Google Scholar]
- 12.Gallo EM, Cante-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 13.Winslow MM, Neilson JR, Crabtree GR. Calcium signalling in lymphocytes. Curr Opin Immunol. 2003;15:299–307. doi: 10.1016/s0952-7915(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 14.Baus E, Andris F, Dubois PM, Urbain J, Leo O. Dexamethasone inhibits the early steps of antigen receptor signaling in activated T lymphocytes. J Immunol. 1996;156:4555–4561. [PubMed] [Google Scholar]
- 15.Lowenberg M, Verhaar AP, Bilderbeek J, Marle J, Buttgereit F, Peppelenbosch MP, et al. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO Rep. 2006;7:1023–1029. doi: 10.1038/sj.embor.7400775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, van Deventer S, et al. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood. 2005;106:1703–1710. doi: 10.1182/blood-2004-12-4790. [DOI] [PubMed] [Google Scholar]
- 17.Northrop JP, Crabtree GR, Mattila PS. Negative regulation of interleukin 2 transcription by the glucocorticoid receptor. J Exp Med. 1992;175:1235–1245. doi: 10.1084/jem.175.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paliogianni F, Raptis A, Ahuja SS, Najjar SM, Boumpas DT. Negative transcriptional regulation of human interleukin 2 (IL-2) gene by glucocorticoids through interference with nuclear transcription factors AP-1 and NF-AT. J Clin Invest. 1993;91:1481–1489. doi: 10.1172/JCI116353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vacca A, Felli MP, Farina AR, Martinotti S, Maroder M, Screpanti I, et al. Glucocorticoid receptor-mediated suppression of the interleukin 2 gene expression through impairment of the cooperativity between nuclear factor of activated T cells and AP-1 enhancer elements. J Exp Med. 1992;175:637–646. doi: 10.1084/jem.175.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 21.Distelhorst CW. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ. 2002;9:6–19. doi: 10.1038/sj.cdd.4400969. [DOI] [PubMed] [Google Scholar]
- 22.Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci. 2006;63:60–72. doi: 10.1007/s00018-005-5390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smets LA, Salomons G, van den Berg J. Glucocorticoid induced apoptosis in leukemia. Adv Exp Med Biol. 1999;457:607–614. doi: 10.1007/978-1-4615-4811-9_67. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11:45–55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- 25.Swerdlow S, McColl K, Rong Y, Lam M, Gupta A, Distelhorst CW. Apoptosis inhibition by Bcl-2 gives way to autophagy in glucocorticoid-treated lymphocytes. Autophagy. 2008;4:612–620. doi: 10.4161/auto.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laane E, Tamm KP, Buentke E, Ito K, Kharaziha P, Oscarsson J, et al. Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 2009;16:1018–1029. doi: 10.1038/cdd.2009.46. [DOI] [PubMed] [Google Scholar]
- 27.Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D, et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donnadieu E, Bismuth G, Trautmann A. Calcium fluxes in T lymphocytes. J Biol Chem. 1992;267:25864–25872. [PubMed] [Google Scholar]
- 30.Wang Z, Malone MH, He H, McColl KS, Distelhorst CW. Microarray analysis uncovers the induction of the proapoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J Biol Chem. 2003;278:23861–23867. doi: 10.1074/jbc.M301843200. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Rong YP, Malone MH, Davis MC, Zhong F, Distelhorst CW. Thioredoxin-interacting protein (txnip) is a glucocorticoid-regulated primary response gene involved in mediating glucocorticoid-induced apoptosis. Oncogene. 2006;25:1903–1913. doi: 10.1038/sj.onc.1209218. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Malone MH, Thomenius MJ, Zhong F, Xu F, Distelhorst CW. Dexamethasone-induced gene 2 (dig2) is a novel pro-survival stress gene induced rapidly by diverse apoptotic signals. J Biol Chem. 2003;278:27053–27058. doi: 10.1074/jbc.M303723200. [DOI] [PubMed] [Google Scholar]
- 33.Malone MH, Wang Z, Distelhorst CW. The glucocorticoid-induced gene tdag8 encodes a pro-apoptotic G protein-coupled receptor whose activation promotes glucocorticoid-induced apoptosis. J Biol Chem. 2004;279:52850–52859. doi: 10.1074/jbc.M408040200. [DOI] [PubMed] [Google Scholar]
- 34.deSouza N, Cui J, Dura M, McDonald TV, Marks AR. A function for tyrosine phosphorylation of type 1 inositol 1,4,5-trisphosphate receptor in lymphocyte activation. J Cell Biol. 2007;179:923–934. doi: 10.1083/jcb.200708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis MC, McColl KS, Zhong F, Wang Z, Malone MH, Distelhorst CW. Dexamethasone-induced inositol 1,4,5-trisphosphate receptor elevation in murine lymphoma cells is not required for dexamethasone-mediated calcium elevation and apoptosis. J Biol Chem. 2008;283:10357–10365. doi: 10.1074/jbc.M800269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 39.Van Laethem F, Baus E, Smyth LA, Andris F, Bex F, Urbain J, et al. Glucocorticoids attenuate T cell receptor signaling. J Exp Med. 2001;193:803–814. doi: 10.1084/jem.193.7.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicencio JM, Ortiz C, Criollo A, Jones AW, Kepp O, Galluzzi L, et al. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009;16:1006–1017. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar S, Rubinsztein DC. Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations. Autophagy. 2006;2:132–134. doi: 10.4161/auto.2387. [DOI] [PubMed] [Google Scholar]
- 42.Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, et al. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bian X, Hughes FM, Jr, Huang Y, Cidlowski JA, Putney JW., Jr Roles of cytoplasmic Ca2+ and intracellular Ca2+ stores in induction and suppression of apoptosis in S49 cells. Am J Physiol. 1997;272:1241–1249. doi: 10.1152/ajpcell.1997.272.4.C1241. [DOI] [PubMed] [Google Scholar]
- 44.Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- 45.Kaiser N, Edelman IS. Calcium dependence of glucocorticoid-induced lymphocytolysis. Proc Natl Acad Sci USA. 1977;74:638–642. doi: 10.1073/pnas.74.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam M, Dubyak G, Distelhorst CW. Effect of glucocorticosteroid treatment on intracellular calcium homeostasis in mouse lymphoma cells. Mol Endocrinol. 1993;7:686–693. doi: 10.1210/mend.7.5.8316252. [DOI] [PubMed] [Google Scholar]
- 47.McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989;269:365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- 48.Orrenius S, McConkey DJ, Nicotera P. Role of calcium in toxic and programmed cell death. Adv Exp Med Biol. 1991;283:419–425. doi: 10.1007/978-1-4684-5877-0_57. [DOI] [PubMed] [Google Scholar]
- 49.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rimessi A, Giorgi C, Pinton P, Rizzuto R. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta. 2008;1777:808–816. doi: 10.1016/j.bbabio.2008.05.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campartla M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007;3:381–383. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- 54.Shah OJ, Kimball SR, Jefferson LS. Glucocorticoids abate p70(S6k) and eIF4E function in L6 skeletal myoblasts. Am J Physiol Endocrinol Metab. 2000;279:74–82. doi: 10.1152/ajpendo.2000.279.1.E74. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem. 2006;281:39128–39134. doi: 10.1074/jbc.M610023200. [DOI] [PubMed] [Google Scholar]
- 56.Dos Santos C, Demur C, Bardet V, Prade-Houdellier N, Payrastre B, Recher C. A critical role for Lyn in acute myeloid leukemia. Blood. 2008;111:2269–2279. doi: 10.1182/blood-2007-04-082099. [DOI] [PubMed] [Google Scholar]
- 57.Milano V, Piao Y, LaFortune T, de Groot J. Dasatinib-induced autophagy is enhanced in combination with temozolomide in glioma. Mol Cancer Ther. 2009;8:394–406. doi: 10.1158/1535-7163.MCT-08-0669. [DOI] [PubMed] [Google Scholar]
- 58.Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest. 2009;119:1109–1123. doi: 10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Z, Chang P, Yang JC, Chu C, Wang L, Chen N, et al. Autophagy Blockade Sensitizes Prostate Cancer Cells towards Src Family Kinase Inhibitors. Genes and Cancer. 2010;1:40–49. doi: 10.1177/1947601909358324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harr MW, Caimi PF, McColl KS, Zhong F, Patel SN, Barr PM, et al. Inhibition of Lck enhances glucocorticoid sensitivity and apoptosis in lymphoid cell lines and in chronic lymphocytic leukemia. Cell Death Differ. 2010;17:1381–1391. doi: 10.1038/cdd.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]