Abstract
Reliable and quantitative assays to measure in vivo autophagy are essential. Currently, there are varied methods for monitoring autophagy; however, it is a challenge to measure “autophagic flux” in an in vivo model system. Conversion and subsequent degradation of the microtubule-associated protein 1 light chain 3 (MAP1-LC3/LC3) to the autophagosome associated LC3-II isoform can be evaluated by immunoblot. However, static levels of endogenous LC3-II protein may render possible misinterpretations since LC3-II levels can increase, decrease or remain unchanged in the setting of autophagic induction. Therefore, it is necessary to measure LC3-II protein levels in the presence and absence of lysomotropic agents that block the degradation of LC3-II, a technique aptly named the “autophagometer.” In order to measure autophagic flux in mouse skeletal muscle, we treated animals with the microtubule depolarizing agent colchicine. Two days of 0.4 mg/kg/day intraperitoneal colchicine blocked autophagosome maturation to autolysosomes and increased LC3-II protein levels in mouse skeletal muscle by >100%. the addition of an autophagic stimulus such as dietary restriction or rapamycin led to an additional increase in LC3-II above that seen with colchicine alone. Moreover, this increase was not apparent in the absence of a “colchicine block.” Using this assay, we evaluated the autophagic response in skeletal muscle upon denervation induced atrophy. Our studies highlight the feasibility of performing an “in vivo autophagometer” study using colchicine in skeletal muscle.
Key words: autophagy, rapamycin, skeletal muscle
Introduction
Autophagy is a nonspecific and bulk degradation process which delivers cytoplasmic materials including damaged organelles, toxic protein aggregates and intracellular pathogens to lysosomes via double-membraned organelles termed autophagosomes.1 Autophagy is highly dynamic and regulated. Autophagic activity is usually low under basal conditions, but can be markedly increased by stimuli such as nutrient deprivation, hypoxia, cellular stress, infection and pharmacological agents such as rapamycin. Defects in autophagy are associated with a number of disorders, including cancer, neurodegeneration, muscle disease and inflammation. There is a rapidly growing need among scientists to have tools and assays that allow one to accurately detect autophagy and study its function in mammalian systems.2,3 LC3 is a ubiquitin-like molecule that is conjugated to phosphatidylethanolamine by Atg7 to form LC3-II upon autophagic stimulation.1 LC3-II is localized to autophagic structures including phagophores, autophagosomes and autolysosomes. Cellular LC3-II levels correlate with autophagosome number and measuring the conversion of LC3-I to LC3-II by immunoblot is a reliable assay for autophagosome formation.3 However, there are limitations to the interpretation of LC3-II levels since LC3-II is both produced and degraded during autophagy. To circumvent this, it has been proposed that LC3-II levels should only be evaluated in the presence and absence of inhibitors which block autophagosome and hence LC3-II degradation.2,4 This method has become the mainstay of LC3-II analysis in cell culture models and is discussed in detail in several review papers.2,4
In order to adapt this technique to an in vivo mammalian model such as mice, we screened several lysomotropic and/or autophagosome-lysosome fusion inhibitors for their ability to increase basal LC3-II protein levels in mouse skeletal muscle. These preliminary studies identified colchicine as a safe, quick-acting and potent compound that increased LC3-II levels >100%. In vivo blockage of LC3-II degradation with colchicine enabled us to quantitate the induction of autophagy following nutrient deprivation or with the mTOR inhibitors rapamycin and temsirolimus in mature skeletal muscle. The following manuscript details how to measure in vivo autophagic flux in mouse skeletal muscle and provides examples of the utility of this assay.
Results
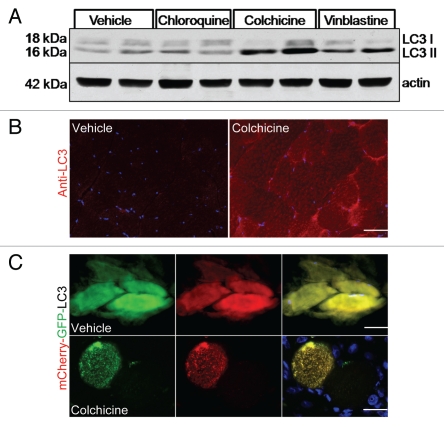
Chronic intoxication of patients and mice with chloroquine, colchicine or vinblastine results in similar vacuolar myopathies.5 The pathogenic mechanism is presumed to be a decrease in lysosomal protein degradation secondary to impaired autophagosome-lysosome fusion, diminished delivery of proteolytic enzymes to the lysosome or deacidification of the lysosome. We reasoned that these drugs may be able to effectively block autophagosome degradation in vivo. With this premise, we treated 3-month-old male mice with intraperitoneal (i.p.) vehicle, 50 mg/kg/day chloroquine, 0.4 mg/kg/day colchicine or 2 mg/kg/day vinblastine for two days and then isolated tibialis anterior (TA) muscle. No weight loss or distress was seen after two days treatment with any of the three drugs. Moreover, there was no evidence of myopathic features or vacuolization in the skeletal muscle by routine histopathology (not shown). The TA was homogenized and the resultant lysate was subjected to SDS-PAGE and immunoblotted with an antibody to LC3. The levels of LC3-II were significantly elevated in the colchicine and vinblastine treated mice (Fig. 1A). This increase in LC3-II correlated with a significant increase in LC3 positive puncta as demonstrated in untreated compared with 2-day colchicine-treated mouse skeletal muscle via immunhistochemistry using an LC3 specific antibody (Fig. 1B). p62 levels were unchanged at this same timepoint (see colchicine-treated animals from blots in Figs. 2A, 3A and D). Longer treatments with all three drugs resulted in only a modest further increase in LC3-II levels and significantly increased levels of p62 (not shown). In addition, longer treatment with vinblastine and chloroquine resulted in significantly more animal toxicity with weight loss and death after 5–7 days. This was not seen with chronic colchicine treatment for an additional 10 days (longest time point evaluated). The addition of vinblastine or chloroquine to i.p. colchicine treatment was also not tolerated by the animals.
Figure 1.
(A) 3-month-old male mice were treated with vehicle, 50 mg/kg/day chloroquine, 0.4 mg/kg/day colchicine or 2 mg/kg/day vinblastine for two days and tibialis anterior (TA) muscle lysates were evaluated by LC3 immunoblot. Actin is shown as a loading control. Blot is representative of 3 independent experiments. (B) TA from similarly treated mice as in (A) was subjected to immunofluorescence using an LC3 antibody. Colchicine treated mice had an increase in LC3 positive puncta. Images were taken at the same camera setting and exposure time. Scale is 40 µM. (C) The TA muscle from 3-month-old male mice was electroporated with a tandemly tagged mCherry-GFP-LC3 reporter construct. Mice were either treated for two days with vehicle or 0.4 mg/kg/day colchicine. Vehicle treated mice had diffuse LC3 fluorescence while colchicine treated mice generated GFP and mCherry positive co-localizing puncta consistent with colchicine impairing autophagosome maturation to an acidic organelle. Scale is 25 µM.
Figure 2.
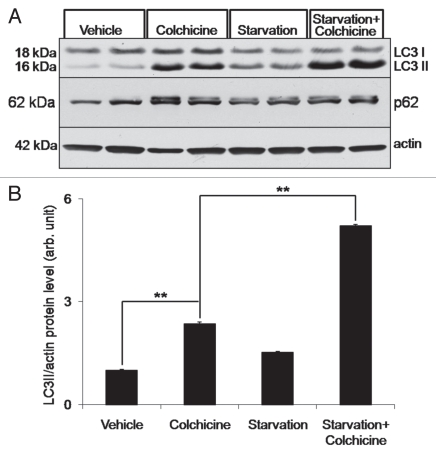
(A) Similar mice were treated with vehicle, 0.4 mg/kg/day colchicine, nutrient deprivation (starvation) or nutrient deprivation plus 0.4 mg/kg/day colchicine for two days. TA muscle lysates were evaluated by LC3 and p62 immunoblot. Note that colchicine treatment significantly increases LC3-II levels. These levels are augmented by nutrient deprivation. Blot is representative of three independent experiments. (B) LC3-II/actin ratios were quantitated via densitometry from six mice per treatment condition. Error bars represent standard error and **denote p values of <0.005 as obtained by student paired t-test.
Figure 3.
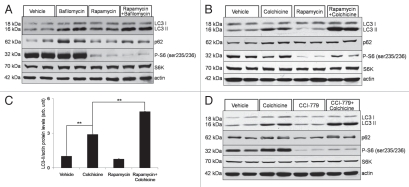
(A) Differentiated C2C12 myotubes were treated with vehicle, 200 nM BafilomycinA1, 10 µg/mL rapamycin or 10 µg/mL rapamycin plus 200 nM BafilomycinA1 for 6 hours. Lysates were subjected to LC3 and p62 immunoblots. Rapamycin did not increase LC3-II levels further than that seen with BafilomycinA1 alone. The same lysates were subjected to immunoblot with antibodies to phospho-S6 (Ser235/236) and S6 kinase demonstrating that rapamycin is capable of mTOR inhibition in these cells. Blot is representative of two independent experiments. (B) 3-month-old male mice were treated with vehicle, 0.4 mg/kg/day colchicine for two days, 10 mg/kg/day rapamycin for 7 days or 10 mg/kg/day rapamycin for 7 days and 0.4 mg/kg/day colchicine for two days beginning on day 5 and TA lysates were subjected to immunoblotting with LC3, p62, phospho-S6, total S6 kinase or actin. Blot is representative of four independent experiments. (C) LC3-II/actin ratios were quantitated via densitometry from eight mice per treatment condition. Error bars represent standard error and **denote p values of <0.005 as obtained by student paired t-test. (D) A similar experiment to (B) was performed using 20 mg/kg/day temsirolimus/CCI-779 instead of rapamycin. Note that the addition of temsirolimus/CCI-779 to colchicine further increased LC3-II levels consistent with an enhancement in autophagic flux.
To confirm that colchicine treatment did indeed block autophagosome degradation (i.e., fusion of the autophagosome with the lysosome), we utilized a tandemly tagged mCherry-GFP-LC3 reporter construct.6 This reporter fluoresces green and red in the neutral environment of an autophagosome and red in the acidic environment of an autolysosome or other acidic organelle. This construct was electroporated into the TA muscle of 3-month-old male mice and allowed to express for seven days. On day 5, mice were treated with saline or 0.4 mg/kg i.p. colchicine for two days. TA muscle was harvested and sectioned. Untreated mouse muscle had diffuse green and red fluorescence throughout the myofiber with no visible puncta as would be expected for an LC3 protein that is not present on an autophagic structure (Fig. 1C). In contrast, two days of colchicine treatment generated myofibers with multiple dual fluorescing GFP and mCherry positive puncta consistent with autophagosomes that have not matured to autolysosomes and become acidified (Fig. 1C). In nonelectroporated myofibers, colchicine induced more LC3 positive puncta than Lamp2 positive puncta and LC3 and Lamp2 did not co-localize (not shown). These data further support that two days of 0.4 mg/kg i.p. colchicine is able to block autophagosome degradation in mouse skeletal muscle and this dose was employed for all subsequent experiments.
To see if the addition of colchicine would allow us to quantitate autophagic flux in vivo, we induced autophagy in mice by depriving them of food for two days. Four different cohorts of mice were used (untreated, colchicine treated, starved and starved plus colchicine treated) and TA, gastrocnemius and quadriceps muscle were harvested. Starved mice lost >10% of their body weight over the two-day period confirming that they were in fact nutrient deprived. Muscle lysates were subjected to SDS-PAGE and LC3 immunoblot. Consistent with our previous experiment, colchicine treatment increased the level of LC3-II by >100%. Starvation alone did not significantly increase LC3-II levels; however, starvation plus colchicine increased LC3-II levels above that seen with colchicine alone in all three muscles (Fig. 2A and B and not shown).
It was previously reported that the mTOR inhibitor rapamycin only modestly enhanced autophagy in cultured myotubes.7 We confirmed this finding in differentiated C2C12 myotubes using an in vitro autophagic flux assay. Differentiated C2C12 cells were treated with 10 µg/mL rapamycin and rapamycin with 200 nM bafilomycinA1. At this dose of rapamycin we inhibited mTOR as assessed by the phosphorylation of ribosomal protein S6 in myotubes, and slightly increased the levels of LC3-II above that seen with bafilomycinA1 alone (Fig. 3A). In contrast, the corollary experiment done in vivo with a colchicine “block” demonstrated a significant increase in LC3-II when mice were treated for seven days with 10 mg/kg/day i.p. rapamycin (Fig. 3B and C). This was similar to that seen when mice were treated for 7 days with 20 mg/kg/day of the rapamycin analog, temsirolimus/CCI-779 (Fig. 3D). Interestingly, in both cases the increase in LC3-II was not apparent without the addition of colchicine for two days. As expected both rapamycin and temsirolimus were able to decrease the phosphorylation of ribosomal protein S6 consistent with their primary effect on the mTOR pathway (Fig. 3B and D).
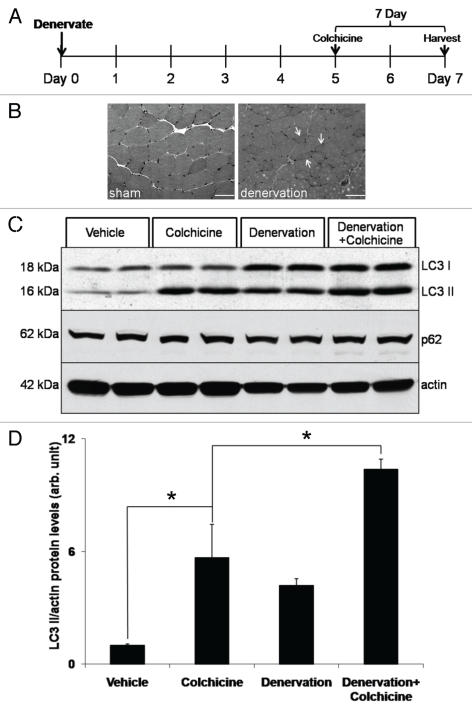
We wondered if the in vivo autophagic flux assay would be able to answer whether autophagy is activated in skeletal muscle following denervation. It has been previously reported that autophagy is upregulated late in denervation-induced atrophy.7,8 Therefore, we evaluated whether autophagic flux was upregulated after seven days neurogenic atrophy. To do this, we performed a sham or complete sciatic nerve transaction in several cohorts of mice treated with and without colchicine for two days (Fig. 4A, outlines experimental design). Denervation-induced atrophy was confirmed via routine muscle histopathology and showed multiple atrophic angular fibers as compared to sham controls (Fig. 4B). TA muscle was harvested seven days post-denervation. Some groups received colchicine for two days prior to animal sacrifice. With this strategy, we confirmed that autophagy is upregulated as assessed by an increase in LC3-I and LC3-II levels and further established that “autophagic flux” is increased in denervation-induced atrophy at seven days (Fig. 4C and D).
Figure 4.
Denervation-induced atrophy enhances autophagic flux. (A) Schematic of experimental design to assess autophagic flux post denervation. Briefly, 3-month-old male mice had a sham or complete sciatic nerve transaction (denervation) for seven days prior to muscle harvesting. Mice were also treated with vehicle or 0.4 mg/kg/day colchicine for two days prior to harvesting. (B) Hematoxylin and eosin staining of TA muscle from 7 day sham or denervated mice. Note an increase in atrophic angular fibers in denervated animal (arrows). Scale is 40 µM. (C) TA muscle lysates from each group were subjected to LC3, p62 or actin immunoblots. Blots are representative of two independent experiments. There was an augmentation of LC3-II levels above that seen with colchicine alone after seven days of denervation. (D) LC3-II/actin ratios were quantitated via densitometry from muscle lysates from two independent experiments with two mice per treatment condition. Error bars represent standard error and *denote p values of <0.05 as obtained by student paired t-test.
Discussion
We describe an in vivo autophagic flux assay that uses colchicine to block autophagosome degradation in skeletal muscle. Although the principles of the assay have been described for cell culture,2,4 this report broadens the utility of an autophagic flux assay to whole animal models. Several key differences in these assays warrant further discussion. One necessary element of the in vitro autophagic flux assay is that autophagosome degradation must be completely blocked. It has been suggested that bafilomycinA1 or other lysomotropic agent be carefully titrated in vitro and that different cell lines may require different doses.4 Moreover, the addition of a second agent such as vinblastine to the optimal established dose of bafilomycinA1 can help confirm a complete “block” in LC3-II degradation.4 We attempted this in vivo. However, increasing doses of colchicine above 0.6 mg/kg/day had lethal consequences as did the addition of other “blocking” agents. Therefore the interpretation of any result using colchicine in vivo should consider the possibility that an increase in LC3-II levels is secondary to an additional blockage in autophagosome maturation or lysosomal degradation. It is important to note that nutrient deprivation and mTOR inhibition via rapamycin or temsirolimus alone failed to increase the levels of LC3-II suggesting that autophagic flux is not impaired by these treatments. In these instances colchicine did not result in an additive effect (as would be expected if starvation or rapamycin resulted in a decrease in autophagic flux) but instead colchicine treatment revealed an increase in autophagic flux that was not readily apparent with rapamycin or starvation alone.
It is notable that p62 levels were unchanged following two days of in vivo colchicine treatment whereas LC3-II levels significantly increased. This difference was not seen in bafilomycinA1 treated myotubes where both p62 and LC3-II levels increased similarly (Fig. 3A). Chronic treatment of mice with colchicine (>five days) did lead to an increase in p62 protein levels (not shown). Perhaps the differential accumulation of LC3-II versus p62 under conditions of autophagic block highlights the distinct autophagic degradation kinetics of these two substrates in mature skeletal muscle as compared to cell culture. For example, p62 associates with myofibrillar proteins such as titin and may turnover more slowly than LC3-II.9
Another critical difference between an in vitro and in vivo autophagic flux assay is the time period of bafilomycinA1 versus colchicine treatment. Whereas in cultured cells one can treat with bafilomycinA1 for short periods of time (e.g., 2–4 hours) and achieve a block in autophagosome-lysosome fusion, this is not feasible in vivo. It is conceivable that the prolonged use of colchicine to block autophagosome-lysosome fusion in vivo compensatorily leads to an upregulation in autophagy. However, this does not seem to be apparent at two days since there is no increase in LC3-I levels. Both LC3-I and LC3-II levels are elevated when mice are treated with colchicine beyond five days suggesting that both an enhancement and blockage of autophagy is occurring with prolonged treatment (not shown).
One important aspect of this assay is the use of colchicine as a blocker of autophagosome degradation. We selected colchicine for several reasons. (1) colchicine is inexpensive and has excellent in vivo pharmokinetics; (2) colchicine is well tolerated by mice unlike chloroquine, bafilomycinA1 and vinblastine which have significant toxicities; and (3) the increase in LC3-II protein levels occurred rapidly in skeletal muscle within two days of colchicine treatment. Colchicine inhibits microtubule polymerization by binding to tubulin.5 This mechanism of action has led to its use as a chemotherapeutic and anti-inflammatory agent since it targets dividing cells and can inhibit neutrophil motility. Chronic treatment of mice or patients with colchicine can cause a reversible vacuolar myopathy.5 Muscle tissue from colchicine myopathy patients characteristically contains vacuoles that label with acid hydrolases suggesting that they are lysosomal in origin.5 This suggests that skeletal muscle may be particularly sensitive to the autophagosome-lysosome fusion effects of colchicine.
Our in vivo autophagic flux assay was able to detect an increase in autophagy following seven days of treatment with the mTOR inhibitors rapamycin or temsirolimus. Previously it had not been well established that these drugs were able to stimulate autophagic flux in skeletal muscle. In fact it had been suggested that rapamycin was not a potent activator of autophagy in skeletal muscle.7 Our data would suggest that mTOR inhibition can activate autophagy similar to that seen with nutrient deprivation. This data raises the possibility that an in vivo autophagic flux may validate the efficacy of autophagy enhancing drugs that fail to significantly induce autophagy in vitro. Finally, our assay was able to detect an increase in autophagic flux following denervation induced atrophy. Consistent with previous reports looking at LC3-II protein levels and autophagy related transcript changes, we found that autophagy was activated after seven days of denervation.7,8 This finding further validates the utility of our in vivo autophagic flux assay.
Materials and Methods
Reagents and antibodies.
Rapamycin (R-5000) and Temsirolimus/CCI-779 (T-8040) were purchased from LC Laboratories (www.LCLabs.com). BafilomycinA1 (B1793), chloroquine diphosphate (C6628), colchicine (C9754) and vinblastine (V1377) were purchased from Sigma-Aldrich (www.sigmaaldrich.com). Anti-LC3B (L7543), p62 (P0067) and actin (A2066) polyclonal antibodies were purchased from Sigma-Aldrich, anti-phospho-S6 (ser235/236) (2211S) and p70 S6K (9202L) polyclonal antibodies were purchased from Cell Signaling Technology (www.cellsignal.com) and anti-Lamp2 (SC-18822) was purchased from Santa Cruz (www.scbt.com). All other reagents were purchased from Sigma-Aldrich. Skeletal muscle histochemistry was performed as previously described.10
In vivo electroporation.
Mice were anesthetized using pentobarbital (50 mg per kg body weight). The skin overlying the tibialis anterior (TA) muscle was shaved, and the animals were injected with 25 µg endotoxin free mCherry-GFP-LC3 expression plasmid (provided by Dr. Abhinav Diwan, Washington University) diluted in sterile PBS to a volume of 50 µl by using a 0.5 ml syringe fitted with a 29-gauge needle. Two-needle array electrodes (450121) (Harvard Apparatus, Holliston, MA) were inserted into the muscle immediately after DNA delivery for electroporation. The distance between the electrodes was 5 mm, and the array was inserted longitudinally relative to the muscle fibers. In vivo electroporation parameters were the following: voltage, 75 V; pulse length, 50 ms; # of pulse, 6 pulses; pulse interval, 200 msec; desired field strength, 200 V/cm, given by a BTX ECM830 Electro Square Porator. Animals were allowed to recover for 5 days at which time they were treated with i.p. vehicle or 0.4 mg/kg/day colchicine for two days. Following treatment, TA muscle was harvested and frozen in liquid nitrogen cooled isopentane. 10 µM sections of TA muscle were affixed to slides, treated for 10 minutes in ice cold acetone and mounted with Prolong Gold + DAPI (Invitrogen; P36931). Specimens were examined using a fluorescent microscope (Nikon 80i upright) and Roper Scientific EZ monochrome CCD camera with deconvolution software analysis (NIS Elements, Nikon). Nonfluorescent images were taken with a 5 megapixel color CCD (Nikon). Image processing and analysis were done with (NIS Elements 4.0) software and Adobe Photoshop CS3. All images were performed at room temperature using a 40X objective.
Activation and blocking autophagy in vivo.
All animal experimental protocols were approved by the Animal Studies Committee of Washington University School of Medicine. Male C57BL6/J mice (Jackson Laboratory) at 11∼12 weeks of age were used for all experiments. Upon arrival to Washington University School of Medicine, mice were housed in a temperature-controlled environment with 12 hr light/dark cycles where they received food and water ad libitum for a minimum of 72 hours prior to any experimentation. Rapamycin and temsirolimus were dissolved in 100% ethanol for a 62.5 mg/ml stock solution and stored at −20°C. Immediately prior to injection, rapamycin and temsirolimus were diluted to 2.5 mg/ml in vehicle solution (5% PEG 400, 5% Tween 80 and 4% ethanol). 10 mg/kg/day rapamycin, 20 mg/kg/day temsirolimus or vehicle was i.p. injected to mice daily for seven days. Nutrient deprivation was performed by removing food for 48 hr. Mice had free access to drinking water. Mice were weighed daily to confirm starvation. 0.4 mg/kg/day colchicine was dosed i.p. in the last two days of the experiment. Colchicine was dissolved in water and stored at −20°C as a stock solution at a concentration of 4 mg/ml. On the day of treatment, colchicine was diluted to 0.1 mg/ml in water prior to injection. Control mice received an equal volume of i.p. water.
Western blot of muscle tissue.
Animals were anesthetized with an i.p. injection of pentobarbital sodium (0.5 mg/kg) and skeletal muscle was immediately dissected and flash frozen in liquid nitrogen. Frozen muscle was homogenized in 1 mL ice-cold RIPA buffer (150 mM NaCl; 10 mM Tris-HCl, pH 7.2; 0.1% Triton X-100; 1% sodium deoxycholate; 5 mM EDTA) containing protease inhibitor cocktail (Sigma-Aldrich) with glass grinding tubes resting in an ice-water bath. The skeletal muscle homogenate was then centrifuged at 10,000 g for 10 min at 4°C. The protein concentration of the supernatant was quantitated using the bicinchoninic acid kit (Pierce Chemical; 23225) with BSA as a standard and adjusted to 2 µg/µl in RIPA buffer. This yields 800–1,000 µL of lysate. Aliquots of the lysate were further solubilized in Laemmli sample buffer and boiled for 5 min prior to SDS-PAGE. 50 µg of protein was loaded and resolved on 12% SDS-PAGE gels. Gel was transferred to nitrocellulose membranes (Trans-Blot, Bio-Rad) and the membrane was blocked in a solution of 5% nonfat dry milk in Tris-buffered saline containing 0.5% Tween-20 (TBS-T). Membranes were incubated with a 1:2,000 dilution of LC3B antibody in 1% milk and TBS-T, a 1:2,500 dilution of anti-p62 antibody, or a 1:5,000 dilution of anti-actin antibody. Blots were subsequently incubated with peroxidase-conjugated secondary antibodies and immunoreactive proteins were revealed using the ECL detection system (GE Healthcare; RPN2209). Western blot results were quantified by densitometric analysis using ImageJ software (NIH). Western blots for phosphorylated (Ser235/236) and p70 S6K were performed by a method analogous to that described above.
Cell culture.
The mouse myoblast cell line C2C12 (American Type Culture Collection) was maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle Medium (GIBCO; 11965) supplemented with 10% fetal bovine serum, 50 µg/ml penicillin and 50 µg/ml streptomycin. Cultures were induced to differentiate in DMEM containing 2% horse serum for five days. C2C12 myotubes grown on six-well plates were treated with 10 µg/mL rapamycin with and without 200 nM BafilomycinA1 for 6 hours. Cells were washed with ice-cold PBS and then scraped into RIPA buffer. Lysates were centrifuged at 10,000 g for 10 min. All western blots of C2C12 cells were processed in an analogous manner as mouse muscle tissues and 20 µg of total proteins were subjected to SDS-PAGE.
Acknowledgements
Dr. Weihl is funded by the NIH (R01AG031867) and the Muscular Dystrophy Association.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/12785
References
- 1.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinsztein DC, Cuervo AM, Ravikumar B, Sarkar S, Korolchuk V, Kaushik S, et al. In search of an “autophagomometer.”. Autophagy. 2009;5:585–589. doi: 10.4161/auto.5.5.8823. [DOI] [PubMed] [Google Scholar]
- 5.Kuncl RW, Bilak MM, Craig SW, Adams R. Exocytotic “constipation” is a mechanism of tubulin/lysosomal interaction in colchicine myopathy. Exp Cell Res. 2003;285:196–207. doi: 10.1016/s0014-4827(03)00034-x. [DOI] [PubMed] [Google Scholar]
- 6.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary MF, Hood DA. Denervation-induced oxidative stress and autophagy signaling in muscle. Autophagy. 2009;5:230–231. doi: 10.4161/auto.5.2.7391. [DOI] [PubMed] [Google Scholar]
- 9.Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, et al. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- 10.Ju JS, Fuentealba RA, Miller SE, Jackson E, Piwnica-Worms D, Baloh RH, Weihl CC. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J Cell Biol. 2009;187:875–888. doi: 10.1083/jcb.200908115. [DOI] [PMC free article] [PubMed] [Google Scholar]