Abstract
Parkinson disease (PD) is the second most prevalent neurodegenerative disorder, and thus elucidation of the pathogenic mechanism and establishment of a fundamental cure is essential in terms of public welfare. Fortunately, our understanding of the pathogenesis of two types of recessive familial PDs—early-onset familial PD caused by dysfunction of the PTEN-induced putative kinase 1 (PINK1) gene and autosomal recessive juvenile Parkinsonism (ARJP) caused by a mutation in the Parkin gene—has evolved and continues to expand.
Key words: PINK1, parkin, ubiquitin, mitochondria, autophagy, mitophagy, membrane potential, quality control
Since the cloning of PINK1 and Parkin, numerous papers have been published about the corresponding gene products, but the mechanism by which dysfunction of PINK1 and/or Parkin causes PD remain unclear. Parkin encodes a ubiquitin ligase E3, a substrate recognition member of the ubiquitination pathway, whereas PINK1 encodes a mitochondria-targeted serine-threonine kinase that contributes to the maintenance of mitochondrial integrity. Based on their molecular functions, it is clear that Parkin-mediated ubiquitination and PINK1 phosphorylation are key events in disease pathogenesis. The underlying mechanism, however, is not as well defined and claims of pathogenicity, until recently, remained controversial. Although Parkin's E3 activity was clearly demonstrated in vitro, we were unable to show a clear E3 activity of Parkin in cell/in vivo. In addition, despite a predicted mitochondrial localization signal for PINK1, we were unable to detect PINK1 on mitochondria by either immunoblotting or immunocytochemistry. More confusingly, overexpression of nontagged PINK1 mainly localized to the cytoplasm under steady state conditions.
Work by Dr. Youle's group at the National Institutes of Health in 2008, however, offered new insights. They reported that Parkin associated with depolarized mitochondria and that Parkin-marked mitochondria were subsequently cleared by autophagy. Soon after their publication, we also examined the function of Parkin and PINK1 following a decrease in mitochondrial membrane potential. Our findings, described below (Fig. 1), have contributed to the development of a mechanism explaining pathogenicity.
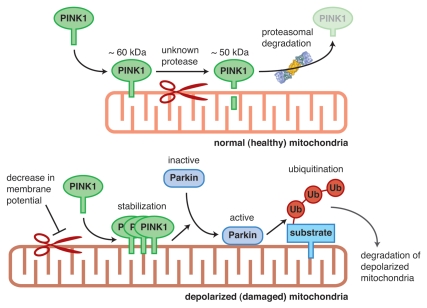
Figure 1.
Model of mitochondrial quality control mediated by PINK1 and Parkin. Under steady-state conditions, the mature 60 kDa PINK1 is constantly cleaved by an unknown protease to a 50 kDa intermediate form that is subsequently degraded, presumably by the proteasome (upper part). The protein, however, is stabilized on depolarized mitochondria because the initial processing event is inhibited by a decrease in mitochondrial membrane potential (lower part). Accumulated PINK1 recruits cytosolic Parkin onto depolarized mitochondria resulting in activation of its E3 activity. Parkin then ubiquitinates a mitochondrial substrate(s). As a consequence, damaged mitochondria are degraded via mitophagy. Ub, ubiquitin.
(1) We sought to determine the subcellular localization of endogenous PINK1, and realized that endogenous PINK1 is barely detectable under steady-state conditions. However, a decrease in mitochondrial membrane-potential following treatment with the mitochondrial uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP) results in the gradual accumulation of endogenous PINK1 on mitochondria. Importantly, when CCCP is washed out, the accumulated endogenous PINK1 rapidly disappears (within 30 min) both in the presence and absence of cycloheximide. These results support the hypothesis that PINK1 is constantly transported to the mitochondria, but is rapidly degraded in a membrane potential-dependent manner (see below for details). We speculate that PINK1 is stabilized by a decrease in mitochondrial membrane potential and as a result accumulates on depolarized mitochondria.
(2) We examined the potential role of PINK1 in the mitochondrial recruitment of Parkin. In control MEFs (PINK1+/+), Parkin is selectively recruited to the mitochondria following CCCP treatment, and subsequently results in the selective disappearance of the mitochondria via autophagy (called mitophagy). In sharp contrast, Parkin is not translocated to the mitochondria in PINK1 knockout (PINK1−/−) MEFs following CCCP treatment, and subsequent mitochondrial degradation is also completely impeded. These results suggest that PINK1 is “a Parkin-recruitment factor” that recruits Parkin from the cytoplasm to damaged mitochondria in a membrane potential-dependent manner for mitophagy.
(3) We monitored the E3 activity of Parkin using an artificial pseudo-substrate fused to Parkin in cells. Parkin's E3 activity was repressed under steady-state conditions; however, we find that Parkin ubiquitinates the pseudo-substrate when it is retrieved to the depolarized mitochondria, suggesting that activation of the latent Parkin E3 activity is likewise dependent on a decrease in mitochondrial membrane potential.
(4) PINK1 normally exists as either a long (approximately 60 kDa) or a short (approximately 50 kDa) protein. Because the canonical mitochondrial targeting signal (matrix targeting signal) is cleaved after import into the mitochondria, the long form has been designated as the precursor and the short form as the mature PINK1. However, our subcellular localization study of endogenous PINK1 following CCCP treatment shows that the long form is recovered in the mitochondrial fraction, suggesting that it is not the pre-import precursor form. Moreover, by monitoring the degradation process of PINK1 following recovery of membrane potential, we realized that the short form of PINK1 transiently appears soon after CCCP is washed out and then later disappears, suggesting that the processed form of PINK1 is an intermediate in membrane-potential-dependent degradation. In conclusion, these results imply that PINK1 cleavage does not reflect a canonical maturation process accompanying mitochondrial import as initially thought, but rather represents constitutive degradation in healthy mitochondria by a two-step mechanism; i.e., first limited processing and subsequent complete degradation probably via the proteasome.
(5) PINK1 accumulation by decrease of membrane potential and subsequent recruitment of Parkin onto mitochondria are presumably etiologically important because they are impeded for the most part by disease-linked mutations of PINK1 or Parkin.
These results, together with reports by other groups, strongly suggest that recessive familial PD is caused by dysfunction of quality control for depolarized mitochondria.
At present, we do not know whether the aforementioned pathogenic mechanism of recessive familial PD can be generalized to prevalent sporadic PD. However, the clinical symptoms of recessive familial PD caused by dysfunction of PINK1 or Parkin resembles that of idiopathic PD except early-onset pathogenesis, and thus it is plausible that there is a common pathogenic mechanism. We accordingly believe that our results provide solid insight into the molecular mechanisms of PD pathogenesis, not only for familial forms caused by Parkin and PINK1 mutations, but also the major sporadic form of PD.
To fully understand the molecular mechanism of PINK1-Parkin-mediated mitophagy, further details need to be addressed including: identifying the protease(s) that processes PINK1 in a mitochondrial membrane-potential dependent manner and that presumably monitors mitochondrial integrity; identifying a physiological substrate(s) of PINK1; determining the molecular mechanism underlying Parkin activation; and identifying the protein(s) linking Parkin-mediated ubiquitination to mitophagy. A detailed mechanism of the aforementioned events will be the focus of future research, however, we feel our conclusion that PINK1 and Parkin function in the removal of depolarized mitochondria is evident and hope that our studies will provide a solid foundation for further studies.
Punctum to: Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13039