Abstract
Autophagy is an intracellular degradative pathway that plays key roles in the homeostatic turnover of long-lived or damaged proteins and organelles, and in the survival of cells during starvation or other stressful conditions. We have uncovered an unexpected link between glutamine (Gln) metabolism and the regulation of autophagy. Our findings indicate that ammonia, generated from Gln deamination in mitochondria, functions as an autocrine- and/or paracrine-acting stimulator of autophagic flux.
Key words: glutamine, autophagy, ammonia, glutaminolysis, tumor metabolism
Cancer cells exhibit significant alterations in metabolic pathways that support cell mass accumulation, nucleic acid biosynthesis and mitotic cell division. Under aerobic conditions, most nontransformed cells metabolize glucose to pyruvate, which is subsequently oxidized to CO2 in the mitochondria, generating ∼36 molecules of ATP per molecule of glucose. In contrast, many cancer cells rely almost exclusively on glycolysis, which yields only two ATP molecules per molecule of glucose and divert pyruvate-derived carbon away from the mitochondria by reducing this metabolite to lactate. Mitochondrial metabolism also undergoes extensive reprogramming in cancer cells, manifested by a shift from ATP production toward the generation of biosynthetic precursor molecules by the tricarboxylic acid (TCA) cycle. Because glycolysis deprives mitochondria of glucose-derived carbon, glutamine largely replaces glucose in rapidly proliferating cells as the alternative carbon source used for the production of biosynthetic building blocks fueled by the TCA cycle.
Glutaminolysis is a mitochondrial pathway that involves the initial deamination of Gln by glutaminase, yielding glutamate (Glu) and ammonia. Glutamate is then converted via a second deamination step to a TCA cycle intermediate, α-ketoglutarate (α-KG). The conversion of Glu to α-KG is catalyzed by either Glu dehydrogenase (yielding a second molecule of ammonia) or one of several transaminases, which convert α-keto acids into their corresponding amino acids. Glutaminolysis also supports the production of molecules, such as glutathione and NADPH, which protect cells from oxidative stress. Our research reveals yet another facet of Gln metabolism that may contribute to cancer development and progression. We discovered an unanticipated link between glutaminolysis and the production of a diffusible stimulator of autophagy that may allow nutritionally replete tumor cells to protect nearby tumor cells from metabolic stress-induced death.
This study began with the relatively predictable observation that proliferating cancer cells showed a robust increase in autophagic flux when maintained in culture for several days without a medium change. Cell-conditioned medium (CCM) obtained from these extended cultures triggers a more rapid increase in autophagic flux when added to secondary cultures of freshly plated cells. The obvious explanation for these results was that CCM was depleted of nutrients during the extended primary culture, and that the increase in autophagy simply reflected a starvation response in the CCM-exposed cells. Surprisingly, however, replenishment of CCM with glucose, amino acids, and other medium components fails to reverse the pro-autophagic activity observed in secondary cell cultures.
In subsequent studies, we explored the possibility that the autophagy-promoting activity in CCM was related to the release of a trans-acting stimulator of the autophagic pathway. Biochemical analyses revealed that this factor has a very low molecular weight, is cationic in nature and is volatile in aqueous solution. We purified the autophagy-inducing factor by distillation and ultimately identified it as ammonia, which is found at low millimolar concentrations (2–4 mM) in CCM. Direct treatment of nutrient-replete cells with equivalent concentrations of ammonia triggers autophagic responses identical to those observed with CCM. However, treatment of cells with a concentration of ammonia (20 mM), which is commonly used to inhibit lysosomal activity, reduces autophagic flux. The biological relevance of these findings is supported by observations that interstitial fluids from human tumor xenografts growing in immunodeficient mice contain ammonia at concentrations identical to those found in CCM.
As described above, glutaminolysis is an ammonia-producing pathway that fuels biosynthetic reactions in cancer cells and normal cells (such as T lymphocytes) that undergo explosive rounds of rapid proliferation. We find that Gln-free culture medium fails to support production of autophagy-promoting CCM. Exposure of cells cultured in Gln-containing complete medium to α-KG (a product of glutaminolysis) diminishes both ammonia production and the generation of autophagy-stimulating activity in CCM. Collectively, these results suggest that Gln is the most significant source of ammonia in these cells, and that the pro-autophagic activity in CCM is largely attributable to glutaminolysis. A noteworthy observation is that ongoing production of ammonia from Gln maintains the steady-state level of autophagy in cultured cancer cells.
We now suggest that Gln plays two seemingly paradoxical roles in cancer cell metabolism. On the one hand, Gln is an important supplier of building blocks that support macromolecular biosynthesis and antioxidant molecules, and in turn, cell mass accumulation and proliferation. On the other hand, Gln represents a transportable depot for ammonia, a bioactive metabolite that stimulates cell catabolism via autophagy. We can rationalize these findings by proposing that cancer cell growth and proliferation occur in a stressful microenvironment (stress can also be induced by cytotoxic chemotherapy), and that autophagy increases stress resistance by eliminating damaged and potentially toxic macromolecules and organelles. Indirect support for this hypothesis comes from experiments demonstrating that cancer cells treated with the death receptor ligand tumor necrosis factor-α are protected by the presence of Gln, due in part to the stimulation of autophagy by the ammonia generated during glutaminolysis.
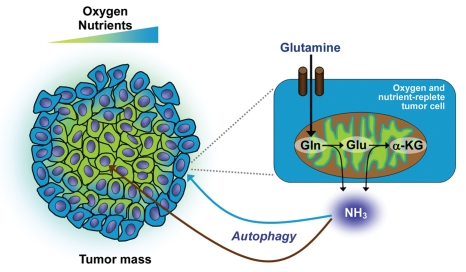
This study raises several important questions that require further investigation. The mechanism of autophagy induction by ammonia remains unknown, although our results indicate that ammonia stimulates autophagic flux through a pathway that does not involve the mTOR complex 1 (mTORC1), a nodal regulator of autophagy in mammalian cells. Furthermore, the observation that tumor tissue-derived interstitial fluids contain autophagy-stimulating concentrations of ammonia suggests that ammonia might transmit pro-autophagic signals in these tissues. We envision that tumor cells residing in well-oxygenated, nutrient-replete microdomains avidly metabolize Gln, and release ammonia that provokes increased autophagy in avascular, nutritionally stressed regions of the tumor (Fig. 1). Hence, Gln not only allows metabolically active tumor cells to grow and reproduce, but also allows these cells to produce a factor (ammonia) that supports their less fortunate neighbors that are coping with severe metabolic stress. Interestingly, the concept of ammonia as a trans-acting messenger has clear precedents in budding yeast and the slime mold, Dictyostelium. Clearly, Gln plays increasingly pervasive roles in both tumor and normal cell biology, a scenario that will undoubtedly provoke intensive research efforts to selectively manipulate Gln metabolism in cancer and other diseases.
Figure 1.
Stimulation of autophagy by Gln-derived ammonia. Gln is metabolized in mitochondria to Glu (by glutaminase) and α-KG (by transaminases or glutamate dehydrogenase). The glutaminase- and glutamate dehydrogenase-catalyzed reactions release ammonia, which diffuses into the local microenvironment and stimulates autophagy in an autocrine fashion (blue arrow). Ammonia also diffuses to other tumor regions to induce autophagy in a paracrine fashion, possibly maintaining the survival of metabolically-stressed cells in these regions (brown arrow).
Punctum to: Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal. 2010;3:ra31. doi: 10.1126/scisignal.2000911.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13082