Abstract
Toll-like receptor 4 (TLR4) signaling triggers autophagy, which has been linked to both adaptive and innate immunity. Engagement of TLR4 recruits to the receptor complex Beclin 1, a key component of a class III phosphatidylinositol 3-kinase complex (PI3KC3) that initiates autophagosome formation. Recently, we found that tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6)-mediates Lys63 (K63)-linked ubiquitination of Beclin 1 is crucial for TLR4-triggered autophagy in macrophages. We identified two TRAF6-binding motifs in Beclin 1 that facilitate the binding of TRAF6 and the ubiquitination of Beclin 1. A lysine located in the Bcl-2 homology 3 (BH3) domain of Beclin 1 serves as a major site for K63-linked ubiquitination. Opposing TRAF6, the deubiquitinating enzyme A20 reduces the extent of K63-linked ubiquitination of Beclin 1 and limits the induction of autophagy in response to TLR4 signaling. Furthermore, treatment of macrophages with either interferong or interleukin-1 triggers the K63-linked ubiquitination of Beclin 1 and the formation of autophagosomes. These results indicate that the status of K63-linked ubiquitination of Beclin 1 plays a key role in regulating autophagy during inflammatory responses.
Key words: autophagy, toll-like receptor, ubiquitin, Beclin 1, A20, TRAF6
Toll-like receptors (TLRs) recognize specific molecular patterns that are present in microbial components. Activated TLRs recruit adaptor proteins such as myeloid differentiation marker 88 (MyD88) and tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6), an E3 ubiquitin ligase and scaffold protein. Ubiquitinated TRAF6 mediates signaling that leads to the translocation of NFκB from the cytoplasm to the nucleus, which triggers the transcription of numerous genes including those that encode inflammatory cytokines. In addition, A20 is an NFκB target gene. A20 possesses both deubiquitinating enzyme and E3 ubiquitin ligase activities. The induction of A20 helps to terminate NFκB triggered gene transcription by removing ubiquitin from TRAF6. Illustrating the importance of A20, mice that lack it exhibit spontaneous inflammation, cachexia and premature death, all of which depend upon the activation of TLRs. In macrophages, both MyD88 and another adaptor protein called TIR domain-containing adaptor inducing interferon-β (TRIF) interact with Beclin 1, thereby linking the TLR signaling pathway to autophagy. Beclin 1, the mammalian homolog of yeast Atg6, is a component of a PI3KC3 that helps to localize autophagy proteins to the preautophagosomal membrane. Exposure of macrophages to a TLR4 ligand also reduces the association between Beclin 1 and Bcl-2, an antiapoptotic protein that binds to the BH3 domain of Beclin 1, acting to limit autophagy.
In a recent study we found that stimulation of the murine macrophage cell line Raw 264.7 or primary human monocytes with the TLR4 ligand lipopolysaccharide (LPS) triggers K63-linked ubiquitination of Beclin 1, which helps promote autophagy. In addition, stimulation of Raw 264.7 cells with IL-1 or interferon. or amino acid starvation of the cells triggers K63-linked ubiquitination of Beclin 1 and the formation of autophagosomes. These results suggest a central role for Beclin 1 ubiquitination in the control of autophagy induced by a variety of signals.
Manipulating the amount of TRAF6 protein expressed supports a role for TRAF6 in the K-63 linked ubiquitination of Beclin 1. An inspection of the human Beclin 1 amino acid sequence reveals two potential TRAF6-binding sites located between residues 54 to 58 and 297 to 301. We show that both sites are needed for an optimal interaction between Beclin 1 and TRAF6 and that the TRAF6-binding mutants of Beclin 1 undergo much less K63-linked ubiquitination than do wildtype Beclin 1. To provide evidence that TRAF6 directly ubiquitinates Beclin 1, we reconstituted the reaction in vitro. We purified FLAG-TRAF6 and FLAG-Beclin 1 and mixed them with recombinant E1 and E2 enzymes, ATP and recombinant hemagglutinin (HA)-tagged ubiquitin. These results show that purified TRAF6 catalyzes the ubiquitination of Beclin 1 in vitro. Whereas TRAF6 ubiquitinates Beclin 1, A20 deubiquitinates it. Supporting this conclusion are several observations. First, reducing A20 protein expression enhances the level of Beclin 1 K63-linked ubiquitination following LPS stimulation of Raw 267.4 cells. Second, overexpression of A20 or an A20 mutant that lacks E3 ligase activity attenuates LPS induced Beclin 1 ubiquitination. Third, recombinant A20 that lacks E3 ligase activity deubiquitinates purified Beclin 1. These data argue that A20 can limit the ubiquitination of Beclin 1 not only by controlling the E3 ligase activity of TRAF6, but also by directly targeting ubiquitinated Beclin 1. Some proteins that undergo ubiquitination also bind ubiquitin. We find that Beclin 1 can bind K63-linked ubiquitin chains, providing a mechanism by which ubiquitinated Beclin 1 can oligomerize.
Beclin 1 contains a conserved BH3 domain that is required for its binding to Bcl-2. The introduction of a L116A mutation in the BH3 domain of Beclin 1 attenuates its binding to Bcl-2 and enhances Beclin 1-triggered autophagy. Adjacent to this residue in Beclin 1 is a conserved lysine residue, which we viewed as a prime candidate to link ubiquitin chains. By comparing the ubiquitination of K117A Beclin 1 to wild-type Beclin 1, we conclude that K117 of Beclin 1 is a major target for linking ubiquitin chains following stimulation of murine macrophages with LPS. We also find that K63-linked ubiquitination facilitates Beclin 1-Beclin 1 interactions and enhances the lipid kinase activity of PI3KC3. These results suggest that the ubiquitination of K117 of Beclin 1 promotes the oligomerization of Beclin 1 and affects the activity of PI3KC3.
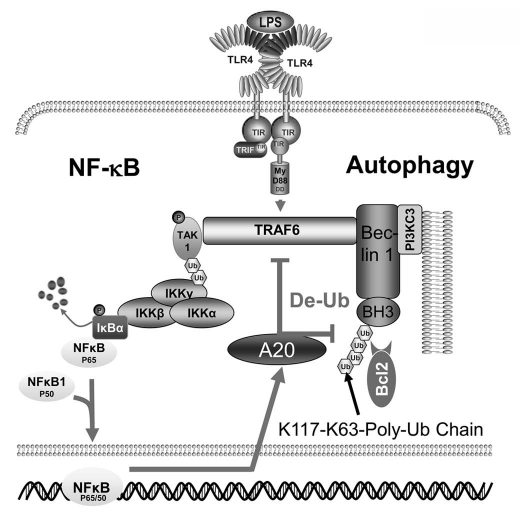
In conclusion, in TLR4-induced autophagy in macrophages, TRAF6 and A20 regulate Beclin 1 in a fashion analogous to their defined roles in controlling the activation of NFκB (Fig. 1). By controlling the extent of ubiquitination of Beclin 1, the TRAF6-A20 axis acts as a rheostat to stimulate or inhibit autophagy. IFNγ exposure and amino acid starvation also cause the ubiquitination of Beclin 1, which suggests that K63-linked ubiquitination of Beclin 1 may be a shared mechanism among the different stimuli that trigger the formation of autophagosomes.
Figure 1.
TRAF6 and A20 control K63-linked ubiquitination of Beclin 1 to trigger autophagy. Exposure of macrophages to LPS activates TLR4 at the plasma membrane. The engaged TLR4 recruits TRIF and MyD88 leading to enhanced TRAF6 E3 ligase activity. One target of TRAF6 is lysine 117 in Beclin 1. The K63-linked ubiquitination of Beclin 1 facilitates the oligomerization of Beclin 1 and the activation of PI 3KC3. This helps trigger the formation of autophagosomes. The engagement of TLR4 also triggers a signaling pathway that leads to the translocation of NFκB to the nucleus. A20 is a direct NFκB target gene and its expression is induced. The expression of A20 limits TLR4-induced autophagy by deubiquitinating TRAF6 and Beclin 1. Lysine 117 in Beclin 1 is strategically located in the binding site for Bcl-2. The interaction of Bcl-2 with Beclin 1 limits the induction of autophagy triggered by TLRs.
Acknowledgements
This research was supported by Intramural Research Program of the National Institutes of Allergy and Infectious Diseases, National Institutes of Health.
Punctum to: Shi CS, Kehrl JH. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci Signal. 2010;3:42. doi: 10.1126/scisignal.2000751.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13288