Abstract
Background
Specific immunotherapy is the only treatment with the potential to prevent progression of the allergic disease and the potential to cure patients. The immunomodulatory ability of SQ-standardized house dust mite (HDM) subcutaneous immunotherapy (SCIT) was investigated in patients with allergic asthma.
Methods
Fifty-four adults with HDM-allergic asthma were randomized 1 : 1 to receive SQ-standardized HDM SCIT (ALK) or placebo for 3 years. At baseline, and after 1, 2 and 3 years of treatment, the lowest possible inhaled corticosteroid dose required to maintain asthma control was determined, followed by determinations of nonspecific and HDM-allergen-specific bronchial hyperresponsiveness, late asthmatic reaction (LAR), immediate and late-phase skin reactions, and immunological response.
Results
SQ-standardized HDM SCIT provided a statistically significantly higher HDM-allergen tolerance (P < 0.05 vs placebo) in terms of a 1.6-fold increase in PD20 (HDM-allergen inhalation challenge), a 60-fold increase in skin test histamine equivalent HDM-allergen concentrations, reduced immediate- and reduced or abolished late-phase skin reactions, as well as fewer patients with LAR. PD20 (methacholine inhalation challenge) increased initially and was similar between groups. House dust mite SCIT induced an initial increase in serum HDM-allergen-specific IgE (P = 0.028 vs placebo), which then declined to baseline value. House dust mite SCIT induced an increase in components blocking IgE binding to allergen [ΔIgE-blocking factor: 0.31; 95% CI of (0.26; 0.37)] after 1 year that remained constant after 2 and 3 years (P < 0.0001 vs placebo).
Conclusion
SQ-standardized HDM SCIT induced a consistent immunomodulatory effect in adults with HDM-allergic asthma; the humoral immune response was changed and the HDM-allergen tolerance in lung and skin increased.
Keywords: allergic asthma, bronchial hyperresponsiveness, house dust mite, immune response, immunotherapy
Asthma prevalence is increasing with an estimate of 300 million individuals affected worldwide (1). Asthma is a chronic inflammatory disorder of the airways associated with airway hyperresponsiveness. This causes recurrent episodes of wheezing, breathlessness, chest tightness and coughing usually associated with airflow obstruction in the lungs (1). Asthma progression often involves airway remodelling (permanent structural changes in airways), which may cause irreversible airflow obstruction and is associated with poorer clinical outcome among patients with asthma (2). Airborne allergens such as house dust mite (HDM) allergens are strongly associated with asthma. House dust mite sensitization commonly initiates the allergic disease manifested as allergic rhinitis that can progress to allergic asthma. Because of the allergic march, only few patients with allergic asthma are still monosensitized as adults (3, 4).
Asthma control is usually achieved by treatment with inhaled corticosteroids (ICS). Prolonged use of high doses of ICS may be associated with a risk of systemic side effects, and although treatment with ICS improves symptoms and inhibits exacerbations, this treatment is not curative (1). Therefore, a treatment is needed that can reduce the use of high doses of ICS, prevent asthma progression to a more severe state and potentially cure patients.
Treatment with specific immunotherapy (SIT) induces immune tolerance to the allergen to which the patient is allergic and is currently the only treatment with the potential to alter the natural course of the disease (5, 6). Specific immunotherapy has been shown to be effective in patients with allergic asthma in terms of reducing asthma symptom score and medication requirements, and improve bronchial hyperresponsiveness (BHR) (7). Specific immunotherapy has also been shown to prevent progression of allergic rhinitis into asthma (8, 9) and to provide sustained effect after treatment cessation in allergic patients (8, 10, 11). As concluded in the recent Cochrane collaboration report, trials are required to elicit the effect of SIT compared with other therapies, as well as the effect of SIT with concurrent steroid therapy in patients with allergic asthma (7).
Previously, we reported that 3 years of SQ-standardized HDM subcutaneous immunotherapy (SCIT) was generally well tolerated and partly replaced the need for ICS treatment to control asthma in adults with HDM-allergic asthma; HDM SCIT had a steroid-sparing effect (12). From the same trial, we now present the difference in treatment efficacy between patients treated with SQ-standardized HDM SCIT plus ICS and patients treated with placebo plus ICS in terms of nonspecific and HDM-allergen-specific BHR, late asthmatic reaction (LAR), lung function, HDM-allergen-specific immediate- and late-phase skin reactions and immunological response (HDM-allergen-specific IgE and IgE-blocking factor).
Materials and methods
Patients
A total of 54 out of 112 screened patients aged 18–60 were included: 32 men and 22 women. Details of inclusion and exclusion criteria are described elsewhere (12). In short, eligible patients had:
HDM allergy and perennial asthma (medical history consistent with HDM allergy).
Regular asthma symptoms requiring long-term treatment with inhaled ICS of at least daily doses of 500–2000 μg fluticasone propionate to control asthma.
A forced expiratory volume in 1 s (FEV1) >70% of predicted value.
Trial design and treatment
Written informed consent was obtained before entering the trial, and the trial was performed in accordance with current GCP standards and the Declaration of Helsinki (13).
Trial design and treatment regimen are described in detail elsewhere (12). In short, it was a 3-year, double-blind, placebo-controlled trial. Patients were randomized 1 : 1 to receive SCIT with Alutard® SQ Dermatophagoides pteronyssinus (D. pteronyssinus; ALK, Hørsholm, Denmark) or placebo.
Treatment included an ‘8-week’ up-dosing (up to 100 000 SQ-U) and a ‘3-year’ maintenance with injection intervals of 6 ± 2 weeks. The placebo group received histamine injections according to the same dose increase and maintenance schedule (0.00001, 0.0001, 0.001 and 0.01 mg histamine/ml).
Patients were also treated with ICS to control their asthma, giving two treatment groups: HDM SCIT plus ICS and placebo SCIT plus ICS. The lowest possible dose of ICS that maintained asthma control in each patient was determined by a stepwise reduction protocol at baseline and after 1, 2 and 3 years of treatment (September–December). Asthma control was defined as ‘the ICS dose one step higher than when patients had uncontrolled symptomatic asthma’. Rescue medication (Salbutamol) was allowed as needed. Tolerability was evaluated by adverse event reporting.
The efficacy measures described in the following paragraph were performed at baseline and after 1, 2 and 3 years of treatment, just after the ICS dose adjustment.
Bronchial challenge tests
Determination of nonspecific BHR. The methacholine bronchial challenge test was carried out with the dosimeter method (14) using a Spira Elektro II™ nebuliser (Respiratory Care Centre, Hameenlinna, Finland). Patients inhaled a fixed amount of solution (nebulization time 0.5 s, start of nebulization at 50 ml, working pressure 2 atm), inspiratory flow of 50 l/s; inspiratory volume of 500–800 ml. FEV1 was measured before provocation and 90 s after each inhalation. Methacholine was administered in doubling doses from 18 to 11 520 μg. Bronchial challenge was terminated when FEV1 decreased at least 20% compared with the patient’s FEV1 measured after a saline inhalation. The decrease in FEV1 was plotted against the methacholine dose (log scale) and the cumulative dose causing a 20% decrease in FEV1 (PD20) was determined. The severity of nonspecific BHR was defined as follows: no BHR, PD20 > 2000 μg; mild BHR, 1000 μg < PD20 ≤ 2000 μg; moderate BHR, 250 μg ≤ PD20 ≤ 1000 μg; severe BHR, PD20 < 250 μg.
Determination of HDM-allergen-specific BHR. The HDM-allergen bronchial challenge test was also carried out with the dosimeter method (14). A dry extract of D. pteronyssinus (Aquagen® SQ, 1 000 000 SQ-U/vial) was diluted to concentrations of 1000; 10 000; and 100 000 SQ-U/ml (ALK). Three breathing steps were used for each allergen concentration (two, four and eight breaths) and the cumulated allergen dose for each breathing step was calculated. Baseline FEV1 was determined 15 min after two initial inhalations with diluent. The maximum allergen dose delivered was 2 breaths of 1000 SQ-U + 2 breaths of 10 000 SQ-U + (2 + 4 + 8 breaths) of 100 000 SQ-U = 1 422 000 SQ-U. FEV1 was measured before and 15 min after each inhalation. Bronchial challenge was terminated when FEV1 decreased at least 20% compared with the patient’s baseline FEV1. The decrease in FEV1 was plotted against the HDM-allergen dose (log scale), and the provocative dose causing a 20% decrease in FEV1 (PD20) determined. PD20 was considered to be 2 844 000 SQ-U if a patient failed to reach a 20% decline in FEV1.
FEV1 was measured according to guideline on a daily calibrated dry wedge spirometer (Vitalograph®, Buckingham, UK) and the percentage of predicted value calculated (15).
Late asthmatic reaction and peak expiratory flow
Patients were provided with an electronic spirometer (Asthma monitor-1; Erish Jaeger GmbH, Hoechberg, Germany) to record their peak expiratory flow (PEF) at home every hour for 24 h after allergen challenge (except during sleep). Late asthmatic reaction was defined as a surplus administration of beta-2 agonist because of asthma symptoms or a fall in PEF of at least 15% from the maximum value after recovery from the early-phase reaction. Morning and evening PEF was recorded over the entire trial.
Skin prick test titration
Dilutions of 1, 10 and 100 Histamine Equivalent in Prick (HEP) of D. pteronyssinus allergen extract (ALK) were used for skin prick test titration (SPTT) on the volar side of the forearm. Positive and negative controls were diluents with or without histamine dihydrochloride (10 mg/ml). Skin reactions (weal area) were measured after 10 min (controls) and after 15 min (HDM-allergen extracts) (New Genius Scanner 4500; Software Genius Inc, Iselin, NJ, USA) (16).
Intradermal allergen challenge
Intradermally, 0.02 ml (20 SQ-U) of Aquagen® SQ D. pteronyssinus (1000 SQ-U/ml; ALK) was injected in the skin of the forearm and a negative control injected in the opposite arm. Immediate-phase skin reactions were determined after 15 min and late-phase skin reactions were determined after 24 h (New Genius Scanner 4500; Genius) (16). The size of the weal area was classified as: (i) no response: <1 cm2; (ii) moderate response: 1–20 cm2; (iii) severe response: >20 cm2.
Immunological response
Serum specific IgE against D. pteronyssinus was determined using Magic Lite® SQ (ALK).
Serum specific IgE-blocking factor against D. pteronyssinus was determined using the ADVIA Centaur immunoassay system (Siemens Medical Solutions, Diagnostics, Tarrytown, NY, USA): IgE-blocking factor was calculated as: 1 – (competed specific IgE/specific IgE); specific IgE is the ordinary determination: the total amount of allergen-specific IgE antibodies that bind to allergen without competing antibodies/components in the solution; competed specific IgE is the total amount of allergen-specific IgE antibodies that bind to allergen in the potential presence of competing components; IgE-blocking factor is the inhibiting capacity of competing components to specific IgE-allergen binding and varies theoretically from 0 (no presence of IgE-blocking components) to 1 (all allergen-specific IgE antibodies are blocked from binding to allergen) (17).
Statistical analysis
Statistical analyses were performed on the full analysis set (FAS) using the available data without imputation of missing values. Endpoints were tested on a 5% significance level, and all tests and confidence intervals (CI) were two-sided. The null hypothesis was no difference between the two groups. There was no adjustment for multiplicity. Doses of methacholine (μg) and HDM-allergen (SQ-U), as well as the concentration of specific IgE (kU/L) were log10-transformed for normality. These data and serum IgE-blocking factor were analysed in anova with change from baseline as response variable, treatment as fixed effect and baseline as covariate. The proportions of patients experiencing a LAR were tested using Fischer’s exact test with exact 95% confidence intervals. In the SPTT, the HDM-allergen dose (in HEP) and skin reactions (weal area in cm2) were log10-transformed for normality. A statistical parallel line regression model was applied to obtain the histamine equivalent HDM-allergen concentration. The immediate- and late-phase skin reactions were analysed with nonparametric Wilcoxon Rank Sum Test. sas® statistical software, Version 8.2 (SAS Institute, Cary, NC, USA) was used.
Results
Patients and tolerability
We included 54 patients with HDM-allergic asthma; 26 patients received HDM SCIT plus ICS (SCIT group) and 28 patients received placebo SCIT plus ICS (placebo group). These two groups were comparable with regard to baseline characteristics (Table 1). Treatment was generally well tolerated; no life-threatening or other serious adverse events related to treatment were reported. Three patients withdrew because of adverse events (two intercurrent illnesses and one worsening of condition) and three patients withdrew because of pregnancies. The trial was completed by 20 patients in the SCIT group and 22 patients in the placebo group. Further details on patient characteristics, safety results and the trial flow according to the CONSORT statement (18) are described elsewhere (12).
Table 1.
Baseline characteristics (FAS)
| HDM SCIT N = 26 | Placebo N = 28 | |
|---|---|---|
| Sex | ||
| Female | 15 | 17 |
| Males | 11 | 11 |
| Age (years, mean ± SD) | 29.8 ± 10.7 | 28.5 ± 7.1 |
| Asthma severity | ||
| Moderate persistent (step 2.2 and 2.3)* | 20 | 22 |
| Severe persistent (step 2.4)† | 6 | 6 |
| Asthma duration (years, mean ± SD) | 14.8 ± 9.7 | 14.1 ± 6.9 |
| Morning PEF‡ (mean ± SD) | 511 ± 111 | 499 ± 80.1 |
| ICS dose (μg/day) | ||
| 500 | 8 | 13 |
| 750 | 1 | 6 |
| 1000 | 11 | 3 |
| 1500 | 4 | 5 |
| 2000 | 2 | 1 |
FAS, full analysis set; HDM, house dust mite; ICS, inhaled corticosteroid; PEF, peak expiratory flow; SCIT, subcutaneous immunotherapy. All patients were caucasians.
According to GINA, asthma severity is moderate when the ICS dose required for asthma control is >500 μg and ≤1000 μg fluticasone propionate/day.
According to GINA, asthma severity is severe when the ICS dose required for asthma control is >1000 μg fluticasone propionate/day.
Baseline PEF was measured during 4 weeks in January. FEV1 was <70% of predicted in all patients.
Bronchial hyperresponsiveness to inhaled methacholine and lung function
At baseline, 42% in the SCIT and 64% in the placebo group experienced moderate to severe BHR to inhaled methacholine (PD20 ≤ 1000 μg methacholine) despite receiving high doses of ICS (500–2000 μg) to control their asthma. PD20 increased from baseline to 1 year in both the SCIT and the placebo groups and stayed constant after 2 and 3 years. The change from baseline in log10(PD20) was similar between treatment groups after 1, 2 and 3 years (Fig. 1A).
Figure 1.
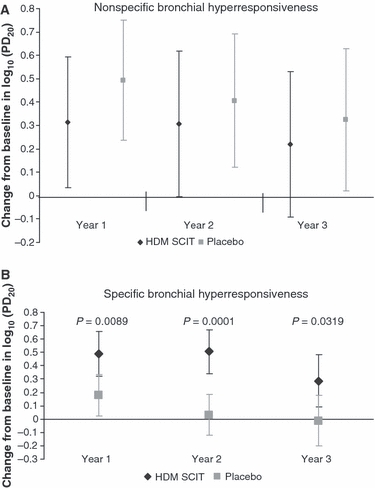
(A) The nonspecific bronchial hyperresponsiveness (BHR) in terms of change from baseline in log10(PD20); estimate and 95% confidence intervals with P-values for the difference between treatment groups. PD20 is the methacholine dose (μg) causing a 20% decline in FEV1. Test: anova with change from baseline as response variable, treatment as fixed effect and baseline as covariate. (B) The house dust mite (HDM)-allergen-specific BHR in terms of change from baseline in log10(PD20); estimate and 95% confidence intervals with P-values for the difference between treatment groups. PD20 is the HDM-allergen dose (SQ-U) causing a 20% decline in FEV1. Test: anova with change from baseline as response variable, treatment as fixed effect and baseline as covariate.
Morning and evening PEF was unchanged in both treatment groups after 1, 2 and 3 years (data not shown).
Bronchial hyperresponsiveness to inhaled HDM-allergen
At baseline, 44 patients (81%) had at least a 20% decline in FEV1 after HDM-allergen challenge despite receiving high doses of ICS (500–2000 μg) to control their asthma.
In the SCIT group, PD20 increased from baseline to 1 year [Δlog10(PD20): 0.49; 95% CI of (0.32; 0.66)], stayed constant after 2 years and the then slightly declined (Fig. 1B). This corresponded to an initial increase by a factor 1.6 from a median PD20 of 552 SQ-U to 857 SQ-U. PD20 slightly increased in the placebo group from baseline to 1 year [Δlog10(PD20): 0.18; 95% CI of (0.020; 0.33)] and then declined to baseline value (Fig. 1B).
The differences in change from baseline between groups were statistically significant, in favour of SCIT, for all 3 years (P = 0.0089; P = 0.0001; P = 0.0319) (Fig. 1B).
Late asthmatic reaction
At baseline, 28 patients (52%) experienced a LAR; 13 of these patients received SCIT and 15 received placebo. After 1 year of treatment, seven patients in the SCIT group [30%; 95% CI of (13; 53)] and 20 patients in the placebo group [77%; 95% CI of (56; 91)] experienced a LAR (Fig. 2; P = 0.0016 vs placebo). The percentage of patients with a LAR in the SCIT group was also lower than in the placebo group after 2 and 3 years (Fig. 3).
Figure 2.
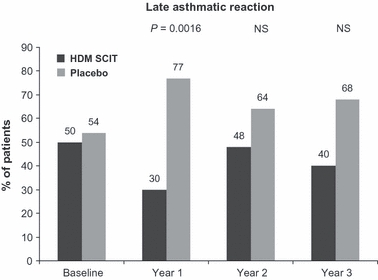
The percentage (%) of patients experiencing a late asthmatic reaction in terms of use of beta-2 agonist in each treatment group with P-values for the difference between treatment groups (Fischer’s exact test). NS, no statistical significance.
Figure 3.
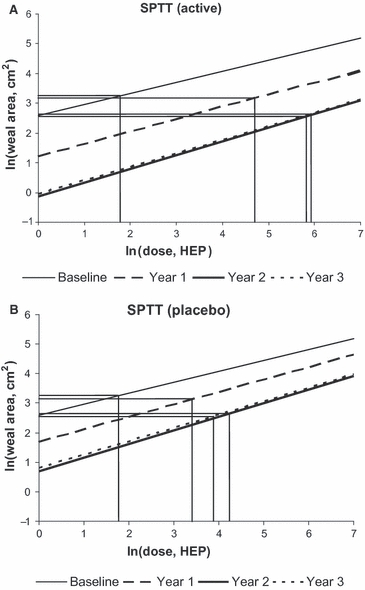
In the skin prick test titration, the house dust mite-allergen dose (in HEP) and skin reactions (weal area in cm2) were ln-transformed. A statistical parallel line regression model was applied. The histamine equivalent Dermatophagoides pteronyssinus allergen concentrations at baseline and after 1, 2, and 3 years of active treatment (A) and placebo treatment (B) are plotted. HEP, Histamine Equivalent in Prick.
Skin prick test titration
At baseline, the estimated HDM-allergen concentration that caused histamine equivalent skin reactions was similar between groups (Fig. 3). This concentration increased from 6 to 377 HEP in the SCIT group and from 6 to 48 HEP in the placebo group after 3 years of treatment (Fig. 3); the difference between groups was statistically significant for all 3 years (P < 0.0001).
Intradermal allergen challenge
At baseline, the immediate-phase skin reactions were similar between the two groups: a median weal area of 24 cm2 in SCIT vs 21 cm2 in placebo. After 1 year of SCIT, the reaction was reduced to a median weal area of 13 cm2, after 2 years to 10 cm2, which remained constant after 3 years of SCIT (11 cm2). This reduction was statistically significantly higher than in the placebo group after all 3 years of treatment (P < 0.04) (Fig. 4A).
Figure 4.
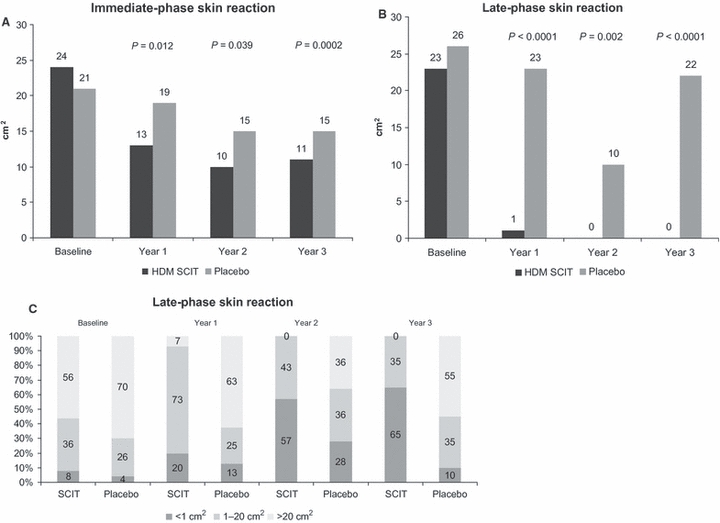
(A) Median areas (in cm2) of the immediate-phase skin reactions for each treatment group at baseline and after 1, 2 and 3 years of treatment with P-values for the difference between treatment groups (Wilcoxon Rank Sum Test). (B) Median areas (in cm2) of the late-phase skin reactions for each treatment group at baseline and after 1, 2 and 3 years of treatment with P-values for the difference between treatment groups (Wilcoxon Rank Sum Test). (C) The percentage (%) of patients without a late-phase skin reaction (weal area <1 cm2), the percentage of patients that experienced intermediate reactions (weal area 1–20 cm2) and the percentage of patients that experienced a severe reaction (weal area >20 cm2) are illustrated at baseline, and after 1, 2 and 3 years of treatment for both house dust mite (HDM) subcutaneous immunotherapy (SCIT) and placebo treatment groups.
At baseline, the late-phase skin reactions were similar between the two treatment groups: a median weal area of 23 cm2 in SCIT vs 26 cm2 in placebo. In the SCIT group, the late-phase skin reaction was reduced to 1 cm2 after 1 year and 0 cm2 (no reaction) after 2 and 3 years. As a contrast, this reaction was unchanged in the placebo group and statistically significantly different from that in the SCIT group after all 3 years (P < 0.002) (Fig. 4B).
In the SCIT group, the percentage of patients without a late-phase skin reaction increased from 8% at baseline to 65% after 3 years; none of the patients had a severe reaction (weal area >20 cm2) after 2 and 3 years (Fig. 4C). In contrast, the percentage of patients with a late-phase skin reaction in the placebo group was constant over 3 years and 55% experienced a severe reaction after 3 years (Fig. 4C).
Immunological response
In the SCIT group, the change from baseline to 1 year in serum specific IgE (D. pteronyssinus) [Δlog10(IgE): 0.048; 95% CI of (−0.017; 0.11)] was statistically significantly different from that in the placebo group [P = 0.028; Δlog10(IgE): −0.051; 95% CI of (−0.11; 0.0080)]. In the SCIT group, specific IgE declined to baseline value after 2 and 3 years, and the difference between treatment groups at year 2 and 3 was statistically insignificant.
In the SCIT group, the increase from baseline to 1 year in serum specific IgE-blocking factor (D. pteronyssinus) [ΔIgE-blocking factor: 0.31; (0.26; 0.37)] remained constant after 2 and 3 years (Fig. 5). There was no change from baseline in the placebo group and a statistically significant difference between treatment groups, in favour of SCIT, was found for all 3 years (P < 0.0001) (Fig. 5).
Figure 5.
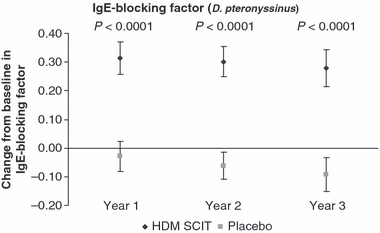
Change from baseline in IgE-blocking factor (Dermatophagoides pteronyssinus); estimate and 95% confidence intervals after 1, 2 and 3 years of treatment with P-values for the difference between treatment groups. IgE-blocking factor is a measure of the inhibitory capacity of competing components to specific IgE-allergen binding. Test: anova with change from baseline as response variable, treatment as fixed effect and baseline as covariate.
Discussion
Treatment with SIT induces immune tolerance to the allergen to which the patient is allergic and is the only treatment with the potential to alter the natural course of the disease (5, 6). Patients with HDM-allergic asthma were treated with HDM SCIT plus ICS or placebo plus ICS for 3 years. Each year, ICS was adjusted to the minimal dose that maintained the patient’s asthma in control and the efficacy and tolerability of SCIT was evaluated accordingly. We previously reported that SCIT reduced the ICS dose that maintained asthma in control and was well tolerated in HDM-allergic patients with moderate asthma (12).
We found that nonspecific BHR was reduced similarly in both groups after 1 year. Methacholine acts as direct stimuli of BHR by binding to specific receptors on the bronchial smooth muscle to cause constriction (19). Regular treatment with ICS is known to progressively reduce the patient’s sensitivity to this stimuli (20), and we found that SCIT fully replaced this effect over 3 years. However, nonspecific BHR was not fully reversed in any of the groups; persistent BHR has been found to correlate significantly with airway remodelling (21), which may be irreversible once established (22). Lung function was also unchanged in both groups after 3 years of treatment. Overall, supporting previous findings that HDM SCIT partly replaced ICS in providing asthma control (12).
Both immediate- and late-phase reactions to HDM allergens were markedly more reduced in SCIT plus ICS treated patients than in patients treated only with ICS. House dust mite-allergens act indirectly by inducing the allergic reaction causing BHR and skin reactions; an immediate IgE-mediated mast-cell-driven response within 15–30 min, and for some, a late-phase allergic inflammation 6–12 h after allergen exposure (19, 23). Measuring allergen-specific BHR and skin reactions to HDM-allergen is, therefore, a direct measure of allergen tolerance in lungs and skin that can indicate if treatment improves the underlying allergic disease manifested as asthma. In this trial, patients treated with SCIT tolerated a higher inhaled HDM-allergen dose when challenged (PD20 increased), fewer patients experienced a LAR to inhaled HDM-allergen challenge, the histamine equivalent concentration was higher at SPTT and the immediate-phase skin reaction was reduced. The late-phase skin reactions were strikingly diminished or abolished in the SCIT group and none of these patients experienced a severe late-phase skin reaction after only 2 years of treatment. Conclusively, HDM SCIT provided improved tolerability to HDM allergens in skin and lungs.
After SCIT, the suppression of early reactions in skin has been found associated with reduction in mast-cell numbers (23–25), and suppression of late-phase skin reaction has been found associated with reduction in the number of infiltrating T cells, eosinophils, basophils and neutrophils and inflammatory mediators (23, 26). Thus, the increased allergen tolerability observed in this trial is probably because of a reduced allergic inflammation as a consequence to SCIT improving the underlying allergic disease.
Allergen-specific IgE is considered a central player in the allergic reaction (27) and is increased in serum of patients with allergy (28). In this trial, SCIT also provided a change in the humoral immune response, which was absent in patients treated only with ICS; an initial increase in serum HDM-allergen-specific IgE antibodies followed by a decline and a marked increase in the effect of components blocking HDM-allergen-specific IgE function. The precise mechanism by which SIT acts remains unclear; however, consistent with the findings in this trial, an effect on IL-10 and TGF-β secreting regulatory T cells (Treg) associated with switching of allergen-specific B-cells towards IgG4 production and suppression of allergen-specific IgE production is the most probable mechanism (23, 25, 29). This was also observed by others (30, 31), and probably reduce the immediate IgE-mediated mast-cell-driven allergic response and thereby also reduce the release of inflammatory mediators that induce the late-phase response, in this trial observed as reduced immediate- and late-phase reactions in lung and skin.
Treg may also directly inhibit the activation of allergen-specific Th2 cells, thereby reducing the production of Th2-cytokines and their multiple effects on cells involved in the allergic response (23). Chen et al. demonstrated that 1 year of SQ-standardized HDM SCIT significantly decreased the serum level of the Th2-cytokine IL-13 (involved in the pathogenesis of airway remodelling) more than did ICS in children with asthma (32). At the same time, the serum level of IL-4 (a Th2-cytokine) decreased and the serum level of IFN-γ (a Th1-cytokine) increased (32). This shift from an allergic ‘Th2 cell predominance’ to a more normal ‘type 1 Treg cell predominance’ immune response to allergen that also involves a shift in the balance of Th2 and Th1 cytokine expression (6, 23, 25, 29) is also observed in allergic rhinitis after SIT, consistent with the idea of a one-airway-one-disease theory (33–35). Therefore, the prevention of disease progression (8, 9) and sustained effect (8, 10, 11) observed in patients with allergic rhinitis after SIT may also be expected treatment outcomes in patients with allergic asthma.
Because of the added benefit over ICS, we recommend that SIT is considered as first-hand medication when treating patients with HDM-allergic asthma. Because of current knowledge and current guidelines on asthma treatment (GINA) (1), initial treatment is suggested to consist of SIT combined with controller medication in terms of inhaled ICS and reliever medication as needed. This trial indicates that over time, SIT will improve the underlying allergic disease in the majority of patients with asthma; the ICS dose can be down-titrated accordingly. For some patients with asthma, SCIT may ultimately become sufficient to control their asthma in combination with reliever medication.
In conclusion, SQ-standardized HDM SCIT induced a consistent immunomodulatory effect in adults with HDM-allergic asthma in terms of a change in the humoral immune response and as increased HDM-allergen tolerance in the lungs and skin of these patients. This strongly indicates that HDM SCIT treats the underlying allergic disease; sustained effect and prevention of disease progression may be expected treatment outcomes for the patient with HDM-allergic asthma.
Acknowledgments
We thank all patients who participated in this trial. We further thank study nurses Jytte Moeller, Ingrid Grothe and Dorte Kristensen for the practical assistance in provocation tests. We also thank Janne Hartmann, Helene Henmar and the Diagnosis Laboratory, ALK, Stenløse, Denmark. Medical writer Christina El-Naaman Gregersen assisted in the drafting of this article. ALK-Abello A/S was the sponsor of the trial.
References
- 1.GINA Executive Committee. Global initiative for asthma; global strategy for asthma management and prevention. Bethesda, Maryland, USA: National Heart, Lung and Blood Institute, National Institute of Health; 2009. [Google Scholar]
- 2.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6:301–305. doi: 10.1513/pats.200808-089RM. [DOI] [PubMed] [Google Scholar]
- 3.Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100(6 Pt 1):S2–S24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 4.Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Curr Opin Allergy Clin Immunol. 2009;9:128–135. doi: 10.1097/aci.0b013e32832678b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousquet J, Lockey RF, Malling HJ. WHO position paper. Allergen immunotherapy: therapeutic vaccines for allergic diseases. Allergy. 1998;53:1–42. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 6.Akdis M. Immune tolerance in allergy. Curr Opin Immunol. 2009;21:1–8. doi: 10.1016/j.coi.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Abramson M, Puy R, Weiner J. Immunotherapy in asthma: an updated systematic review, 2006. Allergy. 2006;54:1022–1041. doi: 10.1034/j.1398-9995.1999.00102.x. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen L, Valovirta E. How strong is the evidence that immunotherapy in children prevents the progression of allergy and asthma? Curr Opin Allergy Clin Immunol. 2007;7:556–560. doi: 10.1097/ACI.0b013e3282f1d67e. [DOI] [PubMed] [Google Scholar]
- 10.Durham SR, Emminger W, Kapp A, Colombo G, De Monchy JG, Rak S, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125:131–138. doi: 10.1016/j.jaci.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 11.Des RA, Paradis L, Knani J, Hejjaoui A, Dhivert H, Chanez P, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. V. Duration of the efficacy of immunotherapy after its cessation. Allergy. 1996;51:430–433. doi: 10.1111/j.1398-9995.1996.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 12.Blumberga G, Groes L, Haugaard L, Dahl R. Steroid-sparing effect of subcutaneous SQ-standardised specific immunotherapy in moderate and severe house dust mite allergic asthmatics. Allergy. 2006;61:843–848. doi: 10.1111/j.1398-9995.2006.01088.x. [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association. The Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects First adopted in 1964 (Helsinki, Finland) and revised in 1975 (Tokyo, Japan), 1983 (Venice, Italy), 1989 (Hong Kong), 1996 (Somerset-West, South Africa) and 2000 (Edinburgh, Scotland). Note of clarification, 2002 (Washington). 1996 Oct.
- 14.Nieminen MM, Lahdensuo A, Kellomaeki L, Karvonen J, Muittari A. Methacholine bronchial challenge using a dosimeter with controlled tidal breathing. Thorax. 1988;43:896–900. doi: 10.1136/thx.43.11.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Report of the working party of the European Community for Coal and Steel: “Standardization of Lung Function Tests”. Bull Europ Physiopath Resp. 1983;19(Suppl. 5):1–95. [PubMed] [Google Scholar]
- 16.Poulsen LK, Liisberg C, Bindslev-Jensen C, Malling HJ. Precise area determination of skin-prick tests: validation of a scanning device and software for a personal computer. Clin Exp Allergy. 1993;23:61–68. doi: 10.1111/j.1365-2222.1993.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 17.Bufe A, Eberle P, Franke-Beckmann E, Funck J, Kimmig M, Klimek L, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–173. doi: 10.1016/j.jaci.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–1194. [PubMed] [Google Scholar]
- 19.O’Byrne PM, Gauvreau GM, Brannan JD. Provoked models of asthma: what have we learnt? Clin Exp Allergy. 2009;39:181–192. doi: 10.1111/j.1365-2222.2008.03172.x. [DOI] [PubMed] [Google Scholar]
- 20.Ward C, Pais M, Bish R, Reid D, Feltis B, Johns D, et al. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002;57:309–316. doi: 10.1136/thorax.57.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covar RA. Bronchoprovocation testing in asthma. Immunol Allergy Clin North Am. 2007;27:633–649. doi: 10.1016/j.iac.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd CM, Robinson DS. Allergen-induced airway remodelling. Eur Respir J. 2007;29:1020–1032. doi: 10.1183/09031936.00150305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James LK, Durham SR. Update on mechanisms of allergen injection immunotherapy. Clin Exp Allergy. 2008;38:1074–1088. doi: 10.1111/j.1365-2222.2008.02976.x. [DOI] [PubMed] [Google Scholar]
- 24.Durham SR, Varney VA, Gaga M, Jacobson MR, Varga EM, Frew AJ, et al. Grass pollen immunotherapy decreases the number of mast cells in the skin. Clin Exp Allergy. 1999;29:1490–1496. doi: 10.1046/j.1365-2222.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- 25.Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of T regulatory cells in immune regulation of allergic diseases. Eur J Immunol. 2010;40:1232–1240. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- 26.Varney VA, Hamid QA, Gaga M, Ying S, Jacobson M, Frew AJ, et al. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J Clin Invest. 1993;92:644–651. doi: 10.1172/JCI116633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 28.Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402(6760 Suppl.):B18–B23. doi: 10.1038/35037014. [DOI] [PubMed] [Google Scholar]
- 29.Frew AJ. Allergen immunotherapy. J Allergy Clin Immunol. 2010;125(2 Suppl. 2):S306–S313. doi: 10.1016/j.jaci.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 30.Malling H-J, Lund L, Ipsen H, Poulsen LK. Safety and immunological changes during specific sublingual immunotherapy with SQ standardized grass allergen tablets. Journal Investig Allergol Clin Immunol. 2006;16:162–168. [PubMed] [Google Scholar]
- 31.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 32.Chen ZG, Li M, Chen YF, Ji JZ, Li YT, Chen W, et al. Effects of Dermatophagoides pteronyssinus allergen-specific immunotherapy on the serum interleukin-13 and pulmonary functions in asthmatic children. Chin Med J (Engl) 2009;122:1157–1161. [PubMed] [Google Scholar]
- 33.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl. 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 34.Grossman J. One airway, one disease. Chest. 1997;111(2 Suppl.):11S–16S. doi: 10.1378/chest.111.2_supplement.11s. [DOI] [PubMed] [Google Scholar]
- 35.Cruz AA, Popov T, Pawankar R, Annesi-Maesano I, Fokkens W, Kemp J, et al. Common characteristics of upper and lower airways in rhinitis and asthma: ARIA update, in collaboration with GA(2)LEN. Allergy. 2007;62(Suppl. 84):1–41. doi: 10.1111/j.1398-9995.2007.01551.x. [DOI] [PubMed] [Google Scholar]


